Abstract
Stiffness-damping balance, high specific mechanical performance, and corrosion resistance are demanded for metals. Here, we developed an enamel-inspired ceramic (EIC) coating strategy involving hydrothermal growth of a tooth enamel-like ZrO₂ nanorods on Zr foil and amorphous ZrO₂ intergranular phase formation via controlled hydrolysis. The EIC coated Zr foil (Zr-EIC) achieved a high viscoelastic figure of merit (4.6 GPa); as well as excellent specific stiffness and hardness owing to the lightweight of ceramics, surpassing the values of Zr and previously reported Zr-based alloys or Zr-based composites. This was due to the vertically oriented stiff nanorods and unique energy dissipation derived from deformation at the crystalline/amorphous interfaces. It demonstrated exceptional NaCl corrosion resistance. This approach showed universality across Ti, Zn, and Cu substrates, enhancing both mechanical properties and corrosion resistance. By constructing an enamel-like hierarchical structure on the metal surface, the strategy overcomes traditional metal limitations without causing an increase in density.
Similar content being viewed by others
Introduction
As widely used engineering materials, metals suffer from certain mechanical inherent trade-offs similar to other engineering materials, including stiffness and damping1,2; they also display relatively low specific mechanical performance compared to ceramics and some biomaterials owing to its relatively high density, such as stiffness and hardness1,3,4. The vulnerability of metals to corrosion also limits their long-term applications, especially in marine environments. Various strategies have been used to address these issues, including the development of specific conventional alloys and high-entropy alloys5,6, three-dimensional (3D) printed architectures7, metal/ceramic composites8, and coating modified metals9. However, these strategies invariably contain compromises, such as an increase in stiffness and strength, but at the expense of damping capacity and toughness, with no net improvement in the overall mechanical properties of the corrosion resistance of the metals10,11,12.
Living organisms have evolved biomaterials with excellent mechanical properties and high stability that often outperform artificial materials. One of the most obvious examples is tooth enamel, which is the outermost layer of teeth. With a thickness of several millimeters, it is composed of 96 wt.% hydroxyapatite (HA) ceramic columns, with amorphous calcium phosphate (ACP) served as amorphous intergranular phases (AIP) in a parallel arrangement in the polymeric matrix. This unusual material combines high hardness and stiffness with substantial damping, and offers outstanding mechanical stability over long periods of time (e.g., over 60 years in the oral cavity)13,14,15. The array structure, composed of regular nanorods and a crystal/amorphous interface, is responsible for the exceptional mechanical performance, despite the fact that it is a nominally brittle material14,16,17,18,19. Strong support provided by the regular arrangement of ceramic nanorods and the mechanical dissipation provided by the crystal/amorphous interface slip are the main reasons for toughening at the outer tooth enamel from micrometer-scale to nanoscale. Some columnar morphologies have been observed in ceramic coatings to improve hardness and thermal insulation of superalloy by physical methods (e.g., electron-beam physical-vapor deposited and pulsed laser deposition)20,21. However, the nanoscale defect, the gap between columnar structure and limitations of the substrate limit the general application of this method and the improvement of mechanical properties. A similar phenomenon can also be observed in ceramic layer-coated titanium implants22. Furthermore, the amorphous intergranular phase with distinctive mechanical dissipative capabilities has yet to be incorporated into the columnar structure, and the method of chemical synthesis in situ design of fine ceramic structures through enamel-inspired concepts on metal surface represents a noteworthy innovation in enhancing the mechanical properties of metals.
Zirconium (Zr) metal is a typical engineering metal which widely used in extreme environments (e.g., high-pressure steam, random vibrations, mechanical impact, and nuclear radiation) due to its high structural stability, relatively good corrosion resistance, and low absorption cross-section for thermal neutrons23,24. Nevertheless, Zr suffers from the inherent trade-off between such properties as stiffness and damping capacity, as well as a vulnerability to corrosion that can be highly detrimental to their durability, particularly in an environment containing corrosive substances. In this study, we successfully coated an enamel-inspired ceramic (EIC) layer on the surface of Zr foil through the in situ growth of zirconia (ZrO2) nanograss on a Zr foil surface to mimic the one-dimensional ordered arrangement of enamel; the amorphous ZrO2 ceramics were subsequently filled into the gaps of the nanograss by controlled hydrolysis processes of metal ions and low-temperature calcination to mimic the amorphous intergranular phases of enamel, forming the EIC coated Zr (Zr-EIC). This ceramic- coated metal shows high stiffness (121.3 GPa), specific modulus (19.4 GPa/(g/cm3)), specific hardness (0.85 GPa/(g/cm3)), and damping (0.038), as well as excellent corrosion resistant (0.015 mg cm−2.day−1). In particular, Zr-EIC exhibits the highest viscoelasticity (4.6 GPa) compared with any metal at room temperature. The EIC layer can not only provide enough mechanical support, but also generating large energy dissipation and preventing the permeation of corrosion ions, contributing to these mechanical and corrosion-resistant improvements. In addition, we have also successfully constructed the enamel-inspired ceramic layer on other metals (Ti, Zn, and Cu) to obtain better mechanical performance and corrosion resistance compared with the corresponding metals, demonstrating the universality of our enamel-inspired strategy for metal reinforcement. We expect that the proposed enamel-inspired structure and phase control design strategy has significant potential for the development of high-performance metallic materials with high stiffness, high damping, and enhanced corrosion resistance.
Results and discussion
Construction of the tooth enamel-inspired ceramic coating
We developed a simple wet-chemical method including one-step hydrothermal, controlled hydrolysis and low temperature calcination to synthesize a high stiff, damping, hard, and corrosion resistant Zr-EIC via the in situ growth of a thin enamel-inspired ceramic layer on the surface of Zr foil (Fig. 1a). Firstly, the ZrO2 nanograss were in situ grown on surface of Zr foil to mimic the array structure of enamel through hydrothermal method. The growth of ZrO2 nanograss has been hardly realized before owing to the isotropic growth tendency of ZrO225. We constructed a strong basicity reaction system for growing one-dimensional ZrO2. The successful synthesis of ZrO2 nanograss was attributed to the formation of an amorphous ZrO2 (A-ZrO2) thin layer under strong basicity at the initial oxidation stage (Supplementary Fig. 1a–c and Supplementary Figs. 2–5), and subsequently local crystallization with oxidation continue. This local crystallization region can serve as the starting point of the growth of crystalline ZrO2, while the surrounding amorphous hinder the growth of crystalline ZrO2, leading to the 1D growth of ZrO2 (Supplementary Fig. 1d–f and Supplementary Fig. 6) and finally forming the C-ZrO2 nanograss (ZrNG) on Zr foil (Zr-ZrNG) surface after 24 h of hydrothermal reaction (Supplementary Fig. 1g and Supplementary Figs. 4–6). The Zr foil was smooth and flat (Supplementary Fig. 7), and the height of the ZrO2 nanograss ranged was ~ 100 nm, while the diameter of the ZrO2 nanograss was ~ 25 nm (Fig. 1b and Supplementary Fig. 8).
a Schematics illustrating the processes by which a Zr-EIC structure is fabricated, including the bare Zr foil, the formation of crystalline ZrO2 (C-ZrO2) nanorod arrays as ZrO2 nanograss (Zr-ZrNG) structures, the formation of an amorphous ZrO2 (A-ZrO2) intergranular phase filling between the Zr-ZrNG structures. b Structural characterization of Zr-EIC. c Cross-sectional transmission electron microscopy (TEM) and High-resolution TEM (HRTEM) images of the tooth enamel. d Cross-sectional TEM and HRTEM images of the Zr-EIC. e The results of uniaxial tensile testing, where the images in the insets present examples of bent and twisted Zr-EIC samples.
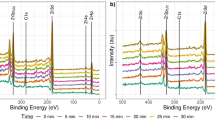
To mimic the crystalline/amorphous interface of natural tooth enamel, the gaps between ZrNG were filled with A-ZrO2 through the controllable hydrolysis of Zr ions. This involved continuous chemical reaction with metal ion absorption, nucleation, and growth in a low-temperature buffer system. After controllable annealing, the EIC layer formed. As shown in Fig. 1b, the entire enamel-inspired coating has no cracks on a large scale, and oxygen was evenly distributed near the Zr-EIC surface. At the same time, it can be seen from the Fig. 1b that the surface roughness of the ceramic coating (~ 10.0 nm) is slightly higher than that of the Zr foil (~ 3.2 nm), indicating that the A-ZrO2 can be deposited into the gap of the ZrNG. In order to support the scalability of our approach, the Raman mapping was also carried out, which revealed that the signal of the samples remains stable throughout the entire test range, with the exception of a few test points (Supplementary Fig. 9). This evidence corroborates the uniformity of the EIC layer on the Zr foil surface. Furthermore, we have compared the structure of Zr-ZrNG with Zr-EIC, founding that the structure retained almost the same before and after amorphous phase infiltration (Supplementary Fig. 8 and the Fig. 1b). The thickness of the Zr-EIC layers was slightly greater than the heights of the ZrO2 nanograss (~ 105 nm), and the oriented nanorod structure exhibited completely filled gaps (Fig. 1b). High-resolution transmission electron microscopy (HRTEM) confirmed that these oriented nanorods were crystalline with an amorphous filler, resulting in a closely connected crystalline/amorphous interface, which is similar to tooth enamel (Fig. 1c, d). Strong chemical interactions between the A-ZrO2 and C-ZrO2 components of the EIC layer were also observed by X-ray photoelectron spectroscopy (XPS), with the results shown in Supplementary Fig. 10. For the Zr-ZrNG, the peaks observed at 181.68 and 183.78 eV correspond to Zr 3d5/2 and Zr 3d3/2. For the A-ZrO2, the peaks observed at 182.18 and 184.58 eV correspond to Zr 3 d5/2 and Zr 3d3/2, which exhibit a shift towards the higher binding energy in comparison to that of crystal ZrO2 in the Zr-ZrNG, indicating Zr exhibits a lower oxidation state26,27. Notably, the Zr 3d spectrum of Zr-EIC shifts toward the lower binding energy compared to that of A-ZrO2, indicating a charge transfer and strong chemical interaction caused by electronic interaction28.
In addition, the uniaxial tensile test results (Fig. 1e) demonstrated that, compared to the properties of the Zr foil, the tensile strength of Zr-EIC increased by ~ 17.9%, while the fracture strain decreased by only ~ 1.9%. This phenomenon can be attributed to the combination of stiff and damping of EIC and the small thickness of EIC, which is also an advantage over traditional ceramic coating strengthening strategies29, that is, the ductility of metals significantly decreasing. Accordingly, the proposed enamel-inspired strategy retained the desirable characteristics of metals, and the satisfactory bending of the Zr-EIC samples, along with their surprising metallic luster (Fig. 1e).
Mechanical performance and enhancement mechanisms
We performed the dynamic mechanical analysis test on the synthetic Zr-EIC to evaluate its energy dissipation ability. Taking the engineering application into consideration, the storage modulus (Eʹ) of the Zr-EIC was measured by dynamic mechanical analysis, according to nanoindentation tests conducted at contact depths of 50–350 nm, to ensure that the nanoindentation process encompassed both the EIC layer and the underlying metallic substrate material; results are presented in Fig. 2a, Supplementary Fig. 11, and Supplementary Table 1. The Eʹ of the Zr-EIC (121.3 GPa) was 1.3 times higher than that of the pure Zr (90.3 GPa). The damping capacity was also assessed in terms of damping coefficient (tanδ), where δ is the phase angle between the stress and strain11. As shown in Fig. 2a, the mean values of tanδ obtained for the Zr-EIC (0.038) was slightly larger than for the Zr foil (0.034). In addition, the degree of viscoelasticity of a material can be typically analyzed according to the product of Eʹ and tanδ, which can be defined as the viscoelastic figure of merit (VFOM). The Zr-EIC has a very high VFOM value of 4.6 GPa, which was the highest value for any metal at room temperature and approximately 7.6 times higher than any other traditional engineering material at room temperature (0.6 GPa)14,30 (Supplementary Fig. 12 and Supplementary Table 2). Meanwhile, we also conducted a macro-DMA test to evaluate the dynamic mechanical behavior of the Zr foil and Zr-EIC, and the results showed that, regardless of the test method used, the enamel-inspired ceramic coating had a similar effect on the mechanical properties of the metal (1.7 times for macro-scale DMA vs 1.48 times for nano-DMA), thereby substantiating the efficacy of our strategy (Supplementary Fig. 13). Hence, this proposed strategy demonstrated a simultaneous increase in both stiffness and damping capacity of the Zr, rather than the usual trade-off between these two properties, with existing reinforcement strategies (alloying, compounding and coating) difficult to achieve30,31,32,33,34,35 (Fig. 2b, Supplementary Fig. 12 and Supplementary Table 2).
a Radar plots of the overall mechanical properties of Zr foil, Zr-EIC and its contrast samples. b Comparison diagram of the storage modulus versus tanδ for the various Zr-based alloys, Zr-based metal composites, ceramic coated metals and samples in this work. c Comparison diagram of the specific modulus versus specific hardness for the various Zr-based alloys, ceramic coated Zr metals, ceramic coated metals and samples in this work.
Hardness indicates a material’s ability to resist penetration by a hard indenter36. The Young’s modulus (EY) and hardness (H) values obtained for the Zr-EIC and Zr foil, as evaluated by quasi-static nanoindentation tests, are shown in Fig. 2a and Supplementary Fig. 14. The results demonstrated that the introduction of EIC layers significantly improved the EY and H of the Zr foil (Fig. 2a and Supplementary Table 1). The specific modulus values reported for various Zr-based alloys, ceramic coated Zr metals, ceramic coated metals, and the Zr-EIC are compared in Fig. 2c and Supplementary Table 3, relative to their specific hardness22,37,38,39,40,41,42,43,44. Although EY and H generally increased with increasing density (ρ) for most engineering metals and composites, the Zr-EIC prepared in this study exhibited a higher specific modulus and specific hardness than that of the Zr-based alloys and ceramic coated Zr metals. The specific modulus values of the Zr foils increased, with its values increasing from 15.0 GPa/(g/cm3) to 19.4 GPa/(g/cm3). In parallel, the specific hardness values of the Zr foils increased from 0.55 GPa/(g/cm3) to 0.85 GPa/(g/cm3), verifying the advantages of our EIC coating strategy for mechanical reinforcement through a relatively lightweight route.
To further illustrate the advantages of the proposed enamel-inspired strategy, the Zr foil with ceramic layers of different structures are obtained by different treatment methods, including (i) Zr foil coated with a ZrO2 nanograss structure (Zr-ZrNG), (ii) Zr foil coated crystalline ZrO2 (C-ZrO2) nanoparticles containing amorphous ZrO2 (A-ZrO2) filler (Zr-AZ/CZ) (Supplementary Fig. 15), and (iii) Zr foil clad with magnetron-sputtered C-ZrO2 nanoparticles (Zr-CZ) (Supplementary Fig. 16). The comparative samples all exhibited a coating thickness of 100-150 nm, similar to the Zr-EIC samples. As a result, the Zr-ZrNG, Zr-AZ/CZ, and Zr-CZ contained C-ZrO2 on the Zr foil surface; however, C-ZrO2 was not present in the form of nanorods in the Zr-AZ/CZ and Zr-CZ, and Zr-ZrNG did not include an intergranular A-ZrO2 phase. Specifically, the Eʹ and tanδ values for the Zr foil and different ceramic layer coated Zr foil samples obtained at contact depths of 50 to 350 nm are shown in Supplementary Fig. 17a, b, with the mean values of the samples compared in Supplementary Fig. 17c. The ZrNG structure and the good contact provided by the intergranular A-ZrO2 phase in the Zr-EIC resulted in a significantly higher mean damping coefficient than the Zr-ZrNG (0.015 ± 0.002), Zr-AZ/CZ (0.014 ± 0.004), and Zr-CZ (0.017 ± 0.002) samples, along with outstanding material stiffness. The importance of the intergranular A-ZrO2 phase on the damping capacity of the material was particularly evident from the results obtained for the Zr-ZrNG. The mean values of EY and H for Zr-EIC were also superior to those obtained for the Zr-ZrNG, Zr-AZ/CZ, and Zr-CZ (Supplementary Fig. 18), with the results indicating that the proposed coating strategy could improve the mechanical performance of the entire metal sample. The overall mechanical performance of this Zr-EIC, relative to the Zr-ZrNG, Zr-AZ/CZ, Zr-CZ, and Zr foils, can be appreciated by the radar plots shown in Fig. 2a, which summarize the five different mechanical parameters measured for these materials. The results showed that the overall performance of this Zr-EIC surpassed all other samples considered (Supplementary Table 4). The Zr-EIC also demonstrated good mechanical stability (Supplementary Fig. 19), with the 100 nm EIC layer protecting the interior of the metal during impact (Supplementary Fig. 20). In summary, the introduction of EIC layer on metal surface can simultaneously enhance the macro and micro mechanical properties of metals, which is closely related to the microstructure of EIC layer, which comprises a parallel nanorods and amorphous intergranular phase, and the microscale mechanical test is particularly advantageous for elucidating the underlying mechanical dissipation mechanism.
The mechanisms responsible for the superior damping capacity of the proposed EIC-coated metal with a high-stiff and high-hardness ceramic coating could be analyzed by the permanently deformed region of the Zr-EIC after nanoindentation, with a Berkovich indenter at a load of 10,000 μN, as shown in Fig. 3a–g. A top scanning electron microscope (SEM) view of the indented region (Supplementary Fig. 21a) demonstrated that the indentation generated a permanently deformed region with a pyramidal morphology, resulting from the Berkovich indenter. Furthermore, the enlarged SEM image (Supplementary Fig. 21b) obtained from the permanently deformed region of the Zr-EIC surface exhibited zig-zag-like cracks that propagated through the deformed regions. Upwarp at the edge of the indentation zone (Supplementary Fig. 21c) also dissipated energy.
a Illustration of the deformed region obtained after nanoindentation with a Berkovich indenter at a load of 10,000 μN. b Cross-sectional TEM image of the permanently deformed region of the Zr-EIC. c Enlarged TEM image of the region marked (c) in (b). d Enlarged TEM image of the region marked (d) in (c). Inset: Fast Fourier Transform (FFT) pattern of the amorphous area from the HRTEM image. e Enlarged TEM image of the region marked (e) in (c). Inset: FFT pattern of the amorphous area from the HRTEM image. f Enlarged TEM image of the region marked (f) in (b). g Mechanical behavior of a Zr-EIC associated with three distinct energy dissipation mechanisms, with the yellow spheres referring to the amorphous region and the blue spheres referring to the crystalline region. Meanwhile, (II)-1 indicates that the C-ZrO2 nanorods undergo lattice distortion, crack propagation and amorphization, while (II)−2 indicates that the C-ZrO2 nanorods break.
The mechanical behavior of the Zr-EIC in the permanently deformed region was also evaluated by cross-sectional TEM imaging, as shown in Fig. 3b–f, and the proposed energy dissipation mechanism was illustrated in Fig. 3g. Crack propagation, without catastrophic failure, was found in the edge of the deformation zone (relatively low indentation stress) after indentation (Fig. 3c), which indicated sliding at the interfaces between the amorphous filler and crystalline nanorods (Fig. 3g(I) and Supplementary Fig. 22a). This resulted in minimal damage due to the relatively high viscoelasticity of the amorphous material45. Increasing the external force (closer to the corner of the deformation zone) resulted in the bending of the C-ZrO2 nanorods, especially under the induction of A-ZrO2, lattice distortion, crack deflection, amorphization (Fig. 3d, e, g(II)−1) and even fracture (Fig. 3e, g(II)−2) can occur inside the nanorod (Supplementary Fig. 22b). Fast Fourier transform (FFT) further confirmed that the internal structure of the crystalline ZrO2 nanorods undergoes amorphization, which is considered an efficient energy-dissipation mechanism similar to those in synthetic and natural ceramics46,47. Furthermore, close-knit interfacial interaction between the amorphous and crystalline ZrO2 structures formed bundles from the adjacent parallel nanorods (Fig. 3f), which served to dissipate energy by confining the fractured nanorods, avoiding collapse and crushing (Fig. 3g(III) and Supplementary Fig. 22c). The simultaneous high stiffness and damping capacity of the Zr-EIC came from the strong support effect of the EIC layer with vertically aligned nanorods, as well as the unique mechanical behavior under load to dissipate energy. In addition, no layering delamination of the coated EIC layer was observed, as indicated by the orange dashed line in Fig. 3f. Meanwhile, by conducting the tensile experiment of adhesion to examine the interfacial connection between the EIC layer and the Zr foil (Supplementary Fig. 23), it can be discovered that in situ grown EIC layer (Zr-EIC, 427.5 N) has a higher delamination force than ex situ sprayed ceramic layer by magnetron sputtering (Zr-CZ, 418.4 N). In addition, the EIC coating could endure larger sliding displacement before be fully peeled out from the metal substrate than that of Zr-CZ, further verifying the strong interfacial connection generated by the in situ grown strategy. This suggested that the EIC layers that grew in situ were well bonded to the Zr substrate, and this good connection was further demonstrated by the scratch test results obtained for the Zr-EIC (Supplementary Fig. 24), which is important for the potential application durability of Zr-EIC. Finally, the cross-sectional TEM images indicating the permanently deformed region of the Zr-EIC in the Zr foil under the EIC layer are shown in Supplementary Fig. 25. These images indicated that energy dissipated through nano-crystallization in the ZrO2 layer and metallic Zr matrix; no catastrophic disruption was observed in these layers, further verifying the mechanical enhancement generated by the EIC layer.
For comparison, Supplementary Fig. 26 presents the mechanical behavior determined from the permanent deformation region of human tooth enamel and reference materials under the same nanoindentation test. Tooth enamel exhibited similar energy dissipation behavior as the observed Zr-EIC, with zig-zag-like cracks and upwarps at the edge (Supplementary Fig. 26a). By contrast, the region in pure Zr foil subjected to nanoindentation (Supplementary Figs. 26b and 27) exhibited only plastic deformation with no cracking, while the Zr-ZrNG, Zr-AZ/CZ, and Zr-CZ exhibited brittle mechanical dissipation behavior (Supplementary Fig. 26c–e). This further demonstrated the advantages of the hierarchical tooth enamel-inspired structure.
Corrosion resistance
A neutral salt spray test was performed using a salt spray composed of a 5 ± 1 wt.% NaCl solution on the Zr-EIC at 24 °C to obtain the corrosion resistance of our samples. As shown in Supplementary Fig. 28, the surface of the pure Zr foil was badly corroded after exposure to salt spray for 15 days (Supplementary Fig. 28a–c), but the Zr-EIC retained its original integrity under the same corrosion process (Supplementary Fig. 28d–f). This excellent corrosion resistance of the Zr-EIC composite was further demonstrated by its outstanding EY and H retained after having undergone a salt spray test for 15 days, while the values of EY and H obtained for the Zr foil, respectively, decreased by 18% and 36% (Supplementary Fig. 28g, h). Furthermore, an average mass loss rate of only 0.002 mg cm−2 day−1 was observed for Zr-EIC, which is some seven times lower than that observed for the Zr foil (0.015 mg cm−2 day−1) (Supplementary Fig. 29).
To better understanding the corrosion resistance of Zr-EIC for a long-time service period, we carried out electrochemical corrosion (EC) testing as an accelerated corrosion test, employing a three-electrode system using 3.5 wt.% NaCl solution as simulated seawater environment48. The surface morphologies of the Zr foil and Zr-EIC obtained under electrochemical corrosion tests were also observed in the optical and SEM images, as shown in Fig. 4a. Characteristics of typical pitting attack were observed on the surface of the Zr foil, while the Zr-EIC retained a pitting-free surface under the same conditions. The most plausible mechanisms proposed for the good corrosion resistance of the Zr-EIC are shown in Fig. 4b; these can be described as follows: (i) The EIC layer and ZrO2 thin layer resisted infiltration by corrosive substances due to the inherent stability of the ceramics49, and (ii) the long-range disordered structure of the A-ZrO2 filler resisted infiltration by corrosive substances. The latter was of particular importance because corrosive substances generally cannot reach the interior of metals unless they follow a zig-zag-like infiltration pathway through the amorphous phase. Therefore, the underlying metal was protected by long-distance and complex infiltration pathways that could trap corrosive substances and inhibit the conversion of metals into Zr-complexes.
a Schematic illustration of EC test and optical and scanning electron microscope (SEM) images of the Zr foil and Zr-EIC before and after the EC tests. b Proposed corrosive substances infiltration pathways for the Zr-EIC. c Zr-L2,3 electron energy loss spectroscopy results of Zr and Zr-EIC obtained from the different regions shown in the high-angle annular dark field scanning transmission electron microscopy image (left), with the white dotted frames referring to the C-ZrO2 nanorods and the gaps between the frames referring to the amorphous A-ZrO2 filler.
To explore the proposed corrosion resistance mechanism, Zr foil and Zr-EIC samples obtained under electrochemical corrosion were further analyzed by high-angle annular dark field scanning TEM (HAADF-STEM), energy dispersive spectroscopy (EDS) mapping (Supplementary Figs. 30 and 31), and Zr-L2,3 electron energy loss spectroscopy (EELS) (Fig. 4c). The results were used to characterize the infiltration pathways of the corrosive Cl− ions in the corroded samples. The Cl− ions were found to preferentially infiltrate from the interface between the A-ZrO2 filler and C-ZrO2 nanorods (Fig. 4b and Supplementary Fig. 31), i.e., along the C-ZrO2/A-ZrO2 interface, which was apparent from the concentrated distribution of Cl−, followed by infiltration from the C-ZrO2 nanorods. Concomitantly, the A-ZrO2 filler exhibited strong resistance to the diffusion of Cl− ions (Supplementary Fig. 31), and these results were consistent with previous theoretical predictions50. In addition, as shown in Fig. 4c, the characteristic Zr-L2,3 EELS peaks of the corroded Zr-EIC sample moved toward higher binding energies, because metallic Zr lost some extranuclear electrons and transformed to Zr cations (Zrx+) during the EC process. Notably, region 1 below A-ZrO2 exhibited the smallest peak shift compared to region 3 below the C-ZrO2/A-ZrO2 interface, as well as region 2 below C-ZrO2, which further indicated that the A-ZrO2 filler was the most effective component in the Zr-EIC that could resist the infiltration of corrosive substances and protect the surface from corrosion. The EELS spectra of C-ZrO2 and A-ZrO2 obtained before and after EC (Supplementary Fig. 32) demonstrated no subsequent shifts, indicating that the EIC layer was stable. Finally, the polarization curves and electrochemical impedance spectra obtained for the Zr foil and Zr-EIC (Supplementary Fig. 33 and Supplementary Table 5) further demonstrated the advantages of the enamel-inspired strategy for enhancing the corrosion resistance of metals.
Universality of the enamel-inspired ceramic strategy
We have demonstrating that constructing an EIC layer on the Zr foil surface can improve the comprehensive mechanical properties and corrosion resistance of Zr foil. To test the universality of this strategy for coating EIC layer on metals to obtain mechanical and corrosion resistant enhancement, we selected three other metals as research objects, including Ti, Zn, and Cu. Among them, Ti has been used as a critical raw material for implants and is widely used in clinical medicine fields due to its biocompatible51. In addition, Zn and Cu exhibit excellent ductility, high thermal and electrical conductivity, and can be used in electronic devices to promote the development of these sophisticated industries52,53.
The in situ growth of metal oxide nanograss on three metal surfaces will occur due to the oxidation of metals in solution under controlled conditions. Ti, Zn, and Cu can be easily oxidated by oxidizing substances in solution and grow in one dimension due to their inherent anisotropic growth tendency54,55,56. The formation process they follow consists of metal oxide nanoparticles nucleating on the metal surface as a growth site and one-dimensional growth of the metal oxide nanorods, and corresponding crystal oxide (TiO2, ZnO, and CuO) nanorods can be obtained by optimizing the reactant concentration and reaction time at a mild oxidation environment (Supplementary Fig. 34). To prepare the crystalline/amorphous interface similar to natural tooth enamel, the gaps between nanograss were filled with amorphous metal oxide through the controllable hydrolysis of metal ions. This involved continuous chemical reaction with metal ion absorption, nucleation, and growth in a low-temperature buffer system (Cu) or through the continuous sol-gel process combined with the dip-coating method (Ti, Zn). After controllable annealing, the EIC layer was formed on the metal surface (Fig. 5a). Through detailed microstructure characterization (Fig. 5b, c and Supplementary Figs. 34 and 35), Raman spectroscopy (Supplementary Fig. 36), and XPS tests (Supplementary Fig. 37), it is proved that EIC layer similar to Zr-EIC has been successfully constructed on the surface of these three metals, and the thickness of these EIC layer were slightly greater than the heights of metal oxide nanograss (Ti, ~ 260 nm; Zn, ~ 270 nm; Cu ~ 160 nm).
a Optical photographs of Ti-EIC, Zn-EIC and Cu-EIC in a top view. Scale bar: 1 cm. b SEM images of Ti-EIC, Zn-EIC and Cu-EIC in a top view. c TEM images of Ti-EIC, Zn-EIC and Cu-EIC in a cross-sectional view (abbreviations for A-TiO2, C-TiO2, A-ZnO, C-ZnO, A-CuO, and C-CuO are amorphous TiO2, crystalline TiO2, amorphous ZnO, crystalline ZnO, amorphous CuO, and crystalline CuO, respectively).
The mechanical properties of these three prepared metal-EIC samples were measured by nanoindentation of both quasi-static and dynamic indentation. As shown in the Fig. 6a, b, Supplementary Figs. 38, 39, and Supplementary Table 1, the storage modulus, damping, Young’s modulus and hardness of the three kinds of metal-EICs are all higher than the corresponding metals. Making a comparison among these three kinds of metal-EICs, we find that the Cu-EIC exhibits the highest VFOM (3.2 GPa), and the Ti-EIC has the best combination of EY and H (139.1 GPa and 5.2 GPa) (Supplementary Table 1). In particular, compared with other metal reinforcement strategy (alloying, compounding and coating), both the stiffness and damping of metal-EICs were improved simultaneously and have obvious advantages8,9,35,57,58,59,60,61,62,63,64,65,66,67 (Fig. 6c and Supplementary Table 2), the Ti-EIC had the highest specific modulus (30.9 GPa/(g/cm3)), and the specific hardness of the Ti-EIC (1.2 GPa/(g/cm3)) reached the level of lightweight metals (e.g., Mg and Al)22,40,41,68,69,70,71,72,73 (Fig. 6d and Supplementary Table 3). Furthermore, the results from the uniaxial tensile testing (Supplementary Fig. 40) revealed that, in comparison to the properties of these three metal foils, the metal-EIC exhibited a mere marginal decrease in fracture strain, accompanied by an enhancement in their tensile strength.
a Storage modulus and tanδ of the Ti foil, Zn foil, Cu foil, Ti-EIC, Zn-EIC, and Cu-EIC. b Young’s modulus and hardness of the Ti foil, Zn foil, Cu foil, Ti-EIC, Zn-EIC, and Cu-EIC. c Comparison diagram of the storage modulus versus tanδ for the various metals, alloys, metal-based composites, coating reinforced metal, and our samples in this work. d Comparison diagram of the specific modulus versus specific hardness for the various metals, alloys, metal-based composites, coating reinforced metal, and our samples in this work. e Optical images of the Ti-EIC, Zn-EIC, and Cu-EIC before and after the EC tests.
For the harsh service environment of metals, the corrosion resistance of these three metals is also discussed. There is significant corrosion on the surface of the Ti, Zn, and Cu foils, evidenced by color changes in the Ti and Cu foils and the appearance of corrosion spots on the Zn foil. However, the Ti-EIC, Zn-EIC, and Cu-EIC samples remained intact after electrochemical corrosion tests (Fig. 6e and Supplementary Figs. 41 and 42). Ultimately, the polarization curves and electrochemical impedance spectra, as obtained for Ti foil, Ti-EIC, Zn foil, Zn-EIC, Cu foil and Cu-EIC (Supplementary Fig. 43 and Supplementary Table 5), underscored the benefits of the enamel-inspired approach in enhancing metal corrosion resistance. Furthermore, the Zr-EIC and Ti-EIC were co-cultured with human gingival fibroblasts, which showed the same trends in morphology, adhesion and cell proliferation to the biocompatible metal (Zr and Ti) surfaces51,74 and natural enamel surface (Supplementary Fig. 44). These results indicate that the enamel-inspired ceramic coated metal have good biocompatibility, thus presenting promising prospects for potential clinical application. Given the above, this enamel-inspired reinforcement strategy demonstrated good universality and could readily form an EIC layer in situ on the surface of the Ti, Zn, and Cu foils besides Zr.
This work addressed one of the limitations of metals as engineering materials, namely their vulnerability to corrosion and the inherent trade-off between stiffness and damping. A strategy was developed to obtain a ZrO2 layer-coated Zr foil with a tooth enamel-inspired structure, including the in situ growth of enamel-like ZrO2 nanograss using a hydrothermal method and infiltration of amorphous ZrO2 intergranular phase between the ZrO2 nanorods by controlled hydrolysis. Extensive analysis of the results indicated that the proposed Zr-EIC simultaneously demonstrated excellent stiffness, hardness, and damping capacity characteristics, exhibiting a high VFOM value of ~ 4.6 GPa compared with other metals and ceramic coated metals. The comprehensive high mechanical performance of these structures could be attributed primarily to the vertical orientation of stiff and hard ZrO2 nanorods, along with the energy dissipation generated by deformation at the crystalline/amorphous ZrO2 interfaces in the enamel-inspired layer. Furthermore, the enamel-inspired layer provided excellent corrosion resistance in saline environments by limiting the infiltration of corrosive substances to the underlying Zr substrate. In particular, this strategy also applies to the strengthening and corrosion resistance of Ti, Zn and Cu, and Ti-EIC exhibits a high specific modulus (30.9 GPa/(g/cm3)). Therefore, this proposed enamel-inspired structure and phase control design strategy offers great potential for the development of high-performance metallic materials with enhanced mechanical and corrosion resistance properties, particularly in the aerospace, military equipment, bioengineering sectors.
Methods
Materials
Zr foil was obtained from You Chuang Metal Co. Ltd. Ti foil, Zn foil, Cu foil, Ethylene glycol (EG), tetrabutyl titanate (C16H36O4Ti) and Tri-hydroxymethyl aminomethane (Tris) were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. Acetone, ethanol and hydrochloric acid (HCl) were purchased from Sinopharm Chemical Reagent Co., China. Sodium hydroxide (NaOH), Octahydrate zirconium chloride oxide (ZrOCl2·8H2O), Ethanolamine (ETA), Diethanolamine (DEA), Ammonium hydroxide solution (NH3·H2O), Zinc nitrate hexahydrate (Zn(NO3)2·6H2O) Zinc acetate dihydrate (C4H6O4Zn·2H2O), ammonium fluoride (NH4F), Ammonium persulfate ((NH₄)₂S₂O₈), Copper nitrate hydrate (Cu(NO3)2·xH2O), Potassium phosphate monobasic (KH2PO4), Potassium phosphate dibasic (K2HPO4) and sodium chloride (NaCl) were acquired from Macklin Chemical Reagent Co., China. All chemicals were received and used without further purification. And human erupted third molars, used in this study, were collected from patients aged between 18 and 40 years at Peking University Health Science Center after signing written informed consent. These are exempt from IRB approval. Only gender and age information were provided to the researchers.
In situ synthesis of ZrNG on the Zr foil
Zr foil was cut to 10 mm × 50 mm, and cleaned respectively in acetone, ethanol and deionized water (DI), each for 10 min, to remove surface pollutants; the foil was then dried under a stream of gaseous nitrogen, and immediately used as a substrate in the ZrO2 nanograss (ZrNG) growth experiment. The cleaned Zr foils were first immersed in a 60 mL solution with various concentrations of NaOH solution (2, 4 and 6 g), and then subjected to hydrothermal synthesis in a sealed Teflon-lined stainless steel autoclave at 180 °C, for 0.5 hr to 36 hr in an electrical dry oven. After synthesis, the autoclave was cooled to room temperature with cold water, with a cooling time of within 15 mins. The resulting ZrNG-coated Zr foil (Zr-ZrNG) was taken out, rinsed extensively with deionized water and ethanol, then allowed to dry in ambient air. In addition, the Zr foil with amorphous layer (Zr-amorphous) is an important intermediate product to induce one-dimensional growth of ZrO2, which can be synthesized by 4 g NaOH for 15 min. Therefore, the optimal dosage is 4 g of NaOH. After 24 h of hydrothermal reaction, a ZrO2 nanograss structure can be obtained.
Constructing the interface of crystalline ZrNG and amorphous ZrO2 filler
By controlling the hydrolysis reaction rate in the wet chemical system, we firstly filled the interstices in the ZrNG with dense amorphous ZrO2, with the intent to develop a flawless ceramic reinforcement layer. Specifically, the solution system (deionized water as solvent with a total volume of 70 mL) was kept at pH = 7.5 with Tris (0.848 g)-HCl (475 μL) buffer reagents. The Zr-ZrNG was then immersed in the solution, and the reaction system cooled to 6 °C in a cold bath. 16 mg ZrOCl2 ∙ 8H2O was added as a Zr source to the low-temperature reaction system with constant stirring for 8 h, so that Zr4+ could be uniformly adsorbed on the ZrNG to fill the interstices in the ceramic reinforcement layer on the Zr foil surface. Afterwards, the EIC layer reinforced Zr foil (Zr-EIC) was calcined for 2 h at 300 °C in a muffle furnace to finish the transformation from the ZrO2 precursor to the amorphous ZrO2 in order to make the reinforcement layer dense.
Preparation of the unordered amorphous ZrO2 filler and crystalline ZrO2 particle coated Zr foil (Zr-AZ/CZ)
ZrO2 crystalline particles can grow in situ on a Zr foil surface under similar conditions to Zr-ZrNG, only 6 g NaOH is selected. Then, the Zr-AZ/CZ can be prepared by the same hydrolysis and calcination process to Zr-EIC, just replacing Zr-ZrNG with crystalline ZrO2 particle-coated Zr foil. The Zr- AZ/CZ included crystalline ZrO2 on the surface (as in the Zr-EIC), but this crystalline ZrO2 was not in the form of nanorods.
Preparation of the crystalline ZrO2 particles-coated Zr foil by magnetron sputtering (Zr-CZ)
Crystalline ZrO2 (CZ) particles were deposited on the Zr foil by radio frequency magnetron sputtering at a base pressure of 1 × 10-4 Pa using a ZrO2 target. A sputtering gas mixture of argon and oxygen was used, with the partial pressure of argon maintained at ~ 0.1 Pa during the film deposition. The crystalline ZrO2 coating was deposited at room temperature, with the thickness of the film controlled at ~ 150 nm. Under such conditions, the growth rate of the ZrO2 coating was ~ 3.3 nm/min. It is worth noting that the crystalline ZrO2 on the Zr-CZ surface did not exist in the form of nanorods, and the Zr-CZ did not include the intergranular amorphous ZrO2 phase.
In situ synthesis of TiNG on the Ti foil
Ti foil was cut to 10 mm × 50 mm, and polished with sandpaper (1000♯, 1600♯, 2000♯), then cleaned respectively in acetone, ethanol and deionized water (DI), each for 5 min, to remove surface pollutant; the foil was then dried under a stream of gaseous nitrogen, and immediately used as a substrate in the TiO2 nanograss (TiNG) growth experiment. The cleaned Ti foils were first immersed in the solution with 10 mL H2O, 9 mL HCl and 0.8 mL C16H36O4Ti, and then subjected to hydrothermal synthesis in a sealed Teflon-lined stainless steel autoclave at 160 °C, for 50 min in an electrical dry oven. After synthesis, the autoclave was cooled to room temperature with cold water, with a cooling time of within 15 mins. The resulting TiNG-coated Ti foil (Ti-TiNG) was taken out, rinsed extensively with deionized water and ethanol, then allowed to dry in ambient air.
Constructing the interface of crystalline TiNG and amorphous TiO2 filler
The filling procedure of amorphous TiO2 was based on a sol-gel method combined with the dip-coating method. Firstly, TiO2 sol solution was prepare by controlled hydrolysis with 0.2 mL C16H36O4Ti, 1.4 mL ETA and 17.5 mL ethanol after adding a mixture of 2.25 mL ethanol and 0.275 mL H2O, then stirred for 1 hr and aged for 2 hr. Subsequently, the Ti-TiNG was immersed in the TiO2 sol solution for 60 s, then slowly pulled out of the solution, and repeated of four times. Afterwards, the EIC layer reinforced Ti foil (Ti-EIC) was calcined for 2 hr at 300 °C in a muffle furnace to finish the transformation from the TiO2 precursor to the amorphous TiO2 in order to make the reinforcement layer dense.
In situ synthesis of ZnNG on the Zn foil
Zn foil was cut to 10 mm × 50 mm, and polished with sandpaper (1000♯, 1600♯, 2000♯), then cleaned respectively in acetone, ethanol and deionized water (DI), each for 5 min, to remove surface pollutant; the foil was then dried under a stream of gaseous nitrogen, and immediately used as a substrate in the ZnO nanograss (ZnNG) growth experiment. The cleaned Zn foils were first immersed in a 20 mL H2O solution with 0.4 mL NH3·H2O and 0.07 g Zn(NO3)2·6H2O, and then subjected to hydrothermal synthesis in a sealed Teflon-lined stainless steel autoclave at 95 °C for 0.5 hr in an electrical dry oven. After synthesis, the autoclave was cooled to room temperature with cold water, with a cooling time of within 15 mins. The resulting ZnNG-coated Zn foil (Zn-ZnNG) was taken out, rinsed extensively with deionized water and ethanol, then allowed to dry in ambient air.
Constructing the interface of crystalline ZnNG and amorphous ZnO filler
The filling procedure of amorphous ZnO was based on a sol-gel method combined with the dip-coating method. Firstly, ZnO sol solution was prepared by controlled hydrolysis with 2.2 g C4H6O4Zn·2H2O, 1 mL Diethanolamine and 25 mL ethanol, then stirred for 1 hr and aged for 24 hr. Subsequently, the Zn-ZnNG was immersed in the ZnO sol solution for 60 s, then slowly pulled out of the solution, and repeated of two times. Afterwards, the EIC layer reinforced Zn foil (Zn-EIC) was calcined for 2 hr at 300 °C in a muffle furnace to finish the transformation from the ZnO precursor to the amorphous ZnO in order to make the reinforcement layer dense.
In situ synthesis of CuNG on the Cu foil
Cu foil was cut to 10 mm × 50 mm, and polished with sandpaper (1000♯, 1600♯, 2000♯), then cleaned respectively in acetone, ethanol and deionized water (DI), each for 5 min, to remove surface pollutant; the foil was then dried under a stream of gaseous nitrogen, and immediately used as a substrate in the CuO nanograss (CuNG) growth experiment. The cleaned Cu foils were immersed in a 9 mL solution with 0.27 g NaOH and 0.685 g (NH₄)₂S₂O₈ at 20 °C for 3 hr. The resulting CuNG-coated Cu foil (Cu-CuNG) was taken out, rinsed extensively with deionized water and ethanol, then allowed to dry in ambient air.
Constructing the interface of crystalline CuNG and amorphous CuO filler
By controlling the hydrolysis reaction rate in the wet chemical system, we firstly filled the interstices in the CuNG with dense amorphous CuO, with the intent to develop a flawless ceramic reinforcement layer. Specifically, the solution system with 20 mL deionized water and 50 mL EG as solvent was kept at pH = ~ 6.0 with KH2PO4 (0.834 g)- K2HPO4 (0.087 g) buffer reagents. The Cu-CuNG was then immersed in the solution, and the reaction system cooled to 2 °C in a cold bath. 10 mg Cu(NO3)2·xH2O was added as a Cu source to the low temperature reaction system with constant stirring for 12 hr, so that Cu2+ could be uniformly adsorbed on the CuNG to fill the interstices in the ceramic reinforcement layer on the Cu foil surface. Afterwards, the EIC layer reinforced Cu foil (Cu-EIC) was calcined for 1 hr at 200°C in a muffle furnace to finish the transformation from the CuO precursor to the amorphous CuO in order to make the reinforcement layer dense.
Natural tooth enamel samples
The enamel samples were sawed along the buccal and lingual tooth surfaces and then polished with 800–5000-grit SiC paper.
Materials characterization
The structure and morphology of different samples, such as the Zr, Zr-EIC, Ti, Ti-EIC, Zn, Zn-EIC, Cu, Cu-EIC, and other prepared samples involved in the discussion, were characterized using a scanning electron microscopy (SEM, FEI-Quanta 250 F). Regions subjected to permanent deformation by nanoindentation were also characterized with energy dispersive spectroscopy (EDS) mapping performed by field-emission scanning electron microscopy (FESEM, JEOL-7500F) equipped with EDS. Cross-sectional transmission electron microscopy (TEM) thin foils of tooth enamel, Zr foil, Zr-ZrNG, and Zr-EIC and other prepared samples were cut by focused ion beam machining (FIB, Helios NanoLab 460HP), prior to the samples being investigated using a JEOL 2010F TEM at 200 kV accelerating voltage; the surface morphology of the Zr foil in hydrothermal initial stage was also examined with this technique. Aberration-corrected high-angle annular dark-field scanning TEM (HAADF-STEM) and electron energy loss spectroscopy (EELS) were performed on a FEI TITAN G2 at an accelerating voltage of 300 kV. The crystal structure of the Zr foil, Zr-amorphous, Zr-ZrNG, Zr-EIC and amorphous ZrO2 were recorded utilizing X-ray diffraction (XRD) (XRD-600, Shimadzu) with Cu Kα radiation. All samples were fixed on an aluminum sample table, and a scan range of 20°–80° carried out at a scan speed of 5°/min. The 3D roughness images of the surfaces of the Zr foil and Zr-EIC were examined with atomic force microscopy, and the scanning range is 2 μm × 2 μm (AFM, Bruker Dimension Icon). Raman spectra of the materials were taken at room temperature using a LabRAM HR800 (Horiba JobinYvon) with an incident laser with a wavelength of 633 nm. Raman mappping was carried out at room temperature using a LabRAM HR800 (Horiba JobinYvon) with an incident laser with a wavelength of 514 nm. The laser power density was ~ 109 W/m2 (laser power: ~ 1.5 mW; laser spot: 1.87 μm2). X-ray photoelectron spectroscopy (XPS) (ESCALab220i-XL, Thermo Scientific) was performed to analyze the surface chemical characteristics of the amorphous metal oxide, NG reinforced metal, and EIC reinforced metal.
Uniaxial tensile experiments
Characterization of the tensile strength and strain of the metal and metal-EIC materials were measured using a Shimadzu AGS-X (100 N) tester. The displacement rate was 1 mm/min, with a gauge length of 5 mm. The specimens were cut into ribbons with a length of 20 mm and a width of 2-3 mm. The thickness of the ribbon was measured using SEM from a cross-sectional view.
Nanoindentation experiments
Characterization of the mechanical properties of our materials – Zr, Zr-EIC, Ti, Ti-EIC, Zn, Zn-EIC, Cu, Cu-EIC, and other prepared samples - were measured at ambient temperatures using a commercial TI950 tribo-indenter (Hysitron) equipped with a Berkovich diamond tip (of radius ~ 100 nm) by a continuous depth-sensing indentation technique. For the standard pattern of metal, metal-EIC and referenced materials, initial and terminal forces of respectively 100 μN and 10,000 μN were used with a loading time of 10 s. For the high load pattern, the limited displacement and load time were set at 1200 nm and 20 s, respectively. To investigate the mechanical properties at different contact depths, EY and H values were measured from force-displacement curves and calculated using the methods of Oliver-Pharr75. Nanodynamic mechanical analysis (nano-DMA measurements) were performed with the same tribo-indenter with the specialized program of nano-DMA and the Berkovich tip. Typically, the load function was set with an initial quasi-static force of 20 μN and a peak quasi-static force of 10,000 μN, with the frequency and dynamic force at 45 Hz and 30 μN, respectively. Similarly, measured values of the storage modulus Eʹ and loss coefficient tanδ were summarized as a function of contact depth; the averages of these data were used for comparison purposes. To explore the specific role of the tooth enamel-inspired ceramic layer, TEM thin slices of the Zr foil and Zr-EIC were cut by FIB following indentation under a quasi-static force of 10,000 μN; these were then characterized in detail by TEM. In addition, the top view of the permanent deformation of tooth enamel and our materials following such indentation was characterized in the SEM. In addition, the nanoscratch testing of Zr-EIC was carried out with the above instrument, operating by increasing the normal depth of 0-75 nm for 10 s first, and then the lateral slip time was 30 s for each test.
Dynamic mechanical analysis
The macroscale dynamic mechanical analysis (macro-DMA) (EPLEXOR®5000 N) measurements were taken in the tension mode. The specific parameters applied are the following: room temperature, dynamic force 40 N, constant part of static force 45 N, frequency 45 Hz, sample length 20 mm, sample width 5 mm, sample thickness 0.01 mm.
Abrasion tests
The adhesive interfaces between each group of ceramic coating and metal substrate were positioned at the center of the shear loading zone, maintaining an interfacial area of 25 mm². Shear testing was performed using a universal testing machine (Instron 5967, USA) equipped with a 10kN load cell and a customized dual-shear fixture system. The system was calibrated according to ISO 7500−1 standards through: (1) three-cycle no-load zeroing operations of the force transducer, (2) laser interferometer verification of displacement accuracy (resolution 0.1 μm), and (3) gauge block alignment for parallelism adjustment (deviation ≤ 5 μm), achieving total system error < 0.5%. Displacement-controlled loading was applied at 0.5 mm/min, with an initial 5 N preload (10 s dwell time) until specimen failure (defined as 80% load drop or 2 mm displacement). Specimens were secured using a sandwich clamping assembly comprising: 3 mm-thick silicone rubber pad (Shore A50 hardness), an aligned specimen, and micro-grooved titanium alloy plate, pressurized uniformly at 0.3 MPa via pneumatic system to prevent slippage. Testing was conducted under controlled conditions (20−25 °C, ≤60% RH). Real-time load-displacement curves were recorded, with the maximum shear force determined from the peak point of the curve.
Neutral salt spray tests
A neutral salt spray test was carried out to evaluate the long-time corrosion resistance of the Zr foil and Zr-EIC following the methods of the ASTM B117 Standard. The total duration of the test, which used a salt spray of 5 ± 1 wt.% NaCl solution controlled at 24 °C, was 15 days and with periodic examinations after 1 day, 2 days, 3 days, 4 days, 6 days, 10 days and 15 days. The exposed area of the Zr foil and Zr-EIC subjected to the salt spray was 100 mm2, with the remainder of the samples covered with epoxy resin. To compare the corrosion resistance of the samples after a neutral salt spray test 15 days, the corrosion morphology of the Zr foil and Zr-EIC were observed by SEM, with mechanical properties of all samples obtained by nanoindentation. In order to obtain the mass loss rate of Zr foil and Zr-EIC, the neutral salt spray test lasted for 45 days.
Electrochemical corrosion tests
In order to speed up corrosion, potentiostatic polarization of the Zr foil and Zr-EIC was carried out at a constant potential of − 600 mV vs. SCE for 300 s in 3.5 wt.% NaCl aqueous solution. The exposed area of the Zr foil and Zr-EIC subjected to the salt solution was 100 mm2, with the remainder of the samples covered with insulated rubber tape. Similarly, the electrochemical corrosion tests of the Ti foil, Zn foil, Cu foil, Ti-EIC, Zn-EIC, and Cu-EIC were carried out in different test conditions (Ti foil and Ti-EIC: 10 V vs. SCE for 7200 s, Zn foil and Zn-EIC: − 920 mV vs. SCE for 20 s, Cu foil and Cu-EIC: − 100 mV vs. SCE for 60 s).
Electrochemical characteristics
The corrosion resistance of the Zr foil and the Zr-EIC were evaluated by electrochemical measurements using a CHI660E electrochemical workstation with a conventional three-electrode system. This system consisted of the sample as the working electrode, a platinum filament as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode in a 3.5 wt.% NaCl solution. Open-circuit potential (OCP) values were recorded for 400 s before potentiodynamic polarization (PDP) and electrochemical impedance spectrum (EIS) measurements. The PDP measurements were taken on the sample polarized at ± 250 mV to measure the corrosion rate close to its OCP by a direct current signal with a scan rate of 0.2 mV/s. The EIS tests were conducted on the sample polarized at ± 10 mV, closing its OCP by an alternating current signal in the frequency range of 106 to 0.01 Hz. All experiments were repeated three times in parallel. Meanwhile, the PDP and EIS of the Ti foil, Zn foil, Cu foil, Ti-EIC, Zn-EIC, and Cu-EIC were carried out at similar test conditions.
Biocompatibility property tests
The biocompatibility of the samples was assessed by co-culturing with human gingival fibroblasts (HGF, ScienCel #2620, USA). HGF cells were maintained in α-MEM basal medium supplemented with 10% fetal bovine serum (FBS, Gibco, China) and 1% penicillin-streptomycin mixture (100 ×, Gibco, China). Sterilized specimens from each group were placed in 48-well plates, followed by seeding HGF cells at a density of 1.0 × 104 cells/well.
-
1)
Cell adhesion morphology visualized using a laser confocal microscope:
After 1, 3, and 5 days of co-culture, the medium was aspirated, and samples were gently rinsed three times with PBS. Cells were fixed with 4% paraformaldehyde for 10 min at room temperature (RT), permeabilized with 0.1% Triton X-100 for 15 min at RT, and blocked with 3% bovine serum albumin (BSA) to minimize nonspecific binding. Sequential incubations were performed as follows: Primary Antibody: Overnight incubation at 4 °C with anti-vinculin primary antibody (1:200 dilution, Abcam ab129002, UK). Secondary Antibody: 1 h incubation at RT with anti-rabbit IgG secondary antibody (1:250 dilution, Abcam ab150081, UK). Cytoskeleton and Nuclear Staining: Sequential incubation with TRITC-phalloidin (1:200 dilution, Solarbio CA1610, China) for 1 h and DAPI solution (5 mg/mL) for 10 min at RT to label F-actin and nuclei, respectively. All steps were interspersed with three PBS washes. Fluorescence images were acquired and analyzed using Image Pro Plus 6.0 software to quantify single-cell spreading area.
-
2)
Cell Proliferation Assay:
Cell proliferation was quantified using a CCK-8 kit (Dojindo Laboratories, Japan). At days 1, 3, and 5, 100 μL of CCK-8 solution was added to each well, followed by 2 h incubation at 37 °C in the dark. Absorbance of the formazan product was measured at 450 nm using a microplate reader.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated in this study are provided in the Supplementary Information/Source Data file. The data used in this study are available in Figshare (https://doi.org/10.6084/m9.figshare.28705358). Source data are provided in this paper.
References
Ashby, M. F. Overview No. 80 On the engineering properties of materials. Acta Met. 37, 1273–1293 (1989).
Woigk, W. et al. Nacre-like composites with superior specific damping performance. Proc. Natl. Acad. Sci. USA 119, e2118868119 (2022).
Sun, J. et al. A Review on binderless tungsten carbide: Development and application. Nano Micro Lett. 12, 13 (2019).
Yue, Y. et al. Hierarchically structured diamond composite with exceptional toughness. Nature 582, 370–374 (2020).
Zhang, M. et al. On the damage tolerance of 3-D printed Mg-Ti interpenetrating-phase composites with bioinspired architectures. Nat. Commun. 13, 3247 (2022).
Lei, Z., Wu, Y., He, J., Liu, X. & Lu, Z. Snoek-type damping performance in strong and ductile high-entropy alloys. Sci. Adv. 6, eaba7802 (2020).
Zhang, M., Yu, Q., Liu, Z., Zhang, J. & Ritchie, R. O. 3D printed Mg-NiTi interpenetrating-phase composites with high strength, damping capacity, and energy absorption efficiency. Sci. Adv. 6, eaba5581 (2020).
Gangwar, S., Patnaik, A. & Bhat, I. K. Tribological and thermomechanical analysis of CaO (quicklime) particulates filled ZA−27 alloy composites for bearing application. Proc. Inst. Mech. Eng. L 232, 20–34 (2015).
Fu, Y., Wu, Q., Yang, W. & Liu, S. Synthesis and properties of hydrogels on medical titanium alloy surface by modified dopamine adhesion. Gels 8, 458 (2022).
Wegst, U. G. K., Bai, H., Saiz, E., Tomsia, A. P. & Ritchie, R. O. Bioinspired structural materials. Nat. Mater. 14, 23–36 (2015).
Lakes, R. High damping composite materials: effect of structural hierarchy. J. Compos. Mater. 36, 287–297 (2002).
Liu, Y. et al. A strong, lightweight, and damping cermet material with a nacre-like ultrafine 3D interpenetrated architecture. Mater. Today 62, 62–70 (2023).
DeRocher, K. A. et al. Chemical gradients in human enamel crystallites. Nature 583, 66–71 (2020).
Yeom, B. et al. Abiotic tooth enamel. Nature 543, 95–98 (2017).
Lew, A. J., Beniash, E., Gilbert, P. U. P. A. & Buehler, M. J. Role of the mineral in the self-healing of cracks in human enamel. ACS Nano 16, 10273–10280 (2022).
Gordon, L. M. et al. Amorphous intergranular phases control the properties of rodent tooth enamel. Science 347, 746–750 (2015).
Li, Y. et al. Mechanically reinforced artificial enamel by Mg2+-induced amorphous intergranular phases. ACS Nano 16, 10422–10430 (2022).
Zhao, H. et al. Multiscale engineered artificial tooth enamel. Science 375, 551–556 (2022).
Balooch, G. et al. Evaluation of a new modulus mapping technique to investigate microstructural features of human teeth. J. Biomech. 37, 1223–1232 (2004).
Padture, N. P., Gell, M. & Jordan, E. H. Thermal barrier coatings for gas-turbine engine applications. Science 296, 280–284 (2002).
Heiroth, S., Ghisleni, R., Lippert, T., Michler, J. & Wokaun, A. Optical and mechanical properties of amorphous and crystalline yttria-stabilized zirconia thin films prepared by pulsed laser deposition. Acta Mater. 59, 2330–2340 (2011).
Crawford, G. A., Chawla, N., Das, K., Bose, S. & Bandyopadhyay, A. Microstructure and deformation behavior of biocompatible TiO2 nanotubes on titanium substrate. Acta Biomater. 3, 359–367 (2007).
Xia, C. et al. Corrosion behavior and wear resistance of Zr-2.5Nb alloy after thermal oxy-nitriding treatment. Surf. Coat. Technol. 446, 128756 (2022).
Xu, L., Xiao, Y., Van Sandwijk, A., Xu, Q. & Yang, Y. Production of nuclear grade zirconium: A review. J. Nucl. Mater. 466, 21–28 (2015).
Kumar, C. S. Nanostructured Oxides. (John Wiley and Sons, 2009).
Ardizzone, S., Cattania, M. G., Lazzari, P. & Lugo, P. Hydrothermal “route” to pure phase ZrO2. Interfacial reactivity by XPS and electrochemical determinations. Colloids Surf. A Physicochem. Eng. Asp. 90, 45–54 (1994).
Yang, N. et al. Amorphous/crystalline hetero-phase Pd nanosheets: One-pot synthesis and highly selective hydrogenation reaction. Adv. Mater. 30, 1803234 (2018).
Tong, S., Zhang, X. & Yang, P. G-C3N4 sheet nanoarchitectonics with island-like crystalline/amorphous homojunctions towards efficient H2 and H2O2 evolution. Environ. Res. 236, 116805 (2023).
Zou, Y. et al. In-situ SEM analysis of brittle plasma electrolytic oxidation coating bonded to plastic aluminum substrate: Microstructure and fracture behaviors. Mater. Charact. 156, 109851 (2019).
Huang, W. et al. A natural impact-resistant bicontinuous composite nanoparticle coating. Nat. Mater. 19, 1236–1243 (2020).
Satish Idury, K. S. N. et al. Room temperature dynamic indentation response of partially crystallized Zr–Cu metallic glass. J. Alloy. Compd. 834, 155161 (2020).
Xue, R. et al. Zr-xNb-4Sn alloys with low Young’s modulus and magnetic susceptibility for biomedical implants. Prog. Nat. Sci. Mater. Int. 31, 772–778 (2021).
Nekouie, V., Kühn, U., Roy, A. & Silberschmidt, V. Quasi-static and dynamic deformation behaviour of Zr-based bulk metallic glass. J. Phys. Conf. Ser. 451, 012009 (2013).
Sharma, A. et al. Investigation of thermophysical properties of Zr-based metallic glass-polymer composite. Metals 11, 1412 (2021).
Shilpa, C., Mahesha, K., Dey, A. & Sachidananda, K. B. Damping performance of alumina and zirconia-based plasma sprayed coatings. Surf. Eng. 36, 1107–1112 (2020).
Mahoney, E., Holt, A., Swain, M. & Kilpatrick, N. The hardness and modulus of elasticity of primary molar teeth:an ultra-micro-indentation study. J. Dent. 28, 589–594 (2000).
Li, N., Liu, L., Chen, Q., Pan, J. & Chan, K. C. The effect of free volume on the deformation behaviour of a Zr-based metallic glass under nanoindentation. J. Phys. D. 40, 6055 (2007).
Hua, N. et al. The effects of normal load on the dry-sliding, micro-scratch and nanoindentation behaviors of the Zr-based bulk glassy alloys. J. Non Cryst. Solids 615, 122427 (2023).
Nezhad, E. Z. et al. Effect of zirconia nanotube coating on the hydrophilicity and mechanochemical behavior of zirconium for biomedical applications. Surf. Interfaces 28, 101623 (2022).
Sreya, P. V. et al. Zinc oxide decorated titania nanostructured layer over Ti metal as a biocompatible and antimicrobial surface for biomedical application. Surf. Interfaces 33, 102275 (2022).
Park, S.-Y., Kim, Y.-C., Ruoff, R. S. & Kim, J.-Y. Incipient plasticity and fully plastic contact behavior of copper coated with a graphene layer. APL Mater. 7, 031106 (2019).
Bai, H. et al. Fabrication of (Ta,W)C surface gradient layer on Ta-10W alloy by in situ solid-phase diffusion. Appl. Surf. Sci. 493, 1317–1325 (2019).
Yang, J. et al. Enhanced Wear Performance of Hybrid Epoxy-Ceramic Coatings on Magnesium Substrates. ACS Appl. Mater. Interfaces 10, 30741–30751 (2018).
Dey, A. et al. Nanoindentation study of microplasma sprayed hydroxyapatite coating. Ceram. Int. 35, 2295–2304 (2009).
Hou, J. et al. An Amorphous peri-implant ligament with combined osteointegration and energy dissipation. Adv. Mater. 33, 2103727 (2021).
Chen, M., McCauley, J. W. & Hemker, K. J. Shock-induced localized amorphization in boron carbide. Science 299, 1563–1566 (2003).
Huang, Z. et al. Uncovering high-strain rate protection mechanism in nacre. Sci. Rep. 1, 148 (2011).
Wang, W., Jenkins, P. E. & Ren, Z. Electrochemical corrosion of carbon steel exposed to biodiesel/simulated seawater mixture. Corros. Sci. 57, 215–219 (2012).
Shan, C. X., Hou, X. & Choy, K.-L. Corrosion resistance of TiO2 films grown on stainless steel by atomic layer deposition. Surf. Coat. Technol. 202, 2399–2402 (2008).
Zhang, B. et al. Unmasking chloride attack on the passive film of metals. Nat. Commun. 9, 2559 (2018).
Kaur, M. & Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 102, 844–862 (2019).
Li, G. et al. Challenges in the use of zinc and its alloys as biodegradable metals: Perspective from biomechanical compatibility. Acta Biomater. 97, 23–45 (2019).
Fateh, A., Aliofkhazraei, M. & Rezvanian, A. R. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 13, 481–544 (2020).
Liu, B. & Aydil, E. S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 131, 3985–3990 (2009).
Yang, H. et al. Large-scale growth of highly oriented ZnO nanorod arrays in the Zn-NH3·H2O hydrothermal system. Cryst. Growth Des. 8, 1039–1043 (2008).
Cao, J. et al. Stable Lithium metal anode achieved by in situ grown CuO nanowire arrays on Cu foam. Energ. Fuel. 34, 7684–7691 (2020).
Jung, T. K. et al. Mechanical properties-graded Ti alloy implants for othopedic applications. Mater. Sci. Forum 631-632, 205–210 (2009).
Xiong, J. Y., Li, Y. C., Hodgson, P. D. & Wen, C. E. Design of a newbiocompatible Ti-based shape memory alloy and its superelastic deformation behaviour. Mater. Sci. Forum 654-656, 2087–2090 (2010).
Wang, W. et al. Reentrant strain glass transition in Ti-Ni-Cu shape memory alloy. Acta Mater. 226, 117618 (2022).
Zhang, J.-S. et al. Ultrafine eutectic TixSny/TiNi composites with high damping capacity. Rare Met. 32, 196–200 (2013).
Mamatha, T. G., Patnaik, A., Biswas, S., Satapathy, B. K. & Redhewall, A. K. Thermo-mechanical and crack position on stress intensity factor in particle-reinforced Zinc–aluminium alloy composites. Comput. Mater. Sci. 55, 100–112 (2012).
Anand, N., Mehrotra, N. & Pal, K. Biodegradable implant application: Electrodeposition of HA/TiO2/ZrO2 coating onto Zn-composite substrates. J. Mech. Behav. Biomed. Mater. 146, 106073 (2023).
Alaneme, K. K. & Umar, S. Mechanical behaviour and damping properties of Ni modified Cu–Zn–Al shape memory alloys. J. Sci. Adv. Mater. Dev. 3, 371–379 (2018).
Ziewiec, K. Properties characterization of the Cu68.5Ni12P19.5 alloy at elevated temperatures. J. Non Cryst. Solids 354, 4019–4023 (2008).
Thokala, N., Kealey, C., Kennedy, J., Brady, D. B. & Farrell, J. B. Characterisation of polyamide 11/copper antimicrobial composites for medical device applications. Mater. Sci. Eng. C 78, 1179–1186 (2017).
Min, U.-S. & Li, J. C. M. The damping properties of dealloyed porous copper and its PMMA composite. J. Mater. Res. 9, 2884–2890 (1994).
Min, U. S. & Li, J. C. M. The damping properties of C6070-copper composite. Nanostruct. Mater. 2, 311–322 (1993).
Li, P. Microstructure and mechanical properties of novel β-type Ti–Co–Zr alloys with high specific strength. Mater. Res. Express 6, 076559 (2019).
Ning, C. Y. et al. Mechanical performances and microstructural characteristics of plasma-sprayed bio-functionally gradient HA–ZrO2–Ti coatings. Surf. Coat. Technol. 200, 2403–2408 (2005).
Chabri, S. et al. Development and characterization of Al2O3 dispersed Al/Mg/Cu/Ti matrix composite. J. Mater. Sci. Technol. 29, 1085–1090 (2013).
Čapek, J. et al. ZnMg0.8Ca0.2 (wt%) biodegradable alloy – The influence of thermal treatment and extrusion on microstructural and mechanical characteristics. Mater. Charact. 162, 110230 (2020).
García-Mintegui, C. et al. Zn-Mg and Zn-Cu alloys for stenting applications: From nanoscale mechanical characterization to in vitro degradation and biocompatibility. Bioact. Mater. 6, 4430–4446 (2021).
Kanders, U. et al. Nanoindentation response analysis of Cu-rich carbon–copper composite films deposited by PVD technique. Surf. Coat. Technol. 280, 308–316 (2015).
Chen, X. et al. Effect of surface roughness of Ti, Zr, and TiZr on apatite precipitation from simulated body fluid. Biotechnol. Bioeng. 101, 378–387 (2008).
Oliver, W. C. & Pharr, G. M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 7, 1564–1583 (1992).
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFA0710403 to L.G.), the National Natural Science Foundation of China (52222203 and 52073008 to H.Z., 52303336 to S.L., 52250119 to L.G.), the Beijing Municipal Science & Technology Commission (221100007422088 to L.G.) and the China Postdoctoral Science Foundation Funded Project (BX20220372 and 2023M730159 to S.L.).
Author information
Authors and Affiliations
Contributions
L.G., H.Z. and R.O.R. conceived this project. S.L., T.W. and B.D. prepared the metal with enamel-inspired ceramic coating and referenced samples. S.L., H.Z., L.G. and J.L. performed the morphology and structural characterization, spectroscopy tests and mechanical tests. J.D. performed the biocompatibility property tests. T.G. prepared conventional ceramic layer coated metals and characterized the microstructure and mechanical properties. L.G., R.O.R., H.Z., T.G. and S.L. analysed the data and wrote this paper. All authors participated in the discussions of the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, S., Deng, J., Zhao, H. et al. Tooth enamel-inspired ceramic coating on metal surface for enhanced mechanical properties and corrosion resistance. Nat Commun 16, 5980 (2025). https://doi.org/10.1038/s41467-025-61060-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61060-1