Abstract
Water and ion channels are crucial for moisture energy harvesting, requiring precise pore design for mass transfer control. However, the key challenge lies in managing the localized assembly process of membrane materials to arrange them orderly, forming confined mass transfer pathways and stable solid-liquid interfaces. This is essential for exploring the interrelationship among channel morphological characteristics, mass transfer dynamics, and device power generation performance. This work proposes the use of freeze-assisted salting-out to meticulously construct hydrogel bilayer membranes with micro-meso-macroporous oriented channels and asymmetric charge characteristics. The produced polyvinyl alcohol/MXene hydrogel devices achieved a Voc × Jsc of 11.4 μW cm−2 (pure hydrovoltaic effect) and 146 μW cm−2 (with active electrodes) at 25 °C, 45%RH, surpassing most moisture-based generators. In addition, the power generation performance is highly consistent with the Hofmeister series, with stronger salting-out effect to obtain more micropores and mesopores, and ice crystal growth can help obtain ordered macropores. It has faster water transport rate, higher ionic conductivity, better ionic selectivity, and stronger channel stability than traditional moisture-based power generation membranes. This relationship between pore tuning from salt ions and device power generation performance provides a design basis for the development of high-performance moisture-based power generators.
Similar content being viewed by others
Introduction
The growing demand for clean and low-carbon energy has driven the rapid development of new green energy. Moisture-enabled electrical generators (MEG) can directly convert atmospheric water cycles into electrical energy, providing an effective pathway for achieving new green energy1,2,3,4. Nanoscale channels adsorb water molecules in humid environments to complete dynamic gas–liquid phase transitions. Utilizing the continuous heat exchange, ion dissociation, and rearrangement of interfacial charges generated during this process, corresponding moisture-based energy devices can be designed5,6,7. Therefore, nanoscale channels are considered ideal structural systems for constructing moisture-based energy devices. However, during interactions with moisture, the dynamic evolution of free ions and water within the channels is challenging to control effectively. For example, nano and sub-nano channel sizes have considerable charge densities, exhibiting excellent ion selectivity. However, they typically result in ion transport channels that are too narrow and too long, thereby reducing ion flux8,9,10,11. Although microscale channels can store water molecules through the network’s swelling effect12, achieving efficient moisture adsorption and heat release to enhance ionic conductivity, the Debye length is much smaller than the channel size, leading to a loss of ion selectivity13,14. On the other hand, dynamic atmospheric humidity changes are accompanied by frequent adsorption and desorption processes15. Small-sized nano channels are unfavorable for water storage, and excessive swelling after hydration may cause a rapid decline in ion selectivity16. Additionally, dynamic adsorption and desorption affect the stability of the nano channel framework, and insufficient mechanical strength can easily lead to channel deformation or collapse17. Therefore, overcoming the trade-off between ion conductivity, ion selectivity, and mechanical stability requires precise structural control of the membrane’s hydrated pore sizes.
Currently, preliminary explorations have been conducted on regulating pore hydration, ion selectivity, and fabricating appropriate pore sizes within hydrated membranes. For example, confining angstrom-sized 2D channels within robust epoxy resins can significantly reduce pore hydration swelling and enhance ion–pore surface charge interactions, thereby maximizing ion diffusion flux to generate moisture-based electric potential18. Introducing hydrophobic side chain groups into polymers to customize the local hydrophobic environment can effectively adjust pore grid sizes at the sub-nanometer scale10, and appropriately sized hydrated micropores are crucial for maintaining ion selectivity during long-term operation. However, the aforementioned confinement manufacturing methods compress the pores to the micropore (below 2 nm) scale, resulting in very long ion transport paths within the membrane material, which increases ion transport resistance10. Highly interconnected micro-meso-macroporous ion transport channels fabricated using pyrolysis, plasma etching, and other processes19,20,21 ensure ion selectivity while greatly shortening the ion transport paths within the membrane. Compared to membranes with a single pore size, they offer superior ion conductivity-selectivity trade-offs22,23,24. However, due to the complexity of those processes, there are still limitations in structural designability and pore customization control. Insufficiently controlled pore structures hinder the study of mass transfer mechanisms and make it difficult to achieve the ultimate fine-tuned synergistic control of ion conductivity, ion selectivity, and mechanical stability required for high-performance MEG.
Inspired by the above work, we used freezing-assisted salting out in poly(vinyl alcohol)/MXene hydrogels to finely construct interconnected multi-scale pores, and explored the relationship between pore tuning due to salt ions and device power generation performance, and found that the synergistic enhancement of ionic conductivity–ionic selectivity–channel stability required for high power densities could be achieved by changing the sequence of salted ions only. Our material and device design involves:
-
1.
Interconnected multilevel network: The hydrogel comprises micron-scale honeycomb pores, nanometer-scale fibrous pores, and sub-nanometer-scale 2D layered pores.
-
2.
Enhanced mechanical stability: The incorporation of MXene nanoscale reinforcement materials and the induction of polymer crystallization through salting-out in PVA both contribute to the enhancement and toughening of the hydrogel, thereby maintaining the mechanical stability of the nanoscale framework.
-
3.
Optimal pore configuration: Utilizing the Hofmeister series allows for the continuous and in situ modulation of polymer–ion interactions over a broad range, facilitating the identification of the optimal pore configuration to ensure a balance between ion conduction and selectivity.
-
4.
Built-in electric fields: By employing an asymmetric grafting modification method on MXene functional groups, we construct a bilayer hydrogel structure with built-in electric fields, further enhancing ion selectivity and energy conversion efficiency.
Besides, the relationship between the hydrogel skeleton structure and surface charge, the water and ion transport kinetics, and the device power generation performance has been explored, providing a design reference for the development of devices in this field.
Results
Design, fabrication, and characterization of hydrogel-based MEGs
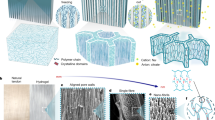
In salt solutions, the type and concentration of salt ions exert a series of effects on the physicochemical properties of proteins, such as solubility and aggregation states. This phenomenon is known as the Hofmeister effect, also referred to as the ion-specific effect25,26. Inspired by the Hofmeister effect, we have designed bilayer hydrogel moisture-enabled electrical generators (BHMEG) fabricated through molecular and structural engineering. As illustrated in Fig. 1a, both layers are composed of a composite of polyvinyl alcohol (PVA) and Ti₃C₂Tx (MXene), with the MXene in one layer undergoing surface grafting with N-[3-(trimethoxysilyl)propyl] ethylenediamine (AEAPTMS), as shown in Fig. 1b. The modified hydrogel (P-MXene@PVA) and the unmodified hydrogel (N-MXene@PVA) form an interfacial bond through self-assembly. Since PVA is relatively neutral and the surface charge of MXene changes from negative to positive upon modification27, a built-in electric field is expected to form at the interface of the two hydrogel layers28,29. This electric field facilitates ion rectification and transport, thereby enhancing energy conversion efficiency. Gold electrodes are placed on the upper and lower surfaces of the hydrogel layers, with the upper gold electrode being perforated to aid the device in more effectively capturing moisture and outputting electrical signals.
a Schematic of the structural composition of BHMEG. The upper electrode is a porous gold electrode, while the two middle layers consist of different charged MXene and PVA composite hydrogels, and the lower electrode is a gold electrode. b Surface grafting modification of Ti3C2Tx MXene. c Flowchart of the process for the preparation of the freeze-assisted salting-out hydrogels, where (i) is the mixing and stirring of MXene nanosheets with the PVA solution, (ii) is the directional freeze molding, (iii) is the salting-out-induced recrystallization process, and (iv) is the SEM image of the surface and cross-section structure of the gel after removing the salting-out ions. d Comparison of the performance of the salting-out BHMEG for power generation with Hofmeister sequence ions. e Comparison of the performance of the BHMEG with that of the reported pure hydrovoltaic MEGs (left)33,34,35,36,37,38,39 and the active electrode assisted MEGs (right)40,41,42,43,44,45,46,47 in the medium humidity range (30%RH–60%RH) at 25 °C. The source data are available in the “Data availability” section.
The preparation process of single-layer hydrogel is illustrated in Fig. 1c. Initially, the prepared MXene suspension is mixed with a PVA solution in a specific ratio and stirred uniformly. The precursor mixture is then poured into a custom-designed mold, which is subsequently placed in liquid nitrogen for freezing. Due to the metallic copper base of the custom mold, ice crystals nucleate at the bottom and grow in the direction of the temperature gradient during the freezing process30, as shown in Fig. 1c(ii). Condensation and close packing of polymer chains during freezing prepare them for subsequent strong aggregation and crystallization induced by salting out31. The completely frozen hydrogel is then soaked in a salt ion solution following the Hofmeister series. During the freeze-thaw process, salt ions participate in the cross-linking of the gel. The soaking process lasts ~24 h, during which salt ions act like needles to tightly stitch the polymer chains together. After gelation, the salt ions are removed by soaking32, as depicted in Fig. 1c(iii). Finally, to enhance the moisture absorption capacity of the hydrogel, the hydrogel is soaked in a LiCl solution for 12 h. The resulting hydrogel exhibits micron-scale honeycomb-like pores on the top surface, remaining after the melting of ice crystals, and the cross-section shows neatly arranged directional channels with a preferential orientation in the vertical direction, as shown in Fig. 1c(iv). According to the power generation performance tests, the output performance correlates with the Hofmeister series (\({{\mbox{Citrate}}}^{-}\), SO42−, CO32−, Ac−, Cl−). Citrate ions, which are at the forefront of the series, produce the optimal electrical signals after salting-out treatment of the hydrogel, as illustrated in Fig. 1d. The optimized citrate sodium salting-out bilayer hydrogel can output an open-circuit voltage (Voc) of 0.8 V and a Voc × Jsc of 11.4 μW cm−2 at 45% RH. When coupled with an active Al electrode, the output reaches up to 146 μW cm−2. The designed bilayer moisture-enabled electric generator demonstrates excellent electrical output performance compared to both pure hydrovoltaic devices33,34,35,36,37,38,39 and devices coupled with active electrodes40,41,42,43,44,45,46,47 under the same temperature and humidity environment (30% RH–60% RH, 25 °C). as shown in Fig. 1e. Detailed data comparisons are presented in Supplementary Tables 1 and 2.
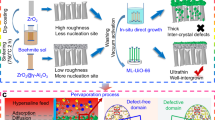
A comprehensive characterization of the hydrogel’s microstructure was conducted. As illustrated in Fig. 2a, the cross-sections of hydrogels treated with various 1 M salt solutions (with the cation fixed as Na⁺ and varying the anion species) were imaged using scanning electron microscopy (SEM). All five types of hydrogels exhibited regular directional pore structures, which facilitate the rapid and directed movement of ions within the charged microchannels. Upon further magnification (Fig. 2b), the surfaces of the micron-scale vertically aligned pore walls revealed dense and abundant nanonetworks. And the microporous aperture distribution maps of the Horvath–Kawazoe (HK) model simulations obtained from the BET test (Supplementary Fig. 1) revealed the existence of microporous pores of less than 2 nm within the sodium citrate salting-out hydrogel. The above series of characterizations confirmed the successful fabrication of a hierarchical porous structure in the hydrogels. This hierarchical structure is advantageous for mass transfer during the moisture-driven process. Notably, the micron-scale pore sizes followed the sequence: Citrate− < \({{\mbox{SO}}}_{4}^{2-}\) < \({{\mbox{CO}}}_{3}^{2-}\) < Ac− <Cl−, exhibiting a systematic variation from tight to sparse. This pattern demonstrates the regular influence of different salt ions on the pore structure of the hydrogel network. This phenomenon originates from the salting-out-induced polymer recrystallization process, where the extensive nucleation of polymer chains due to the abundant formation of crystal grains leads to denser pores48,49.
a SEM images of cross-sectional pores inside different salting-out hydrogels. b SEM images of abundant pores inside sodium citrate salting-out hydrogel skeleton sidewall (top sectional view), inset: cross-sectional view. c Surface SEM images and EDS elemental characterization of modified P-MXene membranes. After modification, new elements of N and Si were added. d The peak fitting results of N1s in the X-ray photoelectron spectroscopy (XPS) analysis of modified P-MXene nanosheets. e ζ-potentials of N-MXene and P-MXene suspensions at different pH. f X-ray diffractograms (XRD) of gels prepared with and without salting out. g Fourier transform infrared (FTIR) spectra of gels prepared with and without salting out. h Compressive strain test curves of gels prepared with and without salting out. The source data are available in the “Data availability” section.
Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy were performed on the modified P-MXene nanosheets after drying, as shown in Fig. 2c. The presence of Si and N elements, which are absent in the original MXene nanosheets, originates from the grafting of AEAPTMS, while the O element is derived from the -OH groups on the original MXene nanosheets. Additionally, X-ray photoelectron spectroscopy (XPS) was conducted on the modified P-MXene nanosheets. As depicted in Fig. 2d, the high-resolution N 1s spectrum was analyzed, revealing fitted peaks at 400.9 eV and 399.1 eV, corresponding to the free and protonated states of nitrogen, respectively. The -NH₃⁺ groups are formed by the combination of -NH₂ with H⁺ from water50, confirming the successful grafting of -NH- and -NH₂ functional groups onto the P-MXene nanosheets. Zeta potential measurements were carried out on both unmodified and modified MXene suspensions (0.1 mg mL−1) before and after modification, as shown in Fig. 2e. Due to the presence of -F, -OH, and -O functional groups on the surface of MXene nanosheets, which inherently carry negative charges, the ζ potential of N-MXene at pH=7 is −27 mV. In contrast, the P-MXene surface, after modification with -NH₃⁺ groups, exhibits a positive surface charge with a ζ potential of 23 mV. This demonstrates the presence of opposite charges within the bilayer hydrogel system.
The size of polymer crystalline grains and the lattice spacing significantly influence the mechanical properties and pore structure of hydrogels. To further investigate the impact of the Hofmeister effect on the internal structure of hydrogels, X-ray diffraction (XRD) was employed to analyze hydrogels subjected to different salting-out treatments. As shown in Fig. 2f, all hydrogel samples exhibited distinct characteristic diffraction peaks near 20°, corresponding to the (101) crystal plane of PVA. Notably, the hydrogel without salting-out exhibited the largest forward shift in the diffraction peak angle (θ). The remaining salting-out hydrogels followed the order: Cl− > Ac− > CO32- > SO42- > Citrate−, with the forward shift in the diffraction peak angle decreasing sequentially. According to Bragg’s law51, the relationship between the diffraction angle (θ) and the interplanar spacing (d) is defined by the following equation:
where n is the number of diffraction levels and λ is the wavelength of the X-rays. When the lattice spacing (d) increases, the diffraction angle (θ) must decrease to maintain the equation balanced, resulting in a shift of the diffraction peak towards smaller angles. A forward shift of the diffraction peak indicates more loosely packed polymer crystallites, corresponding to an increase in the lattice spacing of crystals within the gel. The lattice spacing formed in the polymerization process of the Citrate−-treated hydrogel is the smallest, which originates from its strongest salting out effect, the polymer chains are more tightly aggregated, and ultimately, a crystal structure with smaller lattice spacing is formed, which improves the strength of the material.
Meanwhile, the grain size of the gels (Supplementary Table 3) was calculated by measuring the diffraction peak half width (FWHM) of each gel from Eqs. 2 and 3:
where D is the grain size, K is Scherrer’s constant (usually taken as 0.89), \(\lambda\) is the wavelength of the X-rays, and B is the half-peak width of the diffraction peak converted to the radian system51. The crystalline grain size in the salting-out gels showed regular variation with salting-out ions, and the grain size of the gels treated by sodium citrate salting-out was about 32.295 Å, which was the smallest grain size in the Hofmeister series. The small crystalline grain size of the polymer is favorable to provide more pores and a higher specific surface area.
Figure 2g compares the Fourier transform infrared (FTIR) spectra of various salting-out-induced crosslinked hydrogels, and the peak at 3341 cm−1 reflects the stretching vibration of the -OH group in the hydrogel. The absorption peak of -OH was stronger in the hydrogel without salting-out induction, and the intensity of the absorption peak gradually decreased when a series of salt ion treatments with the same concentration were added. The intensities of the absorption peaks were arranged in the following order: Cl− > Ac−> CO32-> SO42-> Citrate−, and the frequency of the stretching vibration of -OH changed, which led to the weakening of the peaks of -OH in the FTIR spectra. This indicates that the -OH functional group in the hydrogel is involved in the network structure through the formation of hydrogen bonds52. The spectra indicate that more hydrogen bonds are formed within the citrate ion-treated hydrogel, and the hydrogen bonds, as strong interactions, can enhance the mechanical properties and stability of the hydrogel network as well as the water retention ability of the hydrogel, which is of great significance for the improvement of the performance of the MEG. Since the designed and prepared hydrogels have vertically oriented pore structures, the force in the vertical direction best reflects the hydrogel backbone mechanical stability. In order to investigate the effect of different salts on the mechanical properties of the hydrogel, the compressive stress-strain curves of the samples were tested using a tensile machine, and as shown in Fig. 2h, the compressive strength of the Citrate−-treated gel is about seven times that of the Cl− treated hydrogel. We also performed cyclic compression tests on the Citrate− treated gels, as shown in Supplementary Fig. 2, the stress decay of our hydrogels was only 5.1% after 10 compression cycles at a higher strain (50%), with little change in the hysteresis loop area, which demonstrated the good structural stability of sodium citrate salting-out gels.
Analysis of power generation performance and influencing factors
The power generation principle of BHMEG is as follows: The cross-scale pore structure in hydrogels with high specific surface area synergizes with the hygroscopic salt LiCl in the gels to efficiently capture water vapor from the environment. Water molecules undergo the gas–liquid phase change dynamic cycling process of adsorption, liquefaction, and evaporation gasification in the nanochannels, in which the energy accompanying the heat exchange provides power for the subsequent water and ion transport, and the oriented pore structure of the hydrogel facilitates the reduction of mass transfer resistance. On the other hand, due to the double-layer asymmetrically charged hydrogel skeleton, a directional built-in electric field is formed in the internal space of the two-layer gel, which accelerates the separation of anions and cations under the action of the built-in electric field. The anions and cations selectively migrate in opposite directions to form ionic currents. In all, the device converts ambient moisture energy into electrical energy through the close synergy of moisture adsorption and heat exchange in the cross-scale pore structure, fast transport in the oriented pore structure, charge separation at the bilayer heterogeneous interface, and selective migration of ions in the domain-limited EDL.
The power generation performance of the devices was tested in the same humidity environment (45% RH), and all the tested bilayer hydrogels with a volume ratio of MXene to PVA solution of 1:1 were treated with 1 M different salt solutions for freeze-thaw salting. Both bilayer gels were soaked in 3 M LiCl solution to enhance the moisture absorption of the gels. The results are shown in Fig. 3a, b. The magnitude of open-circuit voltage and short-circuit current is in the following order: Citrate− > SO42- > CO32- > Ac− > Cl−, which is in high agreement with the Hofmeister series. The BHMEG with Citrate− has the highest open-circuit voltage of 0.45 V and the highest short-circuit current of 1.47 μA, which visualizes the Hofmeister effect on the excellent regulation of the performance of MEGs.
a Comparison of open-circuit voltage output of BHMEG with different salt additions. b Comparison of short-circuit current output of BHMEG with different salt additions. (All the salting out gels in a and b have been soaked using 3 M LiCl solution.) c Influence of the concentration of sodium citrate solution on the electrical output performance of BHMEG. d Influence of the content of MXene on the electrical output performance of BHMEG. Data represent the mean ± standard deviation (n = 3). e Effect of the concentration of immersed LiCl on the current and the variation of moisture absorption. Data represent the mean ± standard deviation (n = 3). f Effect of different relative humidity on the electrical output performance of BHMEG. g Comparison of the electrical output performance of monolayer gel with that of bilayer gel. h Impedance matching test of BHMEG device. i Output power at different external resistances. The source data are available in the “Data availability” section.
Subsequently, sodium citrate solution was selected as the salting-out inducer to explore other influencing factors. Salting-out treatments were conducted using sodium citrate solutions of varying concentrations, and the electrical output signals of the resulting BHMEG were tested, as shown in Fig. 3c. The results indicated that as the concentration of sodium citrate increased, both the open-circuit voltage and the short-circuit current of the BHMEG initially increased and then decreased, reaching their maximum output at a sodium citrate concentration of 1 M. This suggests that the concentration of sodium citrate directly affects the internal structure of the hydrogel and, consequently, the performance of the BHMEG. When the salt concentration is excessively high, it may lead to over-crosslinking of the hydrogel’s internal structure, resulting in pore channels that are unsuitable for ion transport and thereby diminishing the electrical signal output performance32.
Furthermore, the effect of MXene content within the hydrogel on the performance of the BHMEG was investigated, as depicted in Fig. 3d. In these experiments, the contents of P-MXene and N-MXene within the bilayer hydrogel were varied synchronously. The presence of Ti metal elements and a rich array of active functional groups on the MXene structure imparts high charge characteristics. At low MXene contents, the overall charged hydrogel framework is insufficiently charged, which is unfavorable for ion transport. However, as the MXene content increases, excessive stacking of MXene nanosheets may occur (Supplementary Fig. 3), leading to reduced structural stability of the hydrogel framework (Supplementary Fig. 4) and a consequent decline in electrical output performance53. The results demonstrated that an optimized MXene content of 60% yielded an output Jsc of 6.2 μA cm−2 and Voc of 500 mV.
To improve the moisture absorption capabilities of the BHMEG, the hydrogel is immersed in a lithium chloride (LiCl) solution during the device fabrication process, allowing LiCl to permeate the hydrogel network (Supplementary Fig. 5). In the XRD patterns of MXene@PVA hydrogels containing LiCl, the characteristic peak near 20° disappears. This observation indicates that after soaking in the LiCl solution, Li⁺ ions form complexes with the hydroxyl groups in PVA, disrupting the original hydrogen bonds within PVA. Consequently, characteristic LiCl peaks at (200), (220), and (222) appear in the diffraction spectrum. Subsequently, the influence of LiCl concentration on the moisture absorption performance and electrical output of the BHMEG was further investigated, as shown in Fig. 3e (Supplementary Fig. 6). The moisture absorption performance was tested by drying the gel at 60 °C for removing surface moisture and then weighing and recording it, then placing it in a 45% RH constant humidity flask and taking it out after 12 h and weighing it again, and comparing the weight changes recorded before and after the two times. The results demonstrated that as the concentration of LiCl increased, the moisture absorption capacity of the hydrogel continuously enhanced, approaching saturation at a concentration of 2 M. However, it is noteworthy that the electrical output signals of the BHMEG reached their peak levels at a LiCl concentration of 0.5 M. Beyond this concentration, further increases in LiCl led to a decline in both current and voltage outputs. Excessively high concentrations of LiCl exacerbate the disruption of hydrogen bonds in PVA by Li⁺ ions, diminishing the regularity of PVA molecular chain arrangements. This reduction in structural regularity adversely affects the mobility of water molecules and ions within the hydrogel54. Additionally, an increase in LiCl concentration results in a decreased Debye length, which weakens ion selectivity.
Further investigations were conducted to evaluate the impact of varying humidity levels on the electrical performance of the BHMEG, as illustrated in Fig. 3f. The fabricated BHMEG exhibited distinct responses to different humidity conditions. Specifically, as the relative humidity increased from 15% to 45% RH, the electrical signal output reached its maximum value. Beyond this threshold, with further increases in relative humidity, the hydrogel’s moisture absorption approached saturation, resulting in diminished ion transport efficiency and subsequent reductions in both the Jsc and Voc. Supplementary Fig. 7 demonstrates that the moisture absorption inside the hydrogel is saturated under high humidity and the humidity gradient disappears. Through comparative experiments, it was observed that the electrical generation performance of single-layer hydrogel MEG was markedly inferior to that of bilayer configuration, as depicted in Fig. 3g. This disparity underscores the critical role of the heterojunction formed by the bilayer hydrogel in enhancing the device’s electrical performance. To further validate this finding, the bilayer hydrogel was inverted and the electrodes reconnected, resulting in completely symmetrical current signal outputs (Supplementary Fig. 8). By preparing a BHMEG without oriented pore structure, its electrical signal output performance was compared with that of the oriented pore structure (Supplementary Fig. 9). The current and voltage were significantly lower for BHMEG without the oriented structure designed, i.e., the hydrogel prepared by oriented freezing provided a fast channel for the ion transportation, and greatly enhanced the power generation performance of the BHMEG.
In order to realize the optimal power output of the BHMEG device for practical applications, the relationship between the device and the external load was explored. As shown in Fig. 3h, i, a single BHMEG device was connected in series with a resistor box, and as the load increased from 100 to 1 MΩ, the voltage of the BHMEG increased and the current density decreased. At a load of 10 kΩ, the power output density of the device reaches 3.2 μW cm−2.
Correspondence analysis between power generation performance and pore structure
The power generation performance is mainly limited by ion flux, ion selectivity, and pore stability. First, the relationship between the salting-out effect and the ion flux and ion selectivity is analyzed. Initially, the ion flux is related to the moisture absorption and ion transport path, so it is necessary to do a more detailed analysis of the pore structure under different salting-out effects. Here, specific surface area and N2 adsorption tests were performed on hydrogels with different salt additions. As shown in Supplementary Fig. 10, the overall shapes of the isothermal adsorption and desorption curves of different salting-out hydrogels all belong to the same type of adsorption and desorption, reflecting the existence of both monolayer adsorption and multilayer adsorption stages. The shape of the hysteresis loop also reflects that the pore structure contains slits and cylindrical pores formed by the stacking of lamellar particles, which corresponds to the vertical pores generated by the stacking of two-dimensional lamellar materials of MXene with the assistance of oriented freezing. The isothermal adsorption curves show an increasing trend at low relative pressures, indicating the presence of microporous structures, while a rapid increase at high relative pressures indicates macroporous structures. The adsorption pattern presented by the isothermal curves lays the foundation for the later determination of the pore size distribution within the gel. The adsorption curves of the sodium citrate hydrogel are significantly higher than the rest of the hydrogels at lower relative pressures (0.05–0.35), but the adsorption curves increase relatively flatly as the relative pressure increases and are lower than those of the rest of the hydrogels at higher relative pressures, which confirms that the sodium citrate hydrogel is rich in micropores and mesopores, and that the macroporous pore sizes and quantities are significantly smaller than those of the rest of the hydrogels. In addition, the N2 adsorption curve of the NaCl salting-out gel sample was only lower than that of sodium citrate, and the adsorption amount gradually increased with the increase of the relative pressure, reflecting that the sodium chloride salting-out gel has the widest macroporous pore size distribution, and it can be reasonably deduced that, due to the directional vertical growth of the ice crystals, the effect of the salting-out effect of Cl− in the salting-out-induced crystallization of the polymers is weaker, which produces a larger micrometer-sized pore, the micrometer-sized pore size on the edge of the extrusion of the pores leads to the generation of more slit pores and microporous size defects.
The specific surface areas of different salting-out gels were determined by Brunel–Emmett–Taylor (BET) method, as shown in Fig. 4a, the BET specific surface area of sodium citrate salting-out hydrogel was the largest, which was 38.925 m2 g−1, and the specific surface areas of salting-out ionic hydrogel were decreased in the following order: Citrate− > SO42- > CO32- > Ac− > Cl−. The regularity of the surface area remains consistent with the Hofmeister series. A larger specific surface area implies that the sodium citrate-treated hydrogel may possess a more abundant internal porosity, particularly in mesopores and micropores. Subsequently, the Barrett–Joyner–Halenda (BJH) model was utilized to calculate the pore size distribution in the mesoporous and macroporous scales of the salting-out hydrogels. As in Fig. 4b, the major pore size inside the sodium citrate-treated gel is the smallest, distributed between 50 and 70 nm, while the major pore size distribution of the gels gradually increases with the backward shift of the sequence (distributed between 88 and 160 nm). In Fig. 4c and Supplementary Table 4, sodium citrate-treated hydrogel shows the highest proportion of micropores and mesopores (92.1%), which is advantageous for moisture capture and ion transport. This phenomenon confirms that different salting-out ions systematically regulate the internal pore size of the hydrogels. Furthermore, the hydrophilicity of the surfaces of hydrogels was assessed (Supplementary Fig. 11). The citrate-treated hydrogel demonstrated exceptional hydrophilicity by absorbing a water droplet in merely 0.04 s. In contrast, hydrogels treated with ions positioned later in the Hofmeister series exhibited progressively longer times to fully absorb the water droplets. Specifically, hydrogels treated with chloride (Cl⁻) and acetate (Ac⁻) ions were unable to completely absorb the water droplets.
a Specific surface area of different salting-out gels. b Pore size distributions of mesopores and macropores of different salting-out gels calculated by BJH model simulation. c Pore volume ratios of micropores-mesopores-macropores of different salting-out gels. d Schematic diagram of the effect of salting-out ions on the cross-linking process of gel network. e Comparison of the optical photographs of citrate gels and chloride gels before and after swelling for 24 h. f The swelling rate of different salting-out gels in the aqueous solution to reach equilibrium swelling process. g Comparison of the ionic conductivity values of different salting-out gels. The upper right picture shows the electrochemical impedance spectra of different salting-out gels. h Comparison of ionic rectification ability of different salting-out BHMEG. The illustration in the upper right corner shows the I–V rectification curve of BHMEG that was induced by sodium citrate salting-out. The source data are available in the “Data availability” section.
The rapid diffusion and absorption of water droplets may be affected by the synergistic effect of macro- and micro-meso pores. We performed the same droplet hydrophilicity experiments on the undirected freezing-prepared sodium citrate salting-out hydrogels containing only mesopore and micropore structures, and the experimental results show (Supplementary Fig. 12) that the hydrogels containing only micropores and mesopores, which completely absorbed the droplets for 1.8 s, had a slower rate of absorption than that of the gels containing micro-meso-macro pores. This phenomenon suggests that the composite pore-size hydrogels containing macropores have a better ability to absorb water droplets by diffusion. The macropores provide a fast transport channel for water, which enables water molecules to enter the interior of the hydrogel quickly, while the micropores and mesopores provide a large specific surface area, which can significantly increase the contact area between water molecules and the hydrogel, thus accelerating the adsorption and diffusion of water. The multilevel pore structure with micro-meso-macro pores can realize the best water absorption performance, and the synergistic effect between the pore structures is the source of the hydrogel’s rapid water absorption ability.
Different salt ions themselves affect different attraction and entanglement between polymer molecular chains during cross-linking due to different ionic radius and charge density55 (Fig. 4d). By testing the swelling behavior of different salting-out gels in water, the pore stability of the gels during moisture absorption and removal can be indirectly reflected. The hydrogel with Citrate- for induced cross-linking, showed less volume change after 24 h of swelling compared to the Cl- induced gel (Fig. 4e), suggesting that the Citrate-hydrogel has a more stable pore structure. Further, by comparing the swelling rate of the salting-out gels during reaching the swelling equilibrium, as shown in Fig. 4f, it was demonstrated that the Hofmeister series ion-induced gels had a regular arrangement of their anti-swelling properties, which also reflected the regular effects of different ions on the densification, cross-linking density, and backbone stability of the hydrogel network.
Regular modulation of pore size provides a way to change the ionic conductance. Electrochemical impedance spectroscopy was conducted on BHMEGs, resulting in a series of impedance spectra (Fig. 4g, top right). Utilizing an equivalent circuit model that fits the impedance spectra (Supplementary Fig. 13), the ion impedance (Rb) of BHMEGs with varying salting-out treatments was simulated and calculated using ZSimpWin 3.30 software. The ion conductivity (δ) was determined based on the following ion conductivity equation:
where δ represents the ion conductivity, L is the thickness of the hydrogel, Rb is the ion impedance of the hydrogel, and A is the contact area between the hydrogel surface and the electrodes. The ion conductivity values for BHMEGs subjected to different salting-out ions are presented in Supplementary Table 5. The magnitude of ion conductivity directly reflects the ion flux and ion mobility within the hydrogel. As depicted in Fig. 4g, when citrate ions induce the crosslinking and formation of the hydrogel, the resulting internal ion transport network exhibits the highest ion conductivity of 0.3774 S m−1. The ion transport capabilities decrease in the following order: Citrate− > SO42- > CO32- > Ac− > Cl−. The abundant micropores and mesopores provide more transport pathways and hydrolysis sites for water molecules to penetrate the internal network of the hydrogel. A higher ion conductivity signifies a more robust ion transport capability, facilitating efficient moisture capture and energy conversion.
Ion selectivity refers to the preferential permeability of membrane materials to specific ions. This selectivity is typically associated with factors such as pore size, surface charge, and pore orientation. In the bilayer hydrogel system, the opposite charges of the two layers create a built-in electric field at the interface. This electric field drives the directional transport of cations and anions generated through hydrolysis, thereby generating voltage and current. To evaluate ion selectivity, the rectification ratio was introduced as a metric. A high rectification ratio indicates a significant difference in ion transport efficiency under forward and reverse potentials, which generally signifies higher selectivity. In this study, a bias voltage ranging from −0.5 to 0.5 V was applied to the device, and the asymmetry of the current–voltage (I–V) curves was observed. As depicted in Fig. 4h, the citrate-induced salting-out device achieved a rectification ratio of 30.98. In contrast, the rectification ratios of devices treated with other salting-out ions decreased sequentially following the Hofmeister series. The ion selectivity within the gel can be greatly influenced by precisely adjusting the pore size. The richer micropores and mesopores enhanced the ionic interaction with the pore surface, thus improving the ionic selectivity. In addition, the presence of a built-in electric field, while enabling a similar rectification phenomenon in bilayer hydrogel devices without oriented pore structure (Supplementary Fig. 14), resulted in a significantly lower ion selectivity, illustrating the necessity of an oriented structure.
In addition, we explored the power generation performance and ion transport properties of the PVA/MXene system in which only micropores exist (Supplementary Fig. 15). We utilized secondary vacuum filtration to obtain bilayer heterogeneous microporous films, and the current output performance obtained was significantly lower than that of BHMEG with a multi-scale pore structure system at 45% RH and 25 °C. Although the ionic conductivity was higher (231.6 S m−1), there was no obvious rectification phenomenon, which may be attributed to the fact that the small-sized channels are not favorable for storing water, and the ionic selectivity may rapidly decrease or even disappear after over-expansion upon hydration, thus causing the degradation of the power generation performance.
In all, we verified that directional freezing combined with the salting-out effect can modulate the self-assembly morphology of polymers/MXene over a wide window, leading to an orderly arrangement into an interconnected channel network of microporous-mesoporous-macroporous. The stronger salting-out effect leads to the formation of more micropores and mesopores during the cross-linking process of the hydrogel, which can realize the simultaneous improvement of ion conductivity-ion selectivity-mechanical stability required for high-performance moisture-enabled electric generator.
Application exploration
In practical applications, in order to satisfy the demand for longer power supply and power switching between different appliances, we conducted several cycle tests on the electrical signal output of the BHMEG, as shown in Fig. 5a. For the same BHMEG, under the same humidity (45% RH) and temperature (25 °C), five short-circuit current tests were conducted, each with a testing time of 8000 s. The current was still able to be maintained at 8.7 μA in the last test. When we coupled the device with an active metal electrode and replaced one side electrode with an Al electrode, due to the direct contact of the metal electrode with the hydrogel surface, the effectively reduces the overpotential of the hydrolysis reaction, increases the electrochemical efficiency, and improves the charge collection and transfer by the electrode (Supplementary Fig. 16) the open-circuit voltage and short-circuit current density of a single device with an area of 9 mm × 9 mm are enhanced to 1.04 V and 140.7 μA cm−2, respectively. Connecting multiple devices in series or parallel can effectively amplify the electrical output, as shown in Fig. 5b-c, six parallel-connected devices can reach 650 μA under indoor conditions (~48% RH, 23 °C) and 5.9 V with six devices in series, with the voltage increasing linearly with the current as the number of series or parallel connections increases.
a Current output performance of BHMEGs during 5 cycles of use. b Short circuit current of BHMEG units with different numbers of parallel connections. The purple curve in the figure represents a linear fitting curve, and the fitting equation is: y = 0.97x + 0.13. c Open circuit voltage of BHMEG units with different numbers of series connections. The green curve in the figure is a linear fitting curve, and the fitting equation is: y = 106.63x + 16.47. d Conceptual diagram of integrated BHMEGs serving as a power source for skin detection, smart home appliances, and wearable electronic devices. e Demonstration diagram of power supply to temperature and humidity display by series-parallel connection of integrated BHMEGs. The source data are available in the “Data availability” section.
Our BHMEG devices exhibit excellent adaptability to ambient humidity conditions, enabling them to power a variety of small-scale portable electronic devices, as illustrated in Fig. 5d. Integrated BHMEGs can supply power to compact handheld skin diagnostic instruments, which are particularly compatible with the natural humidity range of human skin (40–60% RH). This synergy with humidity sensors facilitates enhanced monitoring of skin health, allowing for timely replenishment of moisture and nutrients. In other fields, BHMEGs hold potential for powering LED displays, Bluetooth-enabled entertainment devices, and other smart wearable technologies, thereby offering a wide array of future applications. As shown in Fig. 5e, nine BHMEGs are connected in series and parallel in the laboratory to provide power for the temperature and humidity display by continuously absorbing moisture from the air and converting it into energy.
Discussion
Nanoscale channels are considered to be ideal structures for constructing moisture-enabled electric generators, but most of the existing strategies for controlling pore mass transfer are frequently constrained by the inherent trade-offs among ionic conductivity, ion selectivity, and mechanical stability, which hinders the further improvement of the power performance of MEGs. Here, we propose to use freeze-assisted salting-out to precisely control the localized assembly process of MXene and PVA to construct sub-nanometer-nanometer-micrometer cross-scale ordered channel structures. We are surprised to find that the ion ordering (Hofmeister series) in the salting-out effect directly affects several mass transfer and mechanical properties, including polymer crystallinity, pore size, hydrated pore expansion, polymer backbone strength, ionic conductivity, and ion rectification ratio. The power generation performance depends on the mass transfer kinetics, and the power generation performance is also highly consistent with the Hofmeister series, with high-performance devices implying a higher percentage of micropores and mesopores. To further increase the power generation, we employ the design of freeze-assisted salting-out and PN junction structure, which has faster water transport rate, higher ionic conductivity/ionic selectivity, and stronger mechanical backbone compared with conventional membranes. The power density of this device is far higher than that of most MEG devices, and the moderate humidity conditions for power generation are also more suitable for most geographical locations. This salt-ion-associated pore-size tuning design provides substantial insights into the precise control of ion- and water-transporting capabilities of moisture-based power generation membranes: previous studies have more often considered only the positive effects of salt ions on moisture trapping and ionic conductivity, neglecting the tuning of pore structure as a significant influence on power generation performance. It is expected to inspire researchers in the field of moisture-based power generators to emphasize the diverse roles of salt ions introduced during device design.
Methods
Materials preparation
Aluminum titanium carbide (MAX, 98%, Forsman), lithium fluoride (99%, Aladdin), dimethyl sulfoxide (99.5%, Aladdin), hydrochloric acid (Concentration of 38%, Chengdu Kelong Chemical Co., Ltd.), N-[3-(trimethoxysilyl)propyl]ethylenediamine (95%, Aladdin), acetic acid (36%), polyvinyl alcohol (PVA, type 1799, Titan), trisodium citrate dihydrate (99%, Chengdu Kelong Chemical Co., Ltd.), anhydrous sodium sulfate (99%, Aladdin), anhydrous sodium carbonate (99.99%, XFNANO), sodium acetate (AR, Aladdin), sodium chloride (99.5%, Macklin), and anhydrous lithium chloride (98.5%, Chengdu Kelong Chemical Co., Ltd.) pet film. All reagents were used as received without further purification. Deionized water was used for all experiments.
Synthesis and modification of Ti3C2Tx MXene
Ti3C2Tx was prepared by etching with hydrofluoric acid56. Firstly, 1.6 g of LiF was dissolved in 20 mL of 9 M HCl, and stirred in a Teflon container at 25 °C for 15 min. The operation should be carried out in a fume hood and protective measures should be taken. Then 1 g of Ti3AlC2 powder was slowly added to the above solution, and stirred for 24 h in a water bath at a constant temperature of 50 °C and a speed of 32 × g. The post-reaction solution was washed by centrifugation, and the cycle was repeated several times until the pH of the upper clear solution was about 5. The deposits were re-dispersed with deionized water and sonicated for 30 min to make the dispersion homogeneous, so as to make the multilayers of Ti3C2Tx delaminated into monolayers of nanosheets, and, finally, the nanosheets containing the Ti3C2Tx nanosheets were collected by centrifugation using a 3280 × g high-speed centrifugation for 1 h. Ti3C2Tx nanosheets, the supernatant of Ti3C2Tx nanosheets was obtained, and 10 mg mL−1 of Ti3C2Tx MXene suspension (N-MXene) was obtained, with a yield of ~54%.
The prepared Ti3C2Tx MXene suspension was centrifuged at 18,900 × g for 1 h. Excess water was removed to obtain the precipitate, and the precipitated nanosheets were re-dispersed with ethanol pending surface grafting. 3 g of AEAPTMS was added to 20 g of aqueous-alcoholic solution (10/90 wt%) with thorough mixing, and the pH of the solution was adjusted by the addition of acetic acid to pH≈3.5. The mixed solution was added to the ethanol-dispersed Ti3C2Tx solution (mass of Ti3C2Tx was 1 g), and the reaction was carried out by stirring at room temperature at 32 × g for 24 h. 24 h for sufficient grafting reaction. The reacted solution was centrifuged several times with ethanol to wash the precipitate and remove the unreacted silane coupling agent. Finally, the washed precipitate was re-dispersed with deionized water to obtain 10 mg mL−1 of modified Ti3C2Tx solution (P-MXene), with a yield of ~79%.
Preparation of bilayer MXene@PVA hydrogels
Taking N-MXene@PVA hydrogel as an example, N-MXene solution at a concentration of 10 mg mL−1 was mixed and stirred with 10 wt% PVA solution in the ratio of 1:3, 2:3, 1:1, 3:2, and 3:1 by volume at 25 °C. The mixed precursor was poured into a polytetrafluoroethylene (PTFE) mold with brass base, and the mold was placed in liquid nitrogen for directional freezing to promote the vertical growth of ice crystals, and then the frozen gel was immersed in a series of salt solutions (Sodium Citrate, Na2SO4, Na2CO3, NaAc, NaCl) at a concentration of 1 M, freeze-thawed and crosslinked for 24 h, and the molded hydrogel was removed and cleaned of excess salt ions by immersion in DI water. Subsequently, the hydrogel was immersed in 1 M LiCl solution for 12 h to obtain N-MXene@PVA hydrogel. P-MXene@PVA hydrogel only needs to use modified P-MXene solution, and the other steps are identical to the above preparation process. The thickness of each prepared monolayer hydrogel is 3 mm. The N-MXene@PVA hydrogel and P-MXene@PVA hydrogel were self-assembled together to obtain the bilayer MXene@PVA hydrogel.
Preparation of control group hydrogels
Preparation process of non-salting-out MXene@PVA hydrogels: Similar to the standard experimental group, the mixed precursor is poured into a brass-bottomed mold. After immersion in liquid nitrogen for freezing, the mold is transferred to a temperature-controlled, constant temperature and humidity chamber with a preset temperature program. The hydrogels undergo three freeze-thaw cycles, ranging from 25 °C to −40 °C, with each cycle lasting 24 h. Non-directed pore structure hydrogels: The mixed precursor is poured into a pure PTFE mold without a brass base. The mold is then placed in liquid nitrogen for freezing. The frozen hydrogel is subsequently soaked in a sodium citrate solution to induce salting-out crosslinking, resulting in control hydrogels with non-directed pore architectures.
Preparation of bilayer microporous membrane
Add 0.5 mL of 5 mg mL−1 concentration of N-MXene solution with 0.1 mL of 5 wt% PVA solution in 20 mL of DI water, mix and stir for 30 min, pour the mixed solution into a vacuum pumping filter, and use a 0.22 μm PVDF filter membrane for pumping and filtration, to obtain a single layer of N-MXene@PVA microporous membrane, and in the same steps, the P- MXene@PVA microporous membrane was filtered in the upper layer of N-MXene@PVA microporous membrane. After the filtration was completed, the film was removed for drying and immersed in 1 M sodium citrate solution for 24 h, and then put into 1 M LiCl solution for 12 h, and then the bilayer microporous membrane of MXene/PVA system was finally obtained.
Fabrication of bilayer hydrogel moisture-enabled electric generator (BHMEG) devices
Polyethylene terephthalate (PET) substrates were utilized as the foundational material for electrode fabrication. A gold layer with a thickness of 40 nm was electroplated onto the PET substrates to form the electrodes. The upper electrodes were subsequently patterned with an array of perforated holes using laser etching techniques. The bilayer hydrogels were then sandwiched between the upper and lower gold electrodes. To facilitate electrical measurements, carbon fiber wires were connected to the electrodes, ensuring reliable electrical pathways for subsequent testing and analysis.
Characterization methods
Field emission scanning electron microscopy (Thermo Fisher, Apreo 2C, USA) was used to obtain SEM. The elemental distribution of the modified P-MXene nanosheets after drying was analyzed using SEM and energy spectroscopy X-ray imaging. The zeta potential of the MXene suspensions before and after modification was determined using a high-sensitivity zeta potential analyzer (Malvern, Nano ZS, UK). The chemical states of the grafted N elements on the modified MXene surface were measured using an XPS (Thermo Scientific K-Alpha, USA). The characteristic structural peaks of different ion salting-out hydrogels were measured by Fourier transform infrared spectroscopy (Nicolet iS50, Thermo Fisher, USA). X-ray diffractometer (Malvern Panaco 4 kW, China) was used at a scanning rate of 5° min−1 to collect XRD maps. The specific surface area and pore size distribution of the different ion-salting hydrogels were tested using a fully automated specific surface area and pore size analyzer (Micromeritics ASAP2460, USA). The compressive stress-strain curves of the hydrogels were recorded using AGS-X tensile machine.
Electrical measurements
Voc and Isc of the MEG outputs were recorded by a galvanometer (Keithley 6514), a digital multimeter (Keithley DMM7510), and a source meter (Keithley 2450). The I–V rectification curves of the devices were obtained by linear scanning voltammetry using a CHI electrochemical analyzer (CHI760E, CH Instruments, Inc., US), and the impedance profiles of the devices with different salting gels were obtained using AC Impedance measurements (AC Impedance).
Data availability
The data supporting the findings of the study are included in the main text, supplementary information, and the source data files. The source data are available in the Zenodo database under accession code [https://doi.org/10.5281/zenodo.15208206].
References
Zhao, F., Cheng, H., Zhang, Z., Jiang, L. & Qu, L. Direct power generation from a graphene oxide film under moisture. Adv. Mater. 27, 4351–4357 (2015).
Bai, J., Huang, Y., Cheng, H. & Qu, L. Moist-electric generation. Nanoscale 11, 23083–23091 (2019).
Huang, Y., Cheng, H. & Qu, L. Emerging materials for water-enabled electricity generation. ACS Mater. Lett. 3, 193–209 (2021).
Shen, D. et al. Moisture-enabled electricity generation: from physics and materials to self-powered applications. Adv. Mater. 32, 2003722 (2020).
Wang, X. et al. Hydrovoltaic technology: from mechanism to applications. Chem. Soc. Rev. 51, 4902–4927 (2022).
Zhang, X., Liu, H. & Jiang, L. Wettability and applications of nanochannels. Adv. Mater. 31, 1804508 (2019).
Zhang, J. et al. Recent advances in moisture-induced electricity generation based on wood lignocellulose: preparation, properties, and applications. Int. J. Biol. Macromolecules 279, 135258 (2024).
Mo, R.-J. et al. Regulating ion affinity and dehydration of metal-organic framework sub-nanochannels for high-precision ion separation. Nat. Commun. 15, 2145 (2024).
Liu, Y.-C., Yeh, L.-H., Zheng, M.-J. & Wu, K. C.-W. J. S. A. Highly selective and high-performance osmotic power generators in subnanochannel membranes enabled by metal-organic frameworks. Sci. Adv. 7, eabe9924 (2021).
Wang, A. et al. Selective ion transport through hydrated micropores in polymer membranes. Nature 635, 353–358 (2024).
Xin, W. et al. Biomimetic KcsA channels with ultra-selective K+ transport for monovalent ion sieving. Nat. Commun. 13, 1701 (2022).
Wang, A. et al. Ion-selective microporous polymer membranes with hydrogen-bond and salt-bridge networks for aqueous organic redox flow batteries. Adv. Mater. 35, 2210098 (2023).
De France, K. J., Xu, F. & Hoare, T. Structured macroporous hydrogels: progress, challenges, and opportunities. Adv. Healthc. Mater. 7, 1700927 (2018).
Tan, R. et al. Hydrophilic microporous membranes for selective ion separation and flow-battery energy storage. Nat. Mater. 19, 195–202 (2020).
Xiang, C., Yang, X., Deng, F., Chen, Z. & Wang, R. Daytime air–water harvesting based on super hygroscopic porous gels with simultaneous adsorption–desorption. Appl. Phys. Rev. 10, 041413 (2023).
Ozkul, S. et al. Selective adsorption in ion exchange membranes: the effect of solution ion composition on ion partitioning. Water Res. 254, 121382 (2024).
Li, X. & Gong, J. P. Design principles for strong and tough hydrogels. Nat. Rev. Mater. 9, 380–398 (2024).
Kim, S. et al. Neuromorphic van der Waals crystals for substantial energy generation. Nat. Commun. 12, 47 (2021).
Xing, R. et al. Creation of triple hierarchical micro-meso-macroporous n-doped carbon shells with hollow cores toward the electrocatalytic oxygen reduction reaction. Nano Micro Lett. 10, 3 (2017).
Li, X. et al. Construction of angstrom-scale ion channels with versatile pore configurations and sizes by metal-organic frameworks. Nat. Commun. 14, 286 (2023).
Wang, M. et al. Nature-inspired interconnected macro/meso/micro-porous mxene electrode. Adv. Funct. Mater. 33, 2211199 (2023).
Chao, F. et al. Micro‑meso-macroporous FeCo-N-C derived from hierarchical bimetallic FeCo-ZIFs as cathode catalysts for enhanced Li-O2 batteries performance. J. Energy Chem. 35, 212–219 (2019).
Zhu, T. et al. 3D covalent organic framework membrane with fast and selective ion transport. Nat. Commun. 14, 5926 (2023).
Zhang, H. et al. Sub-millisecond lithiothermal synthesis of graphitic meso–microporous carbon. Nat. Commun. 15, 3491 (2024).
Moghaddam, S. Z. & Thormann, E. The Hofmeister series: specific ion effects in aqueous polymer solutions. J. Colloid Interface Sci. 555, 615–635 (2019).
Gregory, K. P. et al. Understanding specific ion effects and the Hofmeister series. Phys. Chem. Chem. Phys. 24, 12682–12718 (2022).
Wang, J. et al. Heterogeneous Two-dimensional lamellar Ti3C2Tx membrane for osmotic power harvesting. Chem. Eng. J. 452, 139531 (2023).
Kim, H. J., Chen, B., Suo, Z. & Hayward, R. C. Ionoelastomer junctions between polymer networks of fixed anions and cations. Sci367, 773–776 (2020).
Duan, J. et al. Tough hydrogel diodes with tunable interfacial adhesion for safe and durable wearable batteries. Nano Energy 48, 569–574 (2018).
Zhang, H. & Cooper, A. I. Aligned porous structures by directional freezing. Adv. Mater. 19, 1529–1533 (2007).
Hua, M. et al. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 590, 594–599 (2021).
Wu, S. et al. Poly(vinyl alcohol) Hydrogels with broad-range tunable mechanical properties via the Hofmeister effect. Adv. Mater. 33, 2007829 (2021).
Gao, X. et al. Electric power generation using paper materials. J. Mater. Chem. A 7, 20574–20578 (2019).
Liu, X. et al. Power generation from ambient humidity using protein nanowires. Nature 578, 550–554 (2020).
Long, Y. et al. Moisture-induced autonomous surface potential oscillations for energy harvesting. Nat. Commun. 12, 5287 (2021).
Moreira, K. S. et al. Flexible, low-cost and scalable, nanostructured conductive paper-based, efficient hygroelectric generator. Energy Environ. Sci. 14, 353–358 (2021).
Tan, J. et al. Self-sustained electricity generator driven by the compatible integration of ambient moisture adsorption and evaporation. Nat. Commun. 13, 3643 (2022).
Xu, T. et al. Electric power generation through the direct interaction of pristine graphene-oxide with water molecules. Small 14, 1704473 (2018).
Zheng, S. et al. Continuous energy harvesting from ubiquitous humidity gradients using liquid-infused nanofluidics. Adv. Mater. 34, 2106410 (2022).
Duan, W. et al. Silicon nanowire/ionic hydrogel-based hybrid moist-electric generators with enhanced voltage output and operational stability. Energy Environ. Sci. 17, 3788–3796 (2024).
Guchait, A., Pramanik, S., Goswami, D. K., Chattopadhyay, S. & Mondal, T. Elastomeric ionic hydrogel-based flexible moisture-electric generator for next-generation wearable electronics. ACS Appl. Mater. Interfaces 16, 46844–46857 (2024).
Mo, J. et al. Sulfated cellulose nanofibrils-based hydrogel moist-electric generator for energy harvesting. Chem. Eng. J. 491, 152055 (2024).
Sun, Z., Wen, X., Wang, L., Yu, J. & Qin, X. Capacitor-inspired high-performance and durable moist-electric generator. Energy Environ. Sci. 15, 4584–4591 (2022).
Wen, X. et al. High-performance fully stretchable moist-electric generator. Adv. Funct. Mater. 34, 2470059 (2024).
Yang, S. et al. Ionic hydrogel for efficient and scalable moisture-electric generation. Adv. Mater. 34, 2200693 (2022).
Zhang, J., Zhuang, J., Lei, L. & Hou, Y. Rapid preparation of a self-adhesive PAA ionic hydrogel using lignin sulfonate–Al3+ composite systems for flexible moisture-electric generators. J. Mater. Chem. A. 11, 3546–3555 (2023).
Zhu, R. et al. Boosting moisture induced electricity generation from graphene oxide through engineering oxygen-based functional groups. Nano Energy 94, 106942 (2022).
He Z., et al. Tuning ice nucleation with counterions on polyelectrolyte brush surfaces. Sci. Adv. 2, e1600345 (2016).
Wu, S. et al. Ion-specific ice recrystallization provides a facile approach for the fabrication of porous materials. Nat. Commun. 8, 15154 (2017).
Riazi, H. et al. Surface modification of a MXene by an aminosilane coupling agent. Adv. Mater. Interfaces 7, 1902008 (2020).
Barla, K., Herino, R., Bomchil, G., Pfister, J. C. & Freund, A. Determination of lattice parameter and elastic properties of porous silicon by X-ray diffraction. J. Cryst. Growth 68, 727–732 (1984).
Mansur, H. S., Sadahira, C. M., Souza, A. N. & Mansur, A. A. P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng.: C. 28, 539–548 (2008).
Hart, J. L. et al. Control of MXenes’ electronic properties through termination and intercalation. Nat. Commun. 10, 522 (2019).
Wang, R. et al. Holistically engineered polymer–polymer and polymer–ion interactions in biocompatible polyvinyl alcohol blends for high-performance triboelectric devices in self-powered wearable cardiovascular monitorings. Adv. Mater. 32, 2002878 (2020).
Cui, W. et al. Strong tough conductive hydrogels via the synergy of ion-induced cross-linking and salting-out. Adv. Funct. Mater. 32, 2204823 (2022).
Murali, G. et al. A review on MXene synthesis, stability, and photocatalytic applications. ACS Nano 16, 13370–13429 (2022).
Acknowledgements
The authors acknowledge the financial support from the Atomic-level Manufacturing Project of Southwest Jiaotong University (R110225H01077), the National Key Research and Development Program of China (No. 2020YFA0711001), Natural Science Foundation of Sichuan Province (No. 2023NSFSC0981, 2023NSFSC1988), the Fundamental Research Funds for the Central Universities of China (Grant Nos. SWJTU 2682024CG012, 2682023QZ004, 2682023ZTPY001, 2682023KJ012, 2682022KJ017). This work was completed at the Research Center for Ultra-precision Surface Manufacturing, Southwest Jiaotong University. The authors would like to thank Shiyanjia Lab (www.shiyanjia.com) for technical Support. The authors would like to thank Ceshigo (www.ceshigo.com) for the SEM analysis. The authors would like to thank Analysis and Testing Center of Southwest Jiaotong University for technical Support.
Author information
Authors and Affiliations
Contributions
T.Y. and C.W. provided the idea for the article. C.W. designed experiments and completed the original draft. T.Y. and L.Q. revised and guided the article. P.D. and Y.H. analyzed the material structure. C.F. and X.L. completed the characterization of photocatalytic materials. Y.Z. analyzed data and discussed the results. T.Y. and L.Q. provided funding support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jinchao Zhang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, C., Duan, P., Huang, Y. et al. Micro-meso-macroporous channels finely tailored for highly efficient moisture energy harvesting. Nat Commun 16, 6568 (2025). https://doi.org/10.1038/s41467-025-61898-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61898-5