Abstract
Efficient and narrowband blue organic afterglow materials are critical components for advanced optoelectronic applications, but so far, have been rarely explored. Herein, we report short-range charge transfer-assisted high efficiency and ultra-narrowband deep blue (<450 nm) afterglow from a series of indolocarbazole-based chromophores. The short-range charge transfer within the fused π-conjugated frameworks enlarges the singlet-triplet energy gap and suppresses vibronic coupling and structural relaxations, thus leading to a slow reverse intersystem crossing rate, a long delayed-fluorescence lifetime of up to 186.48 ms and a high photoluminescence quantum yield of 86.1%. Notably, these afterglow emitters exhibit ultra-narrow full width at half maximum of 18 nm, presenting high colour purity with y colour coordinates below 0.05. Taking advantage of these unique afterglow features, the potential for encrypted light communications and high-resolution afterglow displays are demonstrated. This work not only provides an effective strategy for developing narrowband organic afterglow materials but also extends their applications to advanced fields.
Similar content being viewed by others
Introduction
Deep blue organic light-emitting materials (<450 nm) featuring simultaneously high efficiency and ultra-narrowband emission have become increasingly crucial to the demand for ultrahigh definition displays, high-density data storage, and light communications1,2,3,4. For instance, the exploitation of narrowband and efficient blue emitters has reshaped organic light-emitting diodes (OLEDs) with outstanding external quantum efficiency (>40%), color purity, and operation lifetime5,6,7,8. Meanwhile, these emitters are also ideal light and pumping sources in stacked tandem devices for next-generation commercial displays when integrated with green/red downconverters9,10. In particular, blue emitters exhibiting afterglow are anticipated to mitigate the ubiquitous flicker issue in current OLEDs displays11,12,13. Afterglow materials, showing sustained luminescence from seconds to hours upon the cease of excitation, have garnered increasing attention for their unique photophysical properties and innovative applications14,15,16,17,18,19,20,21,22,23. However, prevalent organic afterglow predominantly originates from phosphorescence governed by radiative decay from low-lying triplet excited states24,25. The inevitably large structural relaxations at triplet excited states, accompanied by strong vibronic coupling between triplet and ground states, generally result in long-wavelength (500–650 nm) and broadband phosphorescence with large full width at half maximum (FWHM > 60 nm) and unsatisfactory color purity26. Additionally, organic long persistent luminescence, as another representative afterglow, arises from the exciplexes formed between donor and acceptor molecules, typically leading to broader FWHM (>100 nm)27,28. Although energy transfer has been used to construct multi-color narrowband afterglow, the emissions are limited to the green-to-red region due to the energy matching constraint29,30. Therefore, achieving high-efficiency and ultra-narrowband deep blue afterglow from purely organic materials still presents a significant challenge (Supplementary Figs. 1–9).
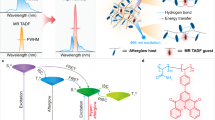
A recently developed design concept of thermally activated delayed fluorescence (TADF) emitters based on the multi-resonance (MR) effect has fulfilled efficient and narrowband luminescence by embedding mutually ortho-positioned electron-donating and electron-withdrawing atoms into a polycyclic aromatic skeleton (Fig. 1a)31,32,33,34,35. In such a manner, the distributions of the highest occupied molecular orbitals (HOMO) and the lowest unoccupied molecular orbitals (LUMO) alternate within the molecular scaffold, affording the unique intramolecular short-range charge transfer (SRCT)36,37 and suppressing the vibronic coupling between excited and ground states. Furthermore, in contrast to the long-range charge transfer (LRCT) in conventional donor-acceptor type TADF emitters with spatially well-separated HOMO-LUMO distribution, SRCT in most MR-TADF chromophores with relatively weak donor-acceptor interactions, such as boron-nitrogen, boron-oxygen, or carbonyl-nitrogen, results in larger singlet-triplet energy gaps (ΔEST, ~0.15-0.25 eV) and slower reverse intersystem crossing (RISC). This leads to delayed fluorescence with longer lifetimes in the μs–ms range (Supplementary Table 1), which provides a precondition for achieving narrowband afterglow38,39. Meanwhile, SRCT shows analogous characteristics to locally excited state (LE), favoring high efficiency and deep blue luminescence38. Therefore, we propose that further increasing ΔEST and slowing down the RISC process in MR-TADF emitters could potentially enable efficient and narrowband deep blue afterglow by incorporating weak electron-accepting/donating moieties onto the large fused-ring framework (Fig. 1b).
a Schematic illustration of the HOMO–LUMO distribution on typical donor–acceptor and MR-type chromophores. b Photophysical processes of room-temperature phosphorescence (RTP) and SRCT-induced thermally activated delayed fluorescence (TADF) afterglow. HOMO and LUMO are the highest occupied molecular orbitals and the lowest unoccupied molecular orbitals, respectively. S0, S1, and T1 represent the ground state, the lowest singlet excited state, and the lowest triplet excited state, respectively; v refers to the vibration energy levels; ISC, Exc. Phos. and non-rad. refer to intersystem crossing, ultraviolet (UV) light excitation, phosphorescence and non-radiative decay, respectively. kp, kRISC, kDF, and knr represent rate constants of phosphorescence, reverse intersystem crossing, delayed fluorescence and non-radiative decay, respectively. kB and T are the Boltzmann constant and temperature, respectively. c Chemical structures of the corresponding narrowband deep blue chromophores.
To validate our hypothesis, a series of indolo[3,2,1-jk]carbazole-based MR chromophores, namely pICz, pICz-Ph, pICz-Me, and pICz-StBu are designed and synthesized through aromatic nucleophilic substitution followed by palladium-catalyzed intramolecular ring cyclization (Fig. 1c and Supplementary Figs. 10 and 11). All the chemical structures of chromophores are confirmed by nuclear magnetic resonance (1H NMR and 13C NMR) spectra and high-resolution mass spectra (Supplementary Figs. 12–29). Carbazole is selected as the building block because of its high singlet/triplet state levels (>3.0 eV), relatively weak electron-donating capability, and planar conformation. The intramolecular ring-closing could further enhance the molecular rigidity and oscillator strength, which are both advantageous for efficient deep blue emission. Moreover, the nitrogen-centered triangle structure surrounded by three π-conjugated rings could induce the HOMO and LUMO distribution on different carbon atoms adjacent to the nitrogen, enabling the MR effect for narrowband emission40. The lack of a strong electron-accepting moiety within the skeleton enables a moderate SRCT nature and a corresponding large ΔEST. Thanks to the large ring-fused conjugated carbazole derivatives, the triplet excited states exhibit nearly pure π–π* configurations, which is conducive to the formation of long-lived triplet excitons41. Meanwhile, polymethyl methacrylate (PMMA) is selected as the polymer matrix to isolate the chromophores, thereby mitigating aggregation-induced spectral broadening and stabilizing the long-lived triplet states of the chromophores42,43. As expected, a bright and ultra-narrowband deep blue afterglow can be observed upon doping these MR chromophores into PMMA. Impressively, the deep blue afterglow shows an ultra-narrow FWHM of 18 nm, a luminescence lifetime of 186.48 ms, and a high photoluminescence quantum yield (PLQY) up to 86.1% (afterglow quantum yield up to 69.39%) under ambient conditions, outperforming most reported organic afterglow in the blue/deep blue region (Supplementary Fig. 1 and Supplementary Table 2).
Results
Photophysical properties of organic chromophores
As a proof-of-concept, pICz-Ph was selected as the model chromophore for photophysical properties investigations. As shown in Fig. 2a, pICz-Ph in toluene solution (10−5 M) exhibited a broad absorption band with multiple fine peaks at 312, 364, and 430 nm. The signals below 400 nm were attributed to the π–π* and n–π* transitions, while the band at 400–450 nm corresponded to the SRCT process36. In sharp contrast, the steady-state photoluminescence (PL) spectrum displayed one narrow fluorescence band at 438 nm with a lifetime of 6.59 ns (Fig. 2a and Supplementary Fig. 30). Notably, the Stokes shift and FWHM were as small as 8 and 16 nm (104.1 meV), respectively, both of which verified that pICz-Ph possessed a rigid π-conjugated framework and negligible structural relaxations between excited and ground states. Similar photophysical properties were also revealed for the 0.1 wt%-doped film of pICz-Ph in PMMA (pICz-Ph/PMMA) under ambient conditions. As shown in Fig. 2a, the bright blue afterglow from the film can be readily observed by the naked eye after removing the 365 nm ultraviolet (UV) lamp (Supplementary Fig. 31 and Supplementary Movie 1). Impressively, the prompt PL and delayed PL (delay time: 8 ms) spectra of pICz-Ph/PMMA were superimposed with an FWHM of 18 nm (118.6 meV), which represents the smallest FWHM among the reported organic afterglow materials, to the best of our knowledge.
a Top: Normalized absorption (red line) and photoluminescence (blue line) spectra of pICz-Ph in dilute toluene solution (10−5 M). Bottom: Normalized photoluminescence (black line) and delayed photoluminescence (blue line, delay time: 8 ms) spectra of pICz-Ph/PMMA (0.1 wt%). Inset: photographs of the pICz-Ph solution or pICz-Ph/PMMA recorded before and after switching off a 365 nm UV lamp. b CIE 1931 chromaticity diagram of afterglow for pICz-Ph/PMMA. The white and black triangles refer to the color gamut standards for displays defined by the Broadcast Service Television 2020 (BT.2020) and the National Television Standards Committee (NTSC), respectively. c Lifetime decay profile of afterglow monitoring at 440 nm for pICz-Ph/PMMA film. d Bar graphs of lifetimes and FWHMs for the afterglow of pICz-Ph/PMMA films with different doping ratios ranging from 0.005% to 0.5%. e Excitation-delayed photoluminescence mapping of pICz-Ph/PMMA (delay time: 8 ms). f Time-resolved delayed photoluminescence spectra of pICz-Ph/PMMA excited by 310 nm. Source data is provided as a Source Data file.
Meanwhile, nearly identical PL spectra measured in dilute solution and PMMA film implied that pICz-Ph chromophores were uniformly isolated within the PMMA matrix, thus mitigating aggregation-induced spectrum broadening and red shift. As a result, narrowband deep blue afterglow with a Commission International de l’Eclairage (CIE) coordinate of (0.156, 0.044) was achieved, which is very close to the pure blue color requirement of (0.131, 0.046) defined in the Broadcast Service Television 2020 (BT.2020) displays standard (Fig. 2b). The color purity of deep blue afterglow from pICz-Ph/PMMA film was quantitatively calculated as high as 95.7% (Supplementary Fig. 32 and Supplementary Table 3). Such small FWHM and high color purity are comparable to those of inorganic quantum dots and perovskite-based emitters44, thus showing great potential in broadening the color gamut of afterglow displays. Additionally, apart from the short nanosecond decay of prompt fluorescence (10.54 ns), pICz-Ph/PMMA also displayed another long decay with a lifetime of 148.26 ms at 440 nm under ambient conditions, proving the delayed fluorescence type afterglow characteristic (Fig. 2c, Supplementary Fig. 33 and Supplementary Table 4). Notably, pICz-Ph/PMMA exhibited a relatively high PLQY of 84.6% under ambient conditions, benefiting from the rigidity of the polycyclic indolocarbazole skeleton and PMMA matrix for suppressed molecular motion-induced nonradiative decay. These results experimentally validated our proposed molecular design strategy for realizing highly efficient and ultra-narrowband deep blue organic afterglow.
We further systematically investigated the stability of the narrowband afterglow performance of pICz-Ph/PMMA film under different conditions. The influence of doping ratios on the photophysical properties was first analyzed. As shown in Fig. 2d, with increasing the concentrations of pICz-Ph from 0.005% to 0.5%, the deep blue afterglow of resultant films exhibited similarly small FWHMs below 22 nm and high color purity, maintaining long lifetimes exceeding 120 ms (Fig. 2d, Supplementary Figs. 34–37 and Supplementary Table 5). Furthermore, as excitation wavelengths varied from 250 to 400 nm, both the prompt and delayed PL spectra remained stable and narrow emission band at 440 nm, suggesting again the isolated molecule emission nature of pICz-Ph within the PMMA matrix (Fig. 2e and Supplementary Figs. 38–39). As revealed in Supplementary Fig. 40, there was also no significant change in the emission peak, FWHM, and color purity of delayed PL spectra as the delayed time increased from 0.1 to 50 ms. Time-resolved emission spectra further confirmed the constant narrowband afterglow feature as time decay (Fig. 2f), suggesting that the afterglow arises from one type of radiation decay. Additionally, after exposure to continuous irradiation of 365 nm UV light for 5 h, the delayed luminescence intensity of pICz-Ph/PMMA film could remain stable as the initial state (Supplementary Fig. 41). All the results demonstrated the high emission stability of our designed narrowband deep blue chromophore.
Mechanism of efficient narrowband deep blue afterglow
To get deep insights into the high-efficiency and ultra-narrowband afterglow from pICz-Ph/PMMA film, a series of photophysical characterizations and theoretical calculations were further carried out. As depicted in Supplementary Fig. 42, pure PMMA film showed an obvious absorption band below 250 nm, while no emission band was observed under the excitation of 225 and 350 nm. After being doped with chromophore, the pICz-Ph/PMMA film displayed nearly identical afterglow spectra and lifetimes under the same excitation wavelengths, proving that the narrowband afterglow intrinsically originated from pICz-Ph rather than energy transfer from PMMA to pICz-Ph. Meanwhile, the delayed PL intensity of pICz-Ph/PMMA film in the air atmosphere decreased by 60% compared to that in the nitrogen (Supplementary Fig. 43), indicating the triplet excited state was involved in the narrowband afterglow generation. Because triplet oxygen in the air could partly quench the triplet excitons of pICz-Ph through the triplet-triplet energy transfer process. This speculation was further verified by the singlet oxygen detection experiment using a typical singlet oxygen chemical tracker, anthracene-9,10-diyl-bis-methylmalonate (ADMA), as revealed in detail in Supplementary Fig. 44.
We subsequently collected the PL and phosphorescence spectra of pICz-Ph in 2-methyltetrahydrofuran (m-THF, 10−5 M) at 77 K. As shown in Supplementary Figs. 45–47, the dilute solution displayed well-resolved fluorescence and phosphorescence emission bands with the maximum peaks at 438 and 494 nm and lifetimes of 9.47 ns and 3.78 s, respectively, wherein the green phosphorescence could be observed for about 20 s by the naked eye after switching off the 365 nm UV lamp (Supplementary Movie 2). This result further certified the long-lived deep blue afterglow derived from delayed fluorescence of pICz-Ph rather than phosphorescence. More importantly, the ultralong phosphorescence lifetime at low temperature was primarily ascribed to the large conjugation of carbazole derivatives featuring π–π* configuration with a low radiative transition rate of triplet excitons, providing the prerequisite for achieving long-lived delayed fluorescence at room temperature. From the temperature-dependent delayed PL spectra of pICz-Ph/PMMA film, fluorescence at 440 nm dominated as temperature decreased from 300 to 220 K, while phosphorescence at 496 nm became prominent as temperature further reduced to 80 K, proving a typical TADF nature (Fig. 3a). Hence, we concluded that the high-efficiency and narrowband deep blue afterglow of pICz-Ph/PMMA film stemmed from TADF through the RISC process.
a Temperature-dependent delayed photoluminescence spectra of pICz-Ph/PMMA film ranging from 80 to 300 K. Delay time: 8 ms. b Solvation effect on the absorption and photoluminescence spectra of pICz-Ph dispersed in different solvents (10−5 M) at room temperature. DCM dichloromethane, DMF N,N-Dimethylformamide. c Solvation effect Lippert–Mataga models of different chromophores 4CzIPN (pink), DtBCz (purple), and pICz-Ph (blue). The dots and lines refer to experimental Stokes shifts and fitting lines, respectively. d The HOMO–LUMO distributions diagram of pICz-Ph. e The Huang-Rhys factor for pICz-Ph. f Spin–orbit coupling matrix element (ξ) at different nuclear coordinates. g Proposed luminescence mechanism of the long-lived, high-efficiency and ultra-narrowband deep blue organic afterglow under ambient conditions. Source data is provided as a Source Data file.
To further reveal the origin of the long lifetime of blue afterglow, the ground and excited state characteristics of pICz-Ph were then investigated through solvation effect measurements. As shown in Fig. 3b, as the solvents’ polarity increased gradually from hexane to N,N-dimethylformamide (DMF), the absorption spectra of pICz-Ph displayed negligible changes, suggesting a very weak charge transfer nature of the ground state. Meanwhile, the corresponding PL spectra exhibited a slight redshift (8 nm), which is much smaller than that of typical TADF emitter 4CzIPN (72 nm) and MR-TADF emitter DtBCz (18 nm) under the same conditions (Supplementary Fig. 48). This result confirmed that pICz-Ph exhibited significantly weaker charge transfer nature in its excited states compared with conventional TADF chromophores, which was also verified by the smaller FWHM (Supplementary Fig. 49). By further fitting the experimental data utilizing the Lippert-Mataga equation, the excited state dipole moments (μe) were calculated to be 1.3 D for pICz-Ph, 6.1 D for DtBCz, and 17.0 D for 4CzIPN, respectively (Fig. 3c and Supplementary Table 6). The large μe of 4CzIPN was attributed to the strong LRCT nature due to the spatially separated donor-acceptor (D-A) configuration, while SRCT is responsible for the small μe of DtBCz and pICz-Ph. Especially, the smallest μe of pICz-Ph resulted in the weakest charge transfer nature, thus leading to the largest ΔEST (0.32 eV) for pICz-Ph than 4CzIPN (83 meV) and DtBCz (0.15 eV). Such a large ΔEST can effectively slow the RISC process, as evidenced by the calculated kRISC rate of 29.24 s−1, several orders of magnitude smaller than the conventional TADF emitters. At the same time, PMMA with high glass transition temperature (Tg = 115.5 °C) and highly tangled network could restrain rotation/vibration of pICz-Ph and block external quenchers for stabilizing the triplet states (Supplementary Figs. 50–52). Additionally, the neat pICz-Ph film without doping into PMMA only showed broadband fluorescence (6.17 ns) with a low PLQY of 22.3% under ambient conditions, suggesting the pivotal role of PMMA matrix in stabilizing triplet excitons of pICz-Ph for achieving efficient and narrowband deep blue afterglow (Supplementary Figs. 53–54).
To better understand the generation of the ultra-narrowband deep blue afterglow of pICz-Ph, density functional theory (DFT) calculations were then performed. As shown in Fig. 3d, the mutually ortho-positioned carbon and nitrogen atoms on the polycyclic framework induced the separated HOMO and LUMO distributions within the central molecular skeleton due to the MR effect, and the resulting nonbonding molecular orbitals minimized the vibrational relaxation and vibronic coupling, thus allowing for the narrowband emission. Indeed, the simulated PL spectrum of pICz-Ph with a narrow FWHM of 15 nm matched well with the experimental one (Supplementary Fig. 55). We further calculated and analyzed the Huang-Rhys (HR) factor to quantify the coupling strength between the vibration and electron transition. As shown in Fig. 3e, the main contribution to HR factors for pICz-Ph arises from the low-frequency region (<200 cm−1), which is associated with the fused-ring structure and primarily involves torsional vibrations with negligible effect on spectral broadening. For example, the torsional vibration mode, with a frequency of 114.89 cm−1, exhibits a very small HR factor of 0.359. Note that the corresponding reorganization energy was found to be as small as 189.72 cm−1 (Supplementary Fig. 56). These results suggested the minor vibronic coupling strength and minimized structural relaxation at the excited state, which is favorable for generating narrow FWHM and long-lived delayed fluorescence (Supplementary Note 1). Additionally, unlike the small oscillator strength (fosc) induced by LRCT in D-A type chromophores, SRCT endowed pICz-Ph with a relatively large fosc of 0.10, which is beneficial for enhancing radiative transitions and achieving high PLQY. Furthermore, to elucidate the mechanism by which an efficient RISC process can occur despite the relatively large ΔEST (0.32 eV) of pICz-Ph, we conducted additional analyses to determine the vibration-dependent spin-orbit coupling matrix elements (ξ) of pICz-Ph across various nuclear coordinates between singlet and triplet states (Fig. 3f, Supplementary Figs. 57–59 and Supplementary Table 7). It was found that the vibration-induced displacement of the fused-ring framework enabled high-lying triplet states to undergo an efficient RISC process, particularly for the T3 → S1 transition with a value of ξ = 0.001 cm−1 at 0 displacement (low-frequency torsional vibration), while a large value of ξ = 0.155 cm−1 at −2 displacement. These results identified the crucial role of spin-vibronic coupling-assisted RISC in planar and symmetrical TADF chromophores with relatively large ΔEST values40. The vibration-induced displacement also decreased the energy gap between triplet states, such as T2 and T3, thereby favoring the reverse internal conversion from T1 to high Tn states. To support this, we further calculated the phosphorescent radiative decay (kp) and reverse internal conversion (RIC, kRIC) rate constants. Notably, due to sufficient thermal energy at room temperature, both kRISC (29.24 s−1) and kRIC (~104–106 s−1) are much larger than the kp (0.26 s−1), allowing the RIC from T1 to higher T2 or T3 and followed efficient RISC from T3 to T1 at room temperature, rather than phosphorescence (Supplementary Fig. 60 and Supplementary Data 1). These theoretical results are consistent with the experimental data, demonstrating the SRCT-induced intrinsically narrowband afterglow of pICz-Ph. Therefore, we concluded that the efficient and ultra-narrowband afterglow stemmed from a synergetic effect of the SRCT within the pICz-Ph chromophore and the triplet state stabilization by the rigid PMMA matrix (Fig. 3g).
The universality of narrowband deep blue afterglow by SRCT
To demonstrate the universality of our strategy, another three MR chromophores with similar fused-ring skeletons were also designed and synthesized, namely pICz-StBu, pICz, and pICz-Me (Fig. 1c). As expected, all these chromophores in dilute toluene solution (10−5 M) showed bright and narrowband deep blue fluorescence with the minimum FWHM and Stokes shift of 18 and 10 nm, respectively (Supplementary Fig. 61). After doping these chromophores into PMMA, long-lived deep blue afterglow could be observed from the resultant hybrid films under ambient conditions (Fig. 4a). Similar to pICz-Ph/PMMA, the films of pICz-StBu/PMMA, pICz/PMMA and pICz-Me/PMMA displayed superimposed steady-state PL and delayed PL spectra with the maximum peaks at 434, 440, and 442 nm, ultra-narrow FWHMs of 21, 19, and 20 nm, and high PLQYs of 84.8%, 86.1%, and 82.3%, respectively. Meanwhile, the corresponding lifetimes for the delayed emission could reach 186.48, 118.20, and 101.22 ms, respectively (Fig. 4a–c, Supplementary Figs. 62–75 and Supplementary Tables 8–11). Impressively, the afterglow all showed high color purity in the deep blue region with CIE coordinates of (0.158, 0.046), (0.155, 0.036), and (0.154, 0.048) for pICz-StBu/PMMA, pICz/PMMA, and pICz-Me/PMMA, respectively, meeting the blue requirement in BT.2020 standard (Fig. 4d, Supplementary Table 3). Additionally, stable emission profiles of the delayed PL spectra were also recorded from the hybrid film with excitation wavelengths varying from 250 to 400 nm (Fig. 4e). These results substantiated the validity of SRCT for developing efficient and ultra-narrowband deep blue organic afterglow materials.
a Photoluminescence (PL, dashed line) and delayed PL (solid line) spectra of chromophores doped in PMMA films (Delay time: 8 ms). Insets show the film photographs before (left) and after (right) turning off a 365 nm UV lamp. b Photoluminescence quantum yields of chromophores doped in PMMA films. c Afterglow lifetime decay profiles of chromophores doped in PMMA films, monitoring at the maximum emission peaks. d CIE chromatic diagram of delayed PL spectra of chromophores doped in PMMA films. The white and black triangles refer to the color gamut standards for displays defined by the Broadcast Service Television 2020 (BT.2020) and the National Television Standards Committee (NTSC), respectively. e Excitation-delayed PL mapping of pICz-StBu/PMMA film. Source data is provided as a Source Data file.
Potential applications of the efficient and narrowband afterglow
Taking advantage of the high quantum yields of the chromophores, we further demonstrated their potential in encrypted light communications. Visible light communication (VLC) is a light-based wireless communication technology with high transport speed, high fidelity, and electromagnetic interference immunity45. Different from traditional manners with optical fiber, VLC depends on the divergent propagation of light in free three-dimensional space. Ideally, indoor light bulbs with VLC capabilities can perform lighting and transmit data simultaneously, while smartphones and personal computers with photodetectors could receive and process data (Fig. 5a). To mimic this process, programmable luminescence carrying specific information data was generated by utilizing efficient chromophores. Then, the luminescence was received by a photodetector and transformed into ciphertext, which was decoded by a given high-fidelity random algorithm. Finally, the true information data was read out. In this context, by regulating the excitation wavelengths from 250 to 400 nm, the luminescence intensity output of pICz-Ph/PMMA could be programmed to four illumination modes: 00, 01, 10, and 11 (Supplementary Fig. 76). The ciphertexts were then decoded by a pre-designed random algorithm to plaintext (with four binary digits to one letter), as shown in Supplementary Table 12. The transmitted information message was next programmed to a string consisting of 36 binary digits of 0100 0011 1101 0100 1011 1011 0010 1001 1000, which was further decrypted as plaintext: I AM IN NWPU (Fig. 5b). Moreover, this encrypted light communication showed high fidelity. When we changed the one-to-one correspondence between binary digits and letters, the same information message string was encrypted to 0011 1110 1000 0011 0100 0100 0111 0010 1100 (Supplementary Fig. 77).
a Schematic illustration of encrypted visible light communications and the corresponding coding/decoding processes. b Signal output of ciphertext generated by programmable luminescence. c Pattern of USAF 1951 test card and photographs of corresponding patterns fabricated by chromophores before and after switching off a 365 nm UV lamp. d Photographs of deep blue afterglow patterns after switching off a 365 nm UV lamp and modulation transfer function (MTF) curve of afterglow photographs. e Flicker issue comparison between conventional (blue line, 4CzIPN) and afterglow chromophores (white line, pICz-Ph). Insets show photographs of pICz-Ph/PMMA film under direct current with an on-off time of 10 ms. Source data is provided as a Source Data file.
Benefiting from the high efficiency and high color purity, these chromophores can also be applied to high-resolution afterglow displays. As illustrated in Fig. 5c, based on a pICz-Ph/PMMA film, the fabricated miniature pattern with the USAF 1951 test card showed intense blue afterglow after switching off the 365 nm UV lamp. Impressively, the lines can be clearly distinguished with a width as small as 84 μm, presenting a high resolution of up to 575 dpi. Moreover, various patterns could be fabricated on the PMMA films through photolithography, including insects, trees, flowers, and so forth (Fig. 5d). Due to the high contrast afterglow, these patterns also showed a high distinguishability of 23.2 lp mm−1. This result is superior to the conventional organic room-temperature phosphorescence (RTP) chromophore (11.6 lp mm−1) with the same emission peak at 440 nm but poorer color purity (FWHM = 82 nm) and lower PLQY (23.5%), as shown in Supplementary Fig. 78. Such high-resolution emitters are potential candidates applied in luminescent tags, multiplexed imaging and sensing. In addition, given the solution-processable nature, the chromophores-PMMA mixture can be fabricated into a uniform and large-area (13 × 13 cm2) luminescent film (Supplementary Fig. 79). Moreover, as shown in Fig. 5e and Supplementary Figs. 80–82, the utilization of afterglow chromophores can reduce the brightness change (Imax–Imin) under the high-frequency and repetitive on-off processes compared to conventional chromophores with short lifetimes (ns–μs), which is expected to mitigate flicker issues in specific scenarios, such as radar scanning displays and emergency signs. Stable and bright blue luminescence was also recorded from an electricity-driven display device under on-off (10 ms) direct current. Therefore, these results demonstrated the potential of highly efficient narrowband afterglow materials in high-resolution and ultrawide-color-gamut afterglow displays.
Discussion
In summary, we have presented an effective strategy to achieve high-efficiency and ultra-narrowband deep blue organic afterglow from a series of indolocarbazole-based chromophores by synergistically combining SRCT with host matrix rigidification. These MR-TADF emitters showed a record-setting FWHM of 18 nm, along with a long lifetime of 186.48 ms and a high PLQY of 86.1% under ambient conditions when doped into PMMA. An ultrapure deep blue organic afterglow (440 nm) was realized, with a CIE coordinate of (0.156, 0.044), closely aligning with the blue color requirement in the BT.2020 standard. Combining theoretical calculations, the SRCT-induced large ΔEST, minimized vibronic coupling and structural relaxations, as well as the stabilization effect of the rigid PMMA matrix, are primarily responsible for the highly efficient narrowband afterglow. Given these superior afterglow characteristics, potential applications in encrypted light communications and high-resolution afterglow displays were successfully demonstrated. This finding not only provides deep insights into the rational design of efficient and narrowband organic afterglow chromophores but also broadens their potential in cutting-edge technologies such as ultrahigh-definition displays, photolithography, data storage, and multiplexed biomedical imaging.
Methods
Reagents and materials
Unless otherwise stated, all raw materials, including reagents and chemicals, used in the experiments were purchased from commercial sources without further purification unless given special instructions. For flash column chromatography, silica gel with 200–300 mesh was used.
Measurements
Nuclear magnetic resonance (1H and 13C NMR) spectra were recorded on a Bruker 500 MHz spectrometer using CDCl3 as the solvent. Chemical shifts were referenced to the internal standard tetramethylsilane (TMS). Gel permeation chromatography (GPC) measurements were performed on an Agilent 1260 HPLC system equipped with a G7110B pump and a G7162A refractive index detector. Tetrahydrofuran was used as the eluent at 0.5 mL min−1 flow rate and PMMA as the standard. Steady-state fluorescence and phosphorescence spectra and lifetimes were measured using Hitachi F-7100 or Edinburgh FLS1000 equipped with a xenon arc lamp (Xe900), a nanosecond hydrogen flash-lamp (nF920), or a microsecond flash-lamp (μF900). The ultraviolet-visible (UV-vis) optical absorption spectra were measured by Hitachi U-3900H. Photoluminescence quantum yields (PLQYs) were collected on a Hamamatsu absolute PL quantum yield spectrometer C11347 (Japan) under ambient conditions. Powder X-ray diffraction patterns were recorded on a Bruker D8 Advance X-ray diffractometer with Cu-Kα radiation. The differential scanning calorimetry (DSC) thermal analysis data were tested by a DSC 214 instrument (NETZSCH, Germany) under a nitrogen atmosphere. HRMS data were collected on Waters Xevo G2-XS Tof (Waters) with acetonitrile as solvent. Photographs were taken by a Canon EOS 850D camera under the irradiation of a hand-held ultraviolet lamp at room temperature (~300 K) or low temperature (77 K).
Preparation of the doped PMMA film
With the 1 wt% pICz-Ph/PMMA film as a model, 1 mg pICz-Ph and 100 mg PMMA were first completely dissolved in 2 mL toluene. Then 200 μL of the above mixture solution was put onto a quartz plate (1.5 × 1.5 cm2) by drop casting, which was then heated in a vacuum oven (75 °C) for 4 h. The obtained film was then used for subsequent characterizations.
Computational details
The equilibrium configuration and harmonic vibrational frequency of the ground (S0) and the lowest singlet excited states (S1), as well as the natural transition orbitals (NTOs) of the low-lying excited states, were performed at the (TD)B3LYP/def2-SVP level implemented in the Gaussian 09 package46. To consider the double excitation effect in these multiple resonance emitters, the ADC2 module in the Turbomole program47 was used to calculate energy levels of the low-lying excited states and oscillator strength (f) of the S1 state, and the spin–orbit coupling matrix elements were evaluated using the ORCA program48. The fluorescence spectrum was simulated by the thermal vibration correlation function rate theory implemented in the MOMAP program49. Note that the Duschinsky rotation effect is not considered.
Data availability
The authors declare that all the data supporting the findings of this study are provided in the manuscript and its supplementary information files, or are available from the corresponding authors on request. Source data are provided with this paper.
References
Sheen, M. et al. Highly efficient blue InGaN nanoscale light-emitting diodes. Nature 608, 56–61 (2022).
Zhu, C. et al. Supramolecular assembly of blue and green halide perovskites with near-unity photoluminescence. Science 383, 86–93 (2024).
García de Arquer, F. P. et al. Semiconductor quantum dots: technological progress and future challenges. Science 373, eaaz8541 (2021).
Ren, A. et al. Emerging light-emitting diodes for next-generation data communications. Nat. Electron. 4, 559–572 (2021).
Chan, C. et al. Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission. Nat. Photon. 15, 203–207 (2021).
Jeon, S. et al. High-efficiency, long-lifetime deep-blue organic light-emitting diodes. Nat. Photon. 15, 208–215 (2021).
Cho, H. H. et al. Suppression of Dexter transfer by covalent encapsulation for efficient matrix-free narrowband deep blue hyperfluorescent OLEDs. Nat. Mater. 23, 519–526 (2024).
Huang, T. et al. Delocalizing electron distribution in thermally activated delayed fluorophors for high-efficiency and long-lifetime blue electroluminescence. Nat. Mater. 23, 1523–1530 (2024).
Hua, T. et al. Deep-blue organic light-emitting diodes for ultrahigh-definition displays. Nat. Photon. 18, 1161–1169 (2024).
Lee, H. D. et al. Valley-centre tandem perovskite light-emitting diodes. Nat. Nanotechnol. 19, 624–631 (2024).
Qiu, W. et al. Afterglow OLEDs incorporating bright closely stacked molecular dimers with ultra-long thermally activated delayed fluorescence. Matter 6, 1231–1248 (2023).
Davis, J., Hsieh, Y. H. & Lee, H. C. Humans perceive flicker artifacts at 500 Hz. Sci. Rep. 5, 7861 (2015).
Kim, M. Assessment of the effect on the human body of the flicker of OLED displays of smartphones. J. Inf. Disp. 22, 269–274 (2021).
Jiang, K. et al. Enabling robust and hour-level organic long persistent luminescence from carbon dots by covalent fixation. Light Sci. Appl. 11, 80 (2022).
Nie, F., Wang, K. Z. & Yan, D. Supramolecular glasses with color-tunable circularly polarized afterglow through evaporation-induced self-assembly of chiral metal–organic complexes. Nat. Commun. 14, 1654 (2023).
Wang, Y. et al. High performance of simple organic phosphorescence host–guest materials and their application in time-resolved bioimaging. Adv. Mater. 33, 2007811 (2021).
Chen, K. et al. Twofold rigidity activates ultralong organic high-temperature phosphorescence. Nat. Commun. 15, 1269 (2024).
Tian, R., Gao, S., Li, K. & Lu, C. Design of mechanical-robust phosphorescence materials through covalent click reaction. Nat. Commun. 14, 4720 (2023).
Liang, Y. et al. Enabling highly robust full-color ultralong room-temperature phosphorescence and stable white organic afterglow from polycyclic aromatic hydrocarbons. Angew. Chem. Int. Ed. 63, e202318516 (2024).
Zhao, Y. et al. Fused-ring pyrrole-based near-infrared emissive organic RTP material for persistent afterglow bioimaging. Angew. Chem. Int. Ed. 63, e202317431 (2024).
Guo, H. et al. Photocured room temperature phosphorescent materials from lignosulfonate. Nat. Commun. 15, 1590 (2024).
Chen, Q. et al. Long lifetimes white afterglow in slightly crosslinked polymer systems. Nat. Commun. 15, 2947 (2024).
Li, J. et al. A direct observation of up-converted room-temperature phosphorescence in an anti-Kasha dopant-matrix system. Nat. Commun. 14, 1987 (2023).
Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869–885 (2020).
Dai, X. Y., Huo, M. & Liu, Y. Phosphorescence resonance energy transfer from purely organic supramolecular assembly. Nat. Rev. Chem. 7, 854–874 (2023).
Ha, J., Hur, S., Pathak, A., Jeong, J. & Woo, H. Recent advances in organic luminescent materials with narrowband emission. NPG Asia Mater. 13, 53 (2021).
Wang, G. et al. Dual-mechanism design strategy for high-efficiency and long-lived organic afterglow materials. J. Am. Chem. Soc. 146, 24871–24883 (2024).
Kabe, R. & Adachi, C. Organic long persistent luminescence. Nature 550, 384–387 (2017).
Zou, X. et al. Narrowband organic afterglow via phosphorescence Förster resonance energy transfer for multifunctional applications. Adv. Mater. 35, 2210489 (2023).
Zhang, X. et al. Multicolor hyperafterglow from isolated fluorescence chromophores. Nat. Commun. 14, 475 (2023).
Kondo, Y. et al. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat. Photon. 13, 678–682 (2019).
Fan, X. C. et al. Ultrapure green organic light-emitting diodes based on highly distorted fused π-conjugated molecular design. Nat. Photon. 17, 280–285 (2023).
Liu, J. et al. Toward a BT.2020 green emitter through a combined multiple resonance effect and multi-lock strategy. Nat. Commun. 13, 4876 (2022).
Yang, M., Park, I. S. & Yasuda, T. Full-color, narrowband, and high-efficiency electroluminescence from boron and carbazole embedded polycyclic heteroaromatics. J. Am. Chem. Soc. 142, 19468–19472 (2020).
Wang, Q., Xu, Y., Yang, T., Xue, J. & Wang, Y. Precise functionalization of a multiple-resonance framework: constructing narrowband organic electroluminescent materials with external quantum efficiency over 40. Adv. Mater. 35, 2205166 (2023).
Huang, Z. et al. Charge transfer excited state promoted multiple resonance delayed fluorescence emitter for high-performance narrowband electroluminescence. J. Am. Chem. Soc. 145, 12550–12560 (2023).
Meng, G. et al. High-efficiency and stable short-delayed fluorescence emitters with hybrid long- and short-range charge-transfer excitations. Nat. Commun. 14, 2394 (2023).
Kim, H. J. & Yasuda, T. Narrowband emissive thermally activated delayed fluorescence materials. Adv. Opt. Mater. 10, 2201714 (2022).
Jiang, H., Jin, J. & Wong, W. Y. High-performance multi-resonance thermally activated delayed fluorescence emitters for narrowband organic light-emitting diodes. Adv. Funct. Mater. 33, 2306880 (2023).
Lee, H. L. et al. Multiple-resonance extension and spin-vibronic-coupling-based narrowband blue organic fluorescence emitters with over 30% quantum efficiency. Adv. Mater. 34, 2202464 (2022).
Ma, H., Peng, Q., An, Z., Huang, W. & Shuai, Z. Efficient and long-lived room-temperature organic phosphorescence: theoretical descriptors for molecular designs. J. Am. Chem. Soc. 141, 1010–1015 (2019).
Thomas, H. et al. Aromatic phosphonates: A novel group of emitters showing blue ultralong room temperature phosphorescence. Adv. Mater. 32, 2000880 (2020).
Garain, S., Sarkar, S., Chandra Garain, B., Pati, S. K. & George, S. J. Chiral arylene diimide phosphors: circularly polarized ambient phosphorescence from bischromophoric pyromellitic diimides. Angew. Chem. Int. Ed. 61, e202115773 (2022).
Han, T. H. et al. A roadmap for the commercialization of perovskite light emitters. Nat. Rev. Mater. 7, 757–777 (2022).
Bao, C. et al. High performance and stable all-inorganic metal halide perovskite-based photodetectors for optical communication applications. Adv. Mater. 30, 1803422 (2018).
Frisch, M. J. et al. Gaussian 09, Revision D.01 (Gaussian Inc., 2009).
Balasubramani, S. G. et al. TURBOMOLE: modular program suite for ab initio quantum-chemical and condensed-matter simulations. J. Chem. Phys. 152, 184107 (2020).
Neese, F. Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 8, e1327 (2018).
Niu, Y. L. et al. Molecular materials property prediction package (MOMAP) 1.0: a software package for predicting the luminescent properties and mobility of organic functional materials. Mol. Phys. 116, 1078–1090 (2018).
Acknowledgements
This research is supported by the National Natural Science Foundation of China (22475172 (L.G.)), (52203242 (L.G.)), (22475098 (Z.A.)), (22275085 (H.M.)), (T2441002 (H.M.)) and (62288102 (W.H.)), Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ23B020004 (L.G.), the National Key R&D Program of China (grant No. 2020YFA0709900 (W.H.)), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
X.Z., Z.A., L.G., and W.H. conceived the experiments. X.Z. and N.G. conducted the experiments and measurements. Y.W. helped with the measurements of differential scanning calorimetry and gel permeation chromatography. X.Z., N.G., and L.G. wrote the paper. Z.L., A.L., and H.M. contributed to the theoretical calculations. W.H. gave suggestions for the paper. All authors contributed to the data analyses.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Ji-Eun Jeong and Chuluo Yang for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zou, X., Gan, N., Lin, Z. et al. Short-range charge transfer for efficient ultra-narrowband deep blue afterglow. Nat Commun 16, 6412 (2025). https://doi.org/10.1038/s41467-025-61513-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61513-7