Abstract
Excitation-dependent multicolor emission from a single-component system, independent of aggregation, remains a fundamental challenge due to inherent difficulties in innovative principles. Herein, we propose a molecular symmetry-breaking strategy to enrich electronic processes, enabling the molecule to exhibit excitation-dependent multicolor emissions from one chemical entity. A star-shaped molecule, 1,3,5-(4-tert-butylphenyl-o-carboranyl-4-phenyl)benzene (Ph-3CP) is designed, where spatial restriction induces inequivalence among three bulky, non-planar branches. This asymmetry gives rise to a broad excitation-dependent emission range of nearly 175 nm across solution, amorphous, and crystalline states. Crystallization from different solvents successfully traps distinct asymmetric conformers of Ph-3CP, providing direct experimental evidence for the predicted symmetry-breaking structures from theoretical calculations. Structure-property relationship studies further reveal two distinct relaxation pathways that dominate the emission behavior of this molecular system. Leveraging these properties, we develop a single-component fluorescence sensor array that enables rapid and selective identification of chlorinated hydrocarbon vapors. This work provides a general strategy for designing multifunctional luminescent materials through symmetry-controlled excited-state engineering.
Similar content being viewed by others
Introduction
Nature’s optical phenomena provide profound insights into materials science1. For example, chameleons achieve dynamic color changes by adjusting the arrangement of nanocrystals in their cells, showcasing the exquisite control of optical properties inherent in natural systems2. This structure-based dynamic optical tuning offers a new pathway for developing intelligent optical materials, usable for multifunctional optical devices and complex environmental applications3,4,5,6.
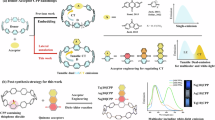
Inspired by nature, scientists have sought to design and develop organic luminescent materials with dynamic responsiveness7,8,9,10, encompassing diverse systems such as fluorescence11,12,13, phosphorescence14,15, and thermally activated delayed fluorescence (TADF)16. Among them, organic fluorescent materials (OFMs) have attracted considerable attention due to their potential for applications in intelligent display17,18, sensors19,20,21,22,23,24, and bioimaging25,26,27. Materials featured diversity and tunability have flourished in the field of OFMs12,28,29,30,31. However, most conventional OFMs exhibit fixed emission spectra, limiting their adaptability for multifunctional applications. In contrast, excitation-dependent (Ex-de) luminescent materials offer dynamic and reversible multicolor emission simply by adjusting the excitation wavelength, significantly expanding the functional range in anti-counterfeiting, encryption, and decryption of OFMs32,33,34. Despite their immense potentials, the development of Ex-de materials faces numerous challenges. Conventional approaches typically rely on co-crystallization or co-polymerization of multiple luminophores, which often suffer from color aging, phase segregation, and compromised material stability (Fig. 1a)35. To overcome these limitations, single-fluorophore systems have been proposed as ideal candidates for Ex-de luminescence. These systems typically achieve tunable emissions by manipulating molecular conformational diversity in the crystalline state (Fig. 1b)36. Additionally, the formation of excimers or aggregates with distinct intermolecular interactions in the solid state has also been reported to realize the Ex-de emission (Fig. 1c)37,38. However, these approaches generally rely on crystalline packing to stabilize the required conformations, the applicability to other states, such as amorphous states, remain difficult. The essence of single component Ex-de multicolor emission is the manipulation of relaxation pathways within excited states. In other words, dynamic molecular structure changes and electronic delocalization are essential for forming diverse electronic states.
Symmetry-breaking strategies have emerged as a design approach for achieving multifunctionality in emissive materials39,40, enabling precise control over energy levels and emission properties by enriching the excited states41,42,43,44. Du and coworkers demonstrated anti-Kasha excited-state luminescence in heterogeneous carbon bisnanohoops (Fig. 1d), where dual excited-state emissions were observed due to the symmetry-breaking structure45. Typically, achieving symmetry breaking involves introducing asymmetric substituents or designing fluorescent groups with twisted molecular conformations. Among the versatile structural motifs, o-carborane can be strategically incorporated to induce structural distortion, and its photophysical properties can be significantly influenced through the introduction of an aryl π-system. Previous studies have reported the integration of o-carborane into 1,3,5-triphenylbenzene-based star-shaped molecules to modulate molecular packing and emission behavior46,47,48,49,50. The flexible C–C bonds within o-carborane, coupled with aryl π-plane rotation, enhance structural diversity, which underlies the formation of charge transfer (CT) states51. Additionally, locally excited (LE) states have also been identified. Zhang and coworkers reported an o-carborane derivative exhibiting Ex-de emission via photoinduced electron transfer, resulting in dual fluorescence52. In these systems, donor-acceptor (D-A) or donor-π-acceptor (D-π-A) architectures are predominantly adopted.
Herein, we design and synthesize a star-shaped compound with three identical “V” type nonplanar branches, 1,3,5-(4-tert-butylphenyl-o-carboranyl-4-phenyl)benzene (Ph-3CP) (Fig. 1e and Supplementary Figs. 1, 2). Unlike conventional D-π-A frameworks, our design breaks symmetry in a tripodal architecture, expanding the diversity of accessible electronic states while facilitating precise control over excited-state relaxation pathways. The asymmetric conformations of Ph-3CP were successfully trapped through crystallization from distinct solvent systems, experimentally confirming the predicted monomeric structure. The molecule exhibited Ex-de multicolor emissions across a wide range in both dilute solution and solid states, achieving this behavior without requiring crystalline stabilization. By combining experimental and theoretical studies, we demonstrated that Ph-3CP stabilizes two distinct excited-state relaxation pathways via intramolecular decoupling, enabling dynamic and tunable emissions independent of external structural constraints. This strategy not only unlocks the potential of asymmetric molecular designs in controlling photophysical properties but also enables a symmetry-breaking fluorophore to achieve single-component Ex-de multicolor emission in solution, amorphous solid, and crystalline states. This breakthrough lays a solid foundation for applying single-fluorophore systems in multifunctional optical devices. As a demonstration, we utilized Ph-3CP to construct a single-component sensor array capable of visually distinguishing volatile organic compounds (VOCs) with varying polarities, such as aromatics and alkanes. Furthermore, structurally similar chlorinated derivatives were also differentiated.
The design strategy based on symmetry breaking in star-shaped molecules offers a viable avenues for the development of single component Ex-de fluorescent materials and paves the way for exploration of their functional applications, such as single molecule sensor array that is hopeful to better meet the requirements of small size, light weight, low power consumption, and low cost (SWaP-C) in the development of chemical, biological, radiative and nuclear materials sensors.
Results
Photophysical properties of Ph-3CP in solutions
Guided by the proposed molecular design, the target compound Ph-3CP and two reference analogs, Ph-3CB and Ph-CP, were synthesized and fully characterized (Fig. 2a, Supplementary Figs. 62–82, Supplementary Fig. 1 and Supplementary Tables 1–3). Structurally, Ph-3CB comprises a triphenylbenzene core bearing three o-carborane units, while Ph-CP incorporates a single tert-butylphenyl group on the same architecture. In contrast, Ph-3CP is decorated with three tert-butylphenyl groups, designed to promote asymmetry and enhance intramolecular CT interactions.
a Molecular structure of reference compounds Ph-3CB (left), Ph-CP (middle) and target compound Ph-3CP (right). α and β correspond to two dihedral angles, respectively. b Emission spectra of Ph-3CP, Ph-3CB, and Ph-CP in toluene (λex = 300 nm). c The excitation spectra of Ph-3CP in toluene (λem = 395–570 nm). d The emission spectra of Ph-3CP in toluene (λex = 290–360 nm). e Boltzmann distributions of Ph-3CP. The structural parameter Δγ is defined as: \(\Delta \gamma=\frac{1}{N}{\sum }_{i}^{N}\left[\left|{\theta }_{i}-{\theta }_{i}^{{C}_{3}}\right|+3\left|{\varphi }_{i}-{\varphi }_{i}^{{C}_{3}}\right|\right]\), where \(\theta\) represents the torsion angle between the central and peripheral benzene rings in triphenylbenzene core, and \(\varphi\) denotes the torsion angle between the central benzene ring and the C–C bond of o-carborane units. These values are extracted from the optimized ground-state geometries of Ph-3CP. A smaller Δγ indicates closer structural resemblance to idealized C3 symmetry. f Superimposed geometries of Ph-3CP illustrating the C3-symmetric conformer (Conf. 9, pink) and the most thermodynamically favored asymmetric conformer (Conf. 1, purple).
To ensure the monomeric state of Ph-3CP in solution, fluorescence spectra were recorded in solvents of varying polarity at 2.0 × 10−5 mol/L (Supplementary Fig. 3). Additionally, two reference compounds, Ph-3CB and Ph-CP, were also measured under identical conditions (Supplementary Figs. 4–10). Upon excitation at 300 nm in toluene, Ph-3CB exhibited obvious LE emission at 330–420 nm (Supplementary Fig. 11), closely resembling the LE band of Ph-3CP at 360–420 nm (Fig. 2b). Importantly, Ph-3CP exhibited a distinct CT emission at 470 nm, absent in Ph-3CB, indicating that tert-butylbenzene groups facilitate CT interactions with the o-carborane acceptor. Single crystal X-ray diffraction (SCXRD) confirmed structural disparities, particularly in o-carborane torsion angles (Supplementary Table 3)51. Ph-3CB exhibited nearly coplanar torsion angles (β) (26.6°, 162.6°, and 2.0°), while Ph-3CP adopted a near-orthogonal conformation (84.5°, 106.0°, and 89.9°), respectively. This deviation promotes vertical π-plane alignment toward the C–C bond in o-carborane, facilitating the CT emission. To validate this hypothesis, Ph-CP, featuring only a single bulky branch, was also examined. Consistently, Ph-CP exhibited a pronounced CT band at 480 nm and a weak LE band at 355 nm upon 300 nm excitation (Supplementary Fig. 12), aligning with the LE and CT1 emissions of Ph-3CP experimentally. Theoretical calculations also reveal that Ph-3CB possesses only one equilibrium S1 state with LE character localized in the triphenylbenzene fragment (Supplementary Table 5), reinforcing the assignment of the LE state in Ph-3CP to the same moiety. And similar CT state distributions were observed in Ph-CP and Ph-3CP, supporting a shared charge-transfer mechanism from benzene rings to bulky o-carborane units. Further steady-state fluorescence confirmed that both references lack the Ex-de behavior and exhibit a single emission pathway (Supplementary Fig. 13).
Importantly, Ph-3CP exhibited Ex-de emissions, indicating distinct intramolecular photophysical behaviors. As mentioned before, upon excitation at 290 nm, dual emissions appeared at 375 and 470 nm first (Fig. 2d). While further shifting the excitation to 360 nm, dominant emission at 395 nm and weak emission at 550 nm were detected. Excitation spectra (see Fig. 2c) scanned from 390 to 570 nm revealed two excitation bands at 310 and 340 nm, suggesting two independent emissive centers. This Ex-de behavior was consistently observed in other solvents, including dichloromethane (DCM), tetrahydrofuran (THF), and N,N-dimethylformamide (DMF) (Supplementary Fig. 14). As expected, polarity-dependent red shifts of the 470 and 550 nm bands indicated the CT characteristics, while the 375 and 395 nm bands remained invariant, corresponding to the LE states with distinct vibrational features (Supplementary Fig. 15). These observations suggest that Ph-3CP exhibits two distinct emission pathways: one involving LE (375 nm, 395 nm) and CT1 (470 nm), and the other comprising LE (375 nm, 395 nm) and CT2 (550 nm). To elucidate the excited-state properties, theoretical calculations were also performed. Three equilibrium S1 states were identified: an LE state localized in the triphenylbenzene fragment and two CT states (CT1 and CT2), as confirmed by hole-electron analysis (Supplementary Tables 4–7). The CT1 state involves a CT from the nonadjacent triphenylbenzene to o-carborane, whereas CT2 arises from an intramolecular CT between adjacent o-carborane and triphenylbenzene units (Supplementary Table 5). To quantify the CT extent, the interfragment CT was conducted. Consistent with expectations, CT1 demonstrates significantly greater CT (86.6%) compared to CT2 (78.2%). This difference is structurally manifested in the elongation of C1–C2 bonds to 2.396 Å (CT1) and 2.512 Å (CT2), respectively.
Asymmetry conformations of Ph-3CP
As seen, the steric crowding from tert-butylbenzene plays a critical role in stabilizing excited states and enabling multiple CT emissions. To validate this, conformational research as well as the geometric optimizations of the ground-state were performed. To quantitatively assess symmetry breaking, we defined Δγ as a metric for the deviation of Ph-3CP from its idealized C3-symmetric geometry. Among the nine lowest-energy conformers identified, only one exhibited C3 symmetry with the small population of 6.8% (Fig. 2e, Supplementary Figs. 16, 17 and Supplementary Tables 8, 9). In contrast, the remaining conformers consistently adopted an asymmetric structural motif, typified by the most stable conformer (Conf. 1, Fig. 2f), in which two of the three bulky substituents orient in the same direction. These findings illustrate that Ph-3CP preferentially adopts a symmetry-breaking conformation in the monomeric state. Additionally, crystal structure simulations also reinforce this preference (Supplementary Table 10), which reveals that the asymmetric conformers of Ph-3CP are also thermodynamically favored, extending the symmetry-breaking behavior from isolated molecules to the solid state.
To further validate the predicted asymmetric characteristics of Ph-3CP and investigate the conformational influence on photophysical behavior, four distinct crystal polymorphs of Ph-3CP were successfully obtained from solvent systems of varying polarity, which include low-polarity toluene/n-hexane (Ph-3CP-Y1) and cyclohexane/n-hexane (Ph-3CP-Y2), medium-polarity THF/n-hexane (Ph-3CP-Y3), and high-polarity chloroform/n-hexane (Ph-3CP-Y4). All polymorphs exhibit a single-molecule conformation within the crystal lattice, with distinct differences in molecular packing and dihedral angles (α and β; Supplementary Table 3) among the polymorphs. Interestingly, despite variations in solvent environments, a conserved structural motif persists across all polymorphs, wherein only two bulky branches consistently orient in the same direction (Fig. 3a). This highly consistent conformational feature mirrors that of the ground-state optimized geometries, strongly suggesting that Ph-3CP inherently adopts this asymmetric conformation, independent of solvent conditions.
a Four distinct crystal polymorphs of Ph-3CP obtained from low-polarity toluene/n-hexane (Ph-3CP-Y1), cyclohexane/n-hexane (Ph-3CP-Y2), medium-polarity tetrahydrofuran/n-hexane (Ph-3CP-Y3), and high-polarity chloroform/n-hexane (Ph-3CP-Y4). Inset: corresponding fluorescence photographs, where blue-emission images were captured under 300 nm UV light, and yellow-emission images under 360 nm UV light. b Emission spectra and fluorescence images of Ph-3CP powder at different excitation wavelengths ranging from 290 to 370 nm. c Corresponding CIE chromaticity coordinates of Ph-3CP powder at different excitation wavelengths from 290 to 370 nm. d Fluorescence decay profiles of Ph-3CP powder monitored at emission wavelengths of 395 nm, 470 nm, and 550 nm, respectively, using EPLED-340 as the excitation source. e, f Fluorescence emission spectra of Ph-3CP powder recorded from 347 to 77 K upon excitation at 300 nm and 360 nm, respectively. Insets in e, f show the ratio between the two emission intensities as a function of temperature.
Photophysical properties of Ph-3CP in solid state
In the solid state, Ph-3CP exhibits strong luminescence with a fluorescence quantum yield (PLQY) of nearly 20% (Supplementary Table 11), which originates from aggregation-induced restriction of intramolecular motion. This restriction suppresses non-radiative decay and promotes radiative transitions, consistent with the aggregation-induced emission mechanism (Supplementary Figs. 18–22 and Supplementary Table 12). Importantly, Ex-de multicolor emission is retained in both crystalline and amorphous states (Fig. 3b, c and Supplementary Figs. 23–25). Short-wavelength excitation yields blue fluorescence, whereas longer-wavelength excitation results in a red-shifted emission toward yellow, demonstrating single-molecule multiple fluorescence independent of molecular aggregation (Supplementary Figs. 26, 27). Structural analysis of four crystal polymorphs reveals that Ph-3CP assembles into discrete packing arrangements, primarily stabilized by weak van der Waals interactions, with the exception of Ph-3CP-Y3, where additional B–H···π interactions are observed (Supplementary Figs. 28–36). The absence of strong interaction sites, coupled with the highly three-dimensional propeller-like structure, minimizes prominent intermolecular forces among Ph-3CP molecules. Hirshfeld surface analysis provided further insights into the nature of these interactions, revealing that C–H···H–B interactions predominately service as the primary driving force in the crystal packing assisted by weak dipole-dipole forces53. The consistent single-molecule fluorescence across all polymorphs suggests a uniform UV/Vis absorption profile. Indeed, despite distinct molecular packing arrangements, all four polymorphs exhibit identical absorption bands centered at 320 nm and 360 nm, differing from the solution spectrum but aligning with the amorphous state of powder (Supplementary Fig. 37). These bands likely originate from the two distinct bulky branches, whose distinct spatial environments are mostly governed by different torsion angles (α). This finding further supports that the multicolor emission of Ph-3CP in the solid state is an intrinsic monomeric property.
In amorphous powder, Ph-3CP exhibited distinct CT1’ emission at 470 nm upon 290–340 nm excitation. This phenomenon is attributed to environmental constraints that suppress non-radiative relaxation pathways, thereby enhancing CT emission. When the excitation wavelengths were extended to 340–370 nm, dual emission characteristics emerged, featuring a weak LE’ band around 395 nm alongside with a strong CT2’ emission centered at 550 nm (Fig. 3b, c). Fluorescence spectra of Ph-3CP taken from the samples embedded in polystyrene (nonpolar, PS) and polymethyl methacrylate (polar, PMMA) films with a dosage of only 5‰ (w/w) also revealed Ex-de multicolor emissions, indicating the monomeric nature (Supplementary Fig. 38). Similar with that observed in solutions, molecular surrounding showed a strong effect upon the relative intensity of the two emissions as well as the CT emission position. Further investigations highlight the high photostability and purity of Ph-3CP, supporting pure compound behavior of the observations no matter in solution or in solid states (Supplementary Figs. 39–41).
To elucidate the relaxation dynamics of Ph-3CP, time-correlated single photon counting (TCSPC) measurements were conducted, focusing on three characteristic emission wavelengths (395, 470, and 550 nm). For the amorphous Ph-3CP powder excited at 340 nm, fluorescence decay profiles displayed triple-exponential behavior, with lifetimes of 0.25 ns, 3.88 ns, and 36.02 ns, corresponding to LE’, CT1’, and CT2’ states, respectively (see Fig. 3d and Supplementary Figs. 42, 43). The LE’ emission at 395 nm showed well-resolved vibrational bands and an ultrafast decay, indicative of an LE transition. To further explore excited-state evolution, time-resolved emission spectroscopy was conducted under selective excitation at 300 and 375 nm. The results revealed a dynamic evolution from LE’ to CT2’ emissions, as evidenced by the gradual decline of the LE’ band and concomitant growth of the CT2’band (Supplementary Figs. 44, 45). In contrast, no such process was observed between LE’ and CT1’, suggesting a preferential LE’ → CT2’ conversion pathway. These observations imply that the LE’ state can surmount a moderate activation barrier at room temperature to evolve into CT2’, likely driven by conformational relaxation. In parallel, the CT1’ band at 470 nm, with a lifetime of 3.88 ns, closely resembled the CT1’ state of Ph-CP (2.89 ns at 470 nm), suggesting the structural origin from the Ph-CP fragment of the molecule (Supplementary Figs. 46, 47). The CT2’ emission at 550 nm displayed a significantly longer population decay of 36.02 ns, reflecting distinct excited-state dynamics and geometric relaxation associated with this state.
Femto-/pico-second transient absorption (fs/ps-TA) measurements further corroborate this inference. Upon selective excitation responsible for the LE’/CT2’ dual emission, the fs/ps-TA spectra of Ph-3CP in toluene exhibit distinct stimulated emission (SE) features associated with the LE state in the 380–420 nm region, alongside with a broad excited-state absorption (ESA) band spanning 500–800 nm (Supplementary Fig. 48). At later delay times, a new positive absorption band appears in the 450–600 nm range, with a well-defined isosbestic point at 550 nm, indicating a redistribution of excited-state populations. This evolving absorption is attributed to the CT state, suggesting that CT2’ can be populated via relaxation from the LE’ state. The expected SE feature of the CT state near 550 nm is not clearly resolved, likely due to spectral overlap with the broad ESA band. In addition, a similar LE’-to-CT2’ dynamic observed in THF (Supplementary Fig. 49) further confirms that the excited-state evolution is intrinsic and independent of aggregation.
Temperature-dependent fluorescence and lifetime measurements provided further insights into the relaxation pathways. Two distinct pathways (I and II) were proposed to account for the observed emission features (Fig. 3e, f and Supplementary Fig. 50). Pathway I, associated with shorter-wavelength excitation, involved transitions between LE’ and CT1’ states. As the temperature dropped from 347 K to 77 K, CT1’ emission intensified while LE’ emission also emerged, resulting in a higher proportion of the LE’ state (see Fig. 3e). Pathway II, linked to lower-energy excitation, involved transitions between LE’ and CT2’ states. For this pathway, CT2’ emission initially increased as the temperature dropped from 347 to 197 K due to the suppression of non-radiative processes such as molecular vibrations and rotations. However, further cooling to 77 K restricted geometry relaxation, resulting in a corresponding reduction in CT2’ emission while LE’ fluorescence increased (see Fig. 3f). This temperature-dependent emission phenomenon could be a result of inhibition of the relevant non-radiative decays as well as restricted geometry relaxation of the excited state structure of the fluorophore. To elucidate the fundamental distinctions between the two de-excitation pathways, root-mean-square deviation (RMSD) calculations were employed to assess conformational changes during the de-excitation process (Supplementary Fig. 51). Path I, characterized by the S4 → CT1 transition with a relatively brief decay time of 3.88 ns, exhibits a reduced RMSD value of 1.115 Å, suggesting minimal conformational rearrangement. Conversely, Path II, involving the S3/S2 → CT2 transition, shows significantly greater intramolecular motion with a larger RMSD value of 2.855 Å/1.261 Å, indicating pronounced structural alterations over a more extended de-excitation process. Consistently, the LE state, with the shortest lifetime, displays the lowest RMSD value of 0.789 Å, further emphasizing the conformational changes are associated with de-excitation process.
Accordingly, these findings confirm that the photophysical behavior of Ph-3CP arises from its monomeric state in both solution and aggregated forms. A dual-channel radiative relaxation mechanism was proposed and verified theoretically (Fig. 4). For short-wavelength excitation (<310 nm), the population of higher excited states in the Franck-Condon region followed by the relaxation to the first singlet excited (S1) state, in accordance with Kasha’s rule. Two distinct S1 states, LE and CT1, were identified, with the LE state involving excitation localized on the triphenylbenzene unit and the CT1 state exhibiting significant CT between neighboring building blocks, resulting in a large dipole moment. These characteristics were consistent with observed solvent-dependent shifts. In a word, the population of higher excited states opened an avenue for relaxation to CT1 states. Under long-wavelength excitation (>310 nm), higher excited states were not populated, resulting in transitions to another two S1 states: LE and CT2 states. The CT2 is characterized by CT within a branch of Ph-3CP, exhibiting a smaller dipole moment, consistent with experimental observations.
CT = charge transfer, LE = localized excitation. CT1 and CT2 represent two distinct conformations arising from structural relaxation of different excited states. Inset: the electronic structures of CT1 and CT2, where the iso-surface of the hole and electron wavefunction distribution is set to 0.001, and the blue and green bubbles represent holes and electrons, respectively. Fluorescence images at the bottom, captured from 297 to 77 K under excitation at 365 nm (left) and 308 nm (right), show the equilibrium among LE, CT1 and CT2 states.
Application in fluorescence sensor array
The argument that the photophysical behavior of Ph-3CP is mainly determined by its molecular structure no matter it is in solution or solid states is further supported by substrate effect studies. As shown in Supplementary Fig. 52, the chemical nature of the substrates showed little effect upon the emission properties of the fluorophore, paving the way for sensing VOCs. As an example, Fig. 5a and the pictures depicted in Supplementary Table 13 show that the Ph-3CP-based silica powder delivered clear visual responses to alkanes, BTEX (benzene, toluene, ethylbenzene, and xylene), and chlorinated hydrocarbons under 365 nm excitation, enabling effective discrimination of them with varying polarities. The dynamic evolution for fluorescence color changes over time in saturated vapor of n-hexane, toluene, and chloroform was comprehensively monitored in Supplementary Fig. 53a–c. The observed fluorescent color transitions also matched well with the results presented in the CIE chromaticity coordinates (Supplementary Fig. 53d).
a Schematic diagram of the response of Ph-3CP silica powder to saturated vapor of alkane, BTEX (benzene, toluene, ethylbenzene, and xylenes) and chloroalkanes, respectively (λex = 365 nm), where it needs to be noted that only the processes with same arrow colors are fully reversible. b Schematic diagram of the homemade single-fluorophore-based sensor array device. c Response traces of the sensor array to six chlorinated hydrocarbons. d Two-dimensional principal component analysis (PCA) score plot for discriminating vapor of the six chlorinated hydrocarbons. e Hierarchical cluster analysis (HCA) heat map of the sensor array for all the tested analytes. A total of 60 trials (two channels, five concentrations for each of the tested samples) were conducted over a concentration range of 80–1284 ppm. DCM, TCM, DCE, TCE, MCB, and DCB refer to dichloromethane, chloroform, 1,2-dichloroethane, 1,1,2,2-tetrachloroethane, chlorobenzene and 1,2-dichlorobenzene, respectively. RST, RCT and k stands for response time, recovery time, and the slope of the plot of signal change against sample concentration.
Distinguishing structural analogs remains a significant challenge, as traditional fluorescence-based sensing arrays typically rely on multiple fluorophores to generate distinct response patterns54,55. However, variations in the photochemical stability of different fluorophores can compromise sensing accuracy and reproducibility. A more robust approach is to construct a single-molecule sensing array, ensuring a uniform and stable response. To achieve this, we developed a homemade sensing array that capitalizes on the Ex-de fluorescence of Ph-3CP. By precisely tuning the excitation wavelength, Ph-3CP exhibits distinct CT state emissions, enabling precise differentiation of chlorinated hydrocarbons. Utilizing a time-to-space conversion strategy, we eliminate the need for multiple fluorophores in sensor array, significantly reducing complexity while enhancing sensing dimensionality and discrimination capability. Guided by these design principles, an innovative dual-optical-path configuration was employed to maximize sensing efficiency. As shown in Fig. 5b and Supplementary Fig. 54, the system integrates orthogonally arranged light sources (308 and 365 nm) and photodiodes (475 and 560 nm) across two optical channels. This dual-channel architecture enables simultaneous signal acquisition from both optical paths, significantly boosting detection speed and overall sensor performance.
Importantly, the sensor array maintained consistent performance under continuous illumination for nearly 140 h (Supplementary Fig. 55). Prominent and consistent response signals were observed across six chlorinated hydrocarbons (CAHs), DCM, chloroform (TCM), 1,2-dichloroethane (DCE), 1,1,2,2-tetrachloroethane (TCE), chlorobenzene (MCB), and 1,2-dichlorobenzene (DCB), verified through the system’s repeatability of TCM tests (Fig. 5c and Supplementary Fig. 56). The response traces, obtained under two excitation wavelengths, revealed significant differences in both response intensity and recovery time, with a sampling time of 25 s (Supplementary Figs. 57, 58). A 360° in situ rotation test confirmed the uniformity of the sensing tube, as response trace differences between two optical paths remained consistent at all angles (Supplementary Fig. 59). For these CAHs at unsaturated vapor concentrations, the presence of the vapors induced a more pronounced microenvironment effect on the excited state of the Ph-3CP molecule, leading to an increase in response intensity. The prolonged recovery time observed in MCB and DCB could be attributed to the lower diffusion efficiencies, likely due to their larger molecular sizes.
To evaluate the discrimination ability of the sensor array, principal component analysis (PCA) and hierarchical cluster analysis (HCA) were performed on the collected sensing data for six analytes at concentrations ranging from 80 to 1284 ppm (Supplementary Figs. 60, 61 and Supplementary Table 14). As shown in Fig. 5d, the PCA score plot, based on the first two principal components, reveals well-separated clusters with no overlap. Similarly, the HCA heatmap also clearly distinguishes all six samples across five concentrations without confusion or error, consistent with the PCA results (Fig. 5e). These findings highlight the excitation wavelength-dependent emission behavior of Ph-3CP, thereby enhancing its capacity to differentiate between various chlorinated hydrocarbons and offering a robust tool for complex analyte identification.
Discussion
In summary, we established a symmetry-breaking molecular design strategy that enables broad Ex-de multicolor emissions from a single-component material in both solution and solid states. By crowding three o-carborane-phenyl units, the resulting molecule, Ph-3CP, adopts highly asymmetric conformations, which are effectively trapped in multiple crystalline polymorphs, experimentally providing direct validation of the predicted solution-phase asymmetry. Comprehensive photophysical characterizations, including SCXRD, TCSPC and fs/ps-TA, alongside theoretical calculations, revealed that Ph-3CP possesses three distinct S1 states and dual relaxation pathways, enabling tunable emissions over a nearly 175 nm range. This Ex-de behavior is retained across all polymorphs and underpins the construction of a dual-channel fluorescent sensor array capable of rapid and selective discrimination of six chlorinated hydrocarbons (DCM, TCM, DCE, TCE, MCB, and DCB) with high sensitivity and robustness, as confirmed by PCA and HCA analyses. This study highlights the potential of symmetry manipulation in fine-tuning photophysical properties at the molecular level. The intramolecular crowding strategy not only enriches the electronic state landscape but also provides a robust platform for designing advanced single-component multi-emissive molecular materials with prominent applications in sensing, bioimaging, and optoelectronic devices.
Methods
Material science
K2CO3 (Macklin, ≥99%), 1-(4-iodophenyl)ethanone (Adamas, ≥99%), 1-(4-bromophenyl)-3,5-diphenylbenzene (Aladdin, ≥98%), decarborane (Yuanli Tech., ≥98%), 4-(tert-butyl)phenylacetylene (Adamas, ≥99%), 1,1’-(Ethyne-1,2-diyl)bis[4-(tert-butyl)benzene] (Yuanli Tech., ≥98%), Pd(PPh3)2Cl2 (TCI, ≥99%) and CuI (Aladdin, ≥99%) were obtained from commercial suppliers. Analytical grade (A.R.) solvents, including DCM, TCM, n-hexane, toluene, DMF, cyclohexane, methanol, THF, acetonitrile, and triethylamine were purchased from National Pharmaceutical Reagent Co. (China). All solvents used for spectroscopic measurements were purified by distillation before use.
Instrumentations
All 1H NMR, 13C NMR, 11B NMR were obtained on Bruker Avance 600 NMR spectrometer using TMS as internal standard. The mass spectra of the compounds were obtained on Bruker MAXIS. UV/Vis absorption spectra were recorded on a UV/Vis spectrophotometer (U-3900, Hitachi). Steady-state fluorescence measurements and the CIE chromaticity coordinates were performed at an FLS1000 spectrometer (Edinburgh Instruments). The fluorescence lifetime measurements were performed at room temperature on a TCSPC unit equipped with the Edinburgh FLS1000 fluorescence spectrometer. During fluorescence lifetime measurements, the photoexcitation sources were picosecond pulse light-emitting diodes. Femto-/pico-second transient absorption (fs/ps-TA) measurements were conducted using a custom-built system combining a PHAROS laser (Light Conversion; 1030 nm, <190 fs pulse duration, 200 µJ/pulse, 100 kHz repetition rate), nonlinear frequency mixing techniques, and a Femto-TA100 spectrometer (Time-Tech Spectra). Samples were excited at their absorption band and the transient absorption changes were probed with a white-light continuum under magic-angle polarization conditions. All the spectra, except temperature-dependent emission spectra, were measured at 298 K. SCXRD measurements were performed using Smart Apex 2 (Bruker). Powder X-ray diffraction measurements were performed on D8 Advance Diffractometer (Bruker). The photo images were taken by an E70D digital camera (Canon).
Synthesis of o-carborane derivative
All o-carborane derivatives were prepared via alkyne insertion reactions using N,N-dimethylaniline as a Lewis base. The structure and purity of Ph-3CP were rigorously verified using nuclear magnetic resonance (NMR) spectroscopy, high-resolution mass spectrometry (HRMS), high-performance liquid chromatography, two-dimensional diffusion-ordered spectroscopy, and SCXRD. All other studied reference compounds, Ph-3CB and Ph-CP, were also comprehensively characterized by NMR, HRMS, and SCXRD with the corresponding data and synthetic details provided in the Supplementary Figs. 62–82 and Supplementary Tables 1–3.
Quantum chemical calculation
Molecular conformation search
The Global Optimizer Algorithm for Torsions implemented in the ORCA program was employed to systematically explore the ground-state conformational space of Ph-3CP. Thousands of conformers were generated, and a comprehensive screening based on relative energies and structural similarity was performed. Representative low-energy conformers were selected for further optimization using density functional theory (DFT) at the B3LYP/def2-SVP level. Boltzmann population analysis at 298 K was subsequently carried out to evaluate the thermodynamic distribution of conformers.
Crystal structure optimization
The crystal structures of Ph-3CP obtained from four different solvent systems, along with a model structure exhibiting idealized C3 symmetry constructed using PolyMorph, were subjected to geometry optimization using the CP2K program. To retain the experimentally observed packing motifs, all optimizations were carried out with fixed unit cell parameters. To assess the impact of molecular symmetry on energetic stability, single-point energy calculations were subsequently performed on the optimized structures using DFT with the B3LYP functional and def2-SVP basis set.
Photophysical mechanisms of absorption and emission
All ground-state (S0) geometries were optimized using the B3LYP functional with the def2-SVP basis set. Excited-state optimizations of Ph-3CP were carried out for several low-lying singlet states (S1–S4) using the Tamm-Dancoff approximation within the TD-DFT framework, combined with state-tracking techniques. Starting from S1 state, a LE singlet state was identified as the lowest-energy excited state. In contrast, optimizations from the S2 and S3 states yielded a distinct CT singlet state, designated as CT1. Similarly, a second CT state (CT2) was accessed via optimization from the S4 state. For comparison, the excited-state properties of Ph-3CB and Ph-CP were also investigated using the same computational protocol. Only one LE-type excited state was identified for Ph-3CB, whereas Ph-CP displayed a single CT-type state. All calculations were performed using the ORCA 6.0.0 program package56,57. Wavefunction analyses were conducted with the Multiwfn program58, and the corresponding hole and electron distributions were visualized using isosurface plots.
Fabrication of single-fluorescent array
Fabrication of sensing film
A freshly prepared solution of Ph-3CP (5.0 × 10−4 mol/L in 2 mL toluene) was mixed with 50 mg of silica gel powder (200–300 mesh). After solvent removal by rotary evaporation at 20 rpm, the resulting Ph-3CP-coated silica particles were packed into a quartz tube (1.5 mm i.d., 4 cm length), forming a sensing tube approximately 3 mm in length. Prior to sensing measurements, the sensing tube was activated by placing it in a dry environment at 313 K for 24 h.
Fabrication of sensing platform
The homemade sensing platform comprises a sample gas supplying system, a sensory unit, and a data processing system. The core component of both the platform and the sensor array is the sensory unit, which adopts a dual-channel architecture that integrates orthogonally arranged light sources (308 and 365 nm) and photodiodes (475 and 560 nm) into two independent optical pathways. To avoid optical interference, the light sources are alternately switched during measurements. The excitation power densities were set at 0.2 mW/cm2 for 308 nm and 0.3 mW/cm2 for 365 nm, respectively. The gas sampling flow rate was set at 20 mL/min, with analyte injection regulated by a solenoid valve operating on a 25 s to ensure analyte saturation. Prior to each measurement, the gas lines were thoroughly purged with clean air to eliminate residual analytes and prevent cross-contamination.
Data availability
The crystallographic data generated in this study have been deposited in the Cambridge Crystallographic Data Centre (CCDC) database under accession code 2433238 (crystal Ph-3CP-Y1), 2367647 (crystal Ph-3CP-Y2), 2367646 (crystal Ph-3CP-Y3), 2367649 (crystal Ph-3CP-Y4), 2367645 (crystal Ph-3CB) and 2367644 (crystal Ph-CP). These data are freely available from the CCDC via https://www.ccdc.cam.ac.uk/data_request/cif. The source data generated from the stability tests has been deposited in the Figshare database under the https://doi.org/10.6084/m9.figshare.29887010. All data supporting the findings of this study are available within the article and its Supplementary Information, and from the corresponding authors upon request. Source data are provided with this paper.
References
Choi, S. H. et al. Bioinspired dynamic colour change. Nat. Rev. Bioeng. 3, 579–595 (2025).
Teyssier, J., Saenko, S. V., Marel, D. & Milinkovitch, M. C. Photonic crystals cause active colour change in chameleons. Nat. Commun. 6, 6368–6375 (2015).
Xu, J. H. et al. Ultrabright molecular scintillators enabled by lanthanide-assisted near-unity triplet exciton recycling. Nat. Photonics 19, 71–78 (2025).
Sun, H. et al. Sulfur lone pairs open avenues for π*→n orange-to-red TADF and OLEDs. J. Am. Chem. Soc. 147, 5432–5439 (2025).
Wang, H. et al. Abnormal thermally-stimulated dynamic organic phosphorescence. Nat. Commun. 15, 2134 (2024).
Wang, H. R. et al. Aggregation-induced emission (AIE), life and health. ACS Nano 17, 14347–14405 (2023).
Li, X.-C., Yan, Y. & Lai, W.-Y. Material design and prospects for color-tunable smart-responsive organic emitters. Sci. Bull. 69, 3329–3333 (2024).
Li, X.-C. et al. Interface preassembly oriented growth strategy towards flexible crystalline covalent organic framework films for OLEDs. Nat. Commun. 16, 3321–3328 (2025).
Xiao, Y. X. et al. Construction of multi-decay pathways and realizing polymer-regulated organic smart luminescent materials. FlexMat 1, 193–202 (2024).
Yan, Y. et al. Single-component color-tunable smart organic emitters with simultaneous multistage stimuli-responsiveness and multimode emissions. Research 6, 0241–0250 (2023).
Wu, X. G., Ni, S. Q., Wang, C.-H., Zhu, W. G. & Chou, P.-T. Comprehensive review on the structural diversity and versatility of multi-resonance fluorescence emitters: advance, challenges, and prospects toward OLEDs. Chem. Rev. https://doi.org/10.1021/acs.chemrev.5c00021 (2025).
Huang, Y. J. et al. Stimuli-fluorochromic smart organic materials. Chem. Soc. Rev. 53, 1090–1166 (2024).
Li, X.-C. et al. Intrinsically stretchable electroluminescent elastomers with self-confinement effect for highly efficient non-blended stretchable OLEDs. Angew. Chem. Int. Ed. 62, e202213749 (2023).
Xing, C., Qi, Z. H., Ma, Y.-J., Yan, D. P. & Fang, W.-H. Dynamic ultralong phosphorescence and optical waveguiding switches in silver-organic complex via reversible single-crystal-to-single-crystal conversion. Angew. Chem. Int. Ed. 64, e202502782 (2025).
You, J. X. et al. Responsive circularly polarized ultralong room temperature phosphorescence materials with easy-to-scale and chiral-sensing performance. Nat. Commun. 15, 7149–7158 (2024).
Zou, Y. et al. Acceleration of reverse intersystem crossing in multi-resonance TADF emitter. Chem 10, 1485–1501 (2024).
Liu, X. Y. et al. Structural rigidification strategy based on self-assembly enabled reversible excited-state conversion of Iridium(III) complexes for multiple-stimulus-responsive data encryption. J. Am. Chem. Soc. 146, 29955–29963 (2024).
Lu, L. et al. Multiple photofluorochromic luminogens via catalyst-free alkene oxidative cleavage photoreaction for dynamic 4D codes encryption. Nat. Commun. 15, 4647–4658 (2024).
Liu, Y. L., Zu, B. Y. & Dou, X. C. Cellulose-based fluorescent materials for chemical sensing applications. Coord. Chem. Rev. 532, 216505–216525 (2025).
Ahmadi, N., Lee, J., Godiya, C. B., Kim, J.-M. & Park, B. J. A single-particle mechanofluorescent sensor. Nat. Commun. 15, 6094–6105 (2024).
Huang, R. R. et al. Molecular design and architectonics towards film-based fluorescent sensing. Chem. Soc. Rev. 53, 6960–6991 (2024).
Uchiyama, S., Fukatsu, E., McClean, G. D. & Silva, A. P. D. Measurement of local sodium ion levels near micelle surfaces with fluorescent photoinduced-electron-transfer sensors. Angew. Chem. Int. Ed. 55, 768–771 (2016).
Yao, X. et al. A phototautomeric 3D covalent organic framework for ratiometric fluorescence humidity sensing. J. Am. Chem. Soc. 147, 9665–9675 (2025).
Wang, Y. H. et al. Zinc(II)-enhanced excimer probe for recognition of MDMB-CA synthetic cannabinoids. Angew. Chem. Int. Ed. 64, e202423576 (2025).
Xu, S. L., Yan, K.-C., Xu, Z.-H., Wang, Y. & James, T. D. Fluorescent probes for targeting the Golgi apparatus: design strategies and applications. Chem. Soc. Rev. 53, 7590–7631 (2024).
Barman, D. et al. Highly efficient color-tunable organic co-crystals unveiling polymorphism, isomerism, delayed fluorescence for optical waveguides and cell-imaging. Nat. Commun. 14, 6648 (2023).
Li, C. B. et al. White-light activatable organic NIR-II luminescence nanomaterials for imaging-guided surgery. Nat. Commun. 15, 5832–5842 (2024).
Navarro-Huerta, A. et al. Optical waveguiding charge-transfer cocrystals: examining the impact of molecular rotations on their photoluminescence. J. Am. Chem. Soc. 147, 8343–8349 (2025).
Li, S. Z. et al. Manipulating dynamic light-driven solid-liquid transition and static reversible photochromism by an organic cocrystal strategy. Angew. Chem. Int. Ed. 64, e202500238 (2025).
Sun, L. S. et al. Light-regulated nucleation for growing highly uniform single-crystalline microrods. Angew. Chem. Int. Ed. 63, e202402253 (2024).
Xu, Q. Y., Zhang, J. Y., Sun, J. Z., Zhang, H. K. & Tang, B. Z. Efficient organic emitters enabled by ultrastrong through-space conjugation. Nat. Photonics 18, 1185–1194 (2024).
Gong, Q. et al. Excitation wavelength dependent fluorescence and phosphorescence from organic ionic crystalline powder. Adv. Opt. Mater. 12, 2401622–2401631 (2024).
Man, Z. W. et al. Excitation-wavelength-dependent organic long-persistent luminescence originating from excited-state long-range proton transfer. J. Am. Chem. Soc. 144, 12652–12660 (2022).
Shen, Y. X., An, Z. F., Liu, H. C., Yang, B. & Zhang, Y. J. Excitation-dependent multicolour luminescence of organic materials: internal mechanism and potential applications. Angew. Chem. Int. Ed. 62, e202214483 (2022).
Gu, L. et al. Color-tunable ultralong organic room temperature phosphorescence from a multicomponent copolymer. Nat. Commun. 11, 944–951 (2020).
Huang, R. R. et al. Single-fluorophore-based organic crystals with distinct conformers enabling wide-range excitation-dependent emissions. Angew. Chem. Int. Ed. 61, e202211106 (2022).
Zhang, Y. J. et al. Excitation wavelength dependent fluorescence of an ESIPT triazole derivative for amine sensing and anti-counterfeiting applications. Angew. Chem. Int. Ed. 58, 8773–8778 (2019).
Shao, Y. T. et al. Unlocking multicolor emissions in the crystalline state through dimerization and configurational transformation of a single fluorophore. Chem. Mater. 36, 3223–3232 (2024).
Liu, Z.-F. et al. Spontaneous symmetry breaking of achiral molecules leading to the formation of homochiral superstructures that exhibit mechanoluminescence. Angew. Chem. Int. Ed. 63, e202318856 (2024).
Wang, Y. P. et al. Narrowband clusteroluminescence with 100% quantum yield enabled by through-space conjugation of asymmetric conformation. Nat. Commun. 15, 6426–6437 (2024).
Qiao, Q. L. et al. Tail-assisted excited-state intramolecular proton transfer (ta-ESIPT) fluorophores: a universal ratiometric platform for hydration sensitive biomolecular imaging and sensing. J. Am. Chem. Soc. 147, 15602–15613 (2025).
Wang, J.-K. et al. Hydrogen-bonded thiol undergoes unconventional excited-state intramolecular proton-transfer reactions. J. Am. Chem. Soc. 146, 3125–3135 (2024).
Ding, N. N. et al. Bi-ortho-carborane unit-riveted perylene monoimides: structure-tuned optical switches for electron transfer and robust thin film-based fluorescence sensors. CCS Chem. 5, 2922–2932 (2023).
Du, M. X. et al. White emissions containing room temperature phosphorescence from different excited states of a D-π-A molecule depending on the aggregate states. Adv. Sci. 9, 2104539–2104546 (2022).
Zhang, X. Y. et al. Anomalous anti-Kasha excited-state luminescence from symmetry-breaking heterogeneous carbon bisnanohoops. Nat. Commun. 15, 2684–2692 (2024).
Dash, B. P. et al. Enhanced π-conjugation and emission via icosahedral carboranes: synthetic and spectroscopic investigation. Inorg. Chem. 50, 5485–5493 (2011).
Juárez-Pérez, E. J. et al. Polyanionic aryl ether metallodendrimers based on cobaltabisdicarbollide derivatives. photoluminescent properties. Macromolecules 43, 150–159 (2010).
Dash, B. P. et al. Facile synthetic routes to phenylene and triazine core based dendritic cobaltabisdicarbollides. Organometallics 29, 5230–5235 (2010).
González-Campo, A. et al. A versatile methodology for the controlled synthesis of photoluminescent high-boron-content dendrimers. Chem. Eur. J. 19, 6299–6312 (2013).
Cabrera-González, J. et al. Blue emitting star-shaped and octasilsesquioxane-based polyanions bearing boron clusters. photophysical and thermal properties. Molecules 25, 1210–1225 (2020).
Ochi, J., Tanaka, K. & Chujo, Y. Recent progress in the development of solid-state luminescent o-carboranes with stimuli responsivity. Angew. Chem. Int. Ed. 59, 9841–9855 (2020).
Yang, H. Y. et al. Multicolour fluorescence based on excitation-dependent electron transfer processes in o-carborane dyads. Angew. Chem. Int. Ed. 61, e202115551 (2022).
Spackman, P. R. et al. CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 54, 1006–1011 (2021).
Liu, K. et al. Precise manipulation of excited-state intramolecular proton transfer via incorporating charge transfer toward high performance film-based fluorescence sensing. J. Am. Chem. Soc. 145, 7408–7415 (2023).
Dou, W.-T. et al. Fluorogenic peptide sensor array derived from angiotensin-converting enzyme 2 classifies severe acute respiratory syndrome coronavirus 2 variants of concern. J. Am. Chem. Soc. 146, 21017–21024 (2024).
Neese, F. Software update: the ORCA program system-version 5.0. WIREs Comput. Mol. Sci. 12, e1606 (2022).
Campetella, M. & García, J. S. Following the evolution of excited states along photochemical reaction pathways. J. Comput. Chem. 41, 1156–1164 (2020).
Lu, T. & Chen, F. W. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Acknowledgements
The authors acknowledge financial support from the Ministry of Science and Technology of China (2022YFA1205502, principal participant: Y.F.), the National Natural Science Foundation of China (22132002, 22427802; PI: Y.F. and 22272101; PI: H.N.P.), and the Fundamental Research Funds for the Central Universities (GK202505032; PI: Y.F. and GK202501003; PI: H.N.P.). The authors wish to acknowledge Dr. Jing Zhang, Dr. Zhaolong Wang, and Dr. Rongrong Huang for their illuminating discussions. The authors also gratefully thank Dr. Nannan Ding from Xi’an Institute of Optics and Precision Mechanics of Chinese Academy of Sciences for her help in the TA measurement and analysis (Time-Tech Spectra LLC Femto-TA100).
Author information
Authors and Affiliations
Contributions
Y.F., H.N.P., L.-Y.P. and S.M.L. performed the data analysis, conceived the research project, and wrote the manuscript. S.M.L. carried out the materials synthesis, structural characterization, and photophysical measurements. H.S.L. and J.C.Z. contributed to the synthetic work. X.B.W., L.-Y.P. and S.W.Y. conducted all theoretical studies. R.J.W. and J.F.M. were responsible for fabricating the sensor device, and S.M.L. performed the sensing tests with their assistance. Y.F. conceived, designed, and supervised the entire study. All authors discussed the results and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Youhong Tang and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, S., Wang, X., Li, H. et al. Single-component-based multicolor emissions enabled by symmetry breaking. Nat Commun 16, 8130 (2025). https://doi.org/10.1038/s41467-025-63519-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63519-7