Abstract
Wireless modules provide an essential platform for power-harvesting and telecommunications enabled by Internet of Things systems. However, substantial interferences in multiple energy interactions and signal transmission may arise due to fluctuations in environmental factors. Here, we report a moist-electromagnetic coupling effect enabled by ionic-electronic polymer diodes for synergistic moist energy harvesting and electromagnetic protection. The thermodynamic and kinetic mechanisms of charge carrier transport in the polymer diodes are effectively manipulated by engineering the molecular interactions within the polyanions, leveraging hydrogen bonding, metal ion coordination, and metal-organic framework modifications, alongside the controlled porous architecture of the polypyrrole polycations. The ion gradient distribution and ionic double layer induced by moist energy endow the films with rectenna effect, leading to optimized impedance matching and enhanced polarization relaxation capabilities, thereby enabling electromagnetic interference shielding. The proposed moist-electric-electromagnetic coupling mechanism demonstrates its operational feasibility through stable power output (480.19 μW·cm-2) and good electromagnetic capability. Our findings provide insight into the environmental adaptability of electromagnetic energy modulation, ensuring the energy and information security of the state-of-the-art self-powered smart wireless electronics.
Similar content being viewed by others
Introduction
The rapid advancement of modern wireless communication and network technologies has led to an inevitable increase in electromagnetic radiation1. This, in turn, generates electromagnetic interference (EMI), which poses potential risks to information security, precision instruments, and even human health2. Significant effort has been devoted to developing EMI shielding materials and electromagnetic wave (EMW) absorption materials (EMAs) to mitigate these effects3. However, the integration of EMI shielding materials and EMAs can introduce uncertainties in the stable operation of wireless energy interaction systems, leading to information distortion4. Moreover, these materials can limit device flexibility and constrain their applicability in diverse scenarios. Consequently, there is an urgent need to explore novel wireless electromagnetic energy modulation strategies and develop advanced electromagnetic protection measures, thereby enabling the self-protection of smart wireless Internet of Things (IoT) accessories, such as nanogenerators, sensors, and actuators, etc5.
Electromagnetic protection was initially based on conductive materials that block the transmission of EMW, which can be traced back to 1836, when Faraday first reported the use of metal enclosures to shield electric fields6. In the past few years, based on Simon’s equation (Supplementary Note 1), materials with higher electrical conductivity have been pursued to achieve higher shielding effectiveness (SE), including metal composites7, carbon materials8, graphene9, MXene10, etc. However, shielding layers based on high conductivity primarily rely on strong surface reflection, which can result in the secondary contamination of electromagnetic radiation, potentially causing additional interference or exacerbating the impact on nearby IoT systems11. To mitigate this drawback, enhancing polarization loss to promote energy attenuation has emerged as an effective strategy for achieving dominance in EMW absorption12. Traditionally, the relationship between the chemical composition, nano-micro structures, and metasurface architectures of materials and their electromagnetic properties, as well as EMW absorption performance, has been extensively studied13. However, under natural conditions, the external environment surrounding a material is invariably subject to dynamic changes, including but not limited to strain, light, heat, moisture, etc., which undoubtedly influence the dielectric properties and electromagnetic response characteristics of the material, ultimately affecting electromagnetic protection performance14. Therefore, the key and difficulty of achieving precise wireless electromagnetic energy modulation lies in exploring the multiple energy coupling mechanism under the synergistic effect of environmental factors and electromagnetic fields, thence establishing dynamic and controllable intelligent electromagnetic protection physical models15.
To implement efficient multiple energy coupling, several challenges must be addressed, including strong EMW absorption, clear charge behavior, energy conversion and transfer mechanism, etc. (i) Dielectric and electrolyte properties should be regulated by the ambient environment for excellent EMW absorption performance, which relies on the good impedance matching and electromagnetic attenuation16. (ii) The migration and delocalization of charge carriers within the material under changing ambient environment need to be well-defined and understood. Precise control over charge dynamics is essential for regulating electromagnetic responses17. (iii) The mechanisms of harvesting, converting, and transmitting electromagnetic energy must be thoroughly explored to effectively modulate electromagnetic energy and achieve efficient electromagnetic protection18. As an emerging technique, nanogenerators offer a promising solution to these challenges, which could induce charge migration and delocalization by harvesting environmental energy, leading to the formation of internal electric fields and currents19. Typically, moist-enabled electricity generation (MEG), driven by surface potential differences and ionic currents, has great potential to induce ionic diode properties, enabling electromagnetic energy modulation20. By harnessing the abundant and ubiquitous chemical potential energy of atmospheric moisture—referred to as moist energy—the osmotic pressure difference arising from ionic concentration gradients can drive the directional transport and asymmetric distribution of ionic charge carriers21. Furthermore, the modulated dielectric and electrolyte properties by MEG, such as diode-like rectifying effects and interfacial micro-capacitor behaviors, provide critical mechanisms for EMW reception and polarization loss22.
In this work, we demonstrate a moist-electromagnetic coupling mechanism that enables synergistic energy harvesting and electromagnetic protection. A series of polymer ionic diodes (PIDs) materials was prepared by coating a hydrophilic polyanion electrolyte on carbon fabric (CF) deposited with hydrophobic polycationic conjugated conducting polymer polypyrrole (PPy). Due to the directional orientation of ion diffusion, the surfaces of the polyanion electrolyte and PPy become negatively and positively charged, respectively, which further induces the formation of an electric double layer at their interface. Leveraging the diode-like rectifying effect, PIDs possess the capability to synergistically modulate the coupled electromagnetic and moist energy, thereby enabling the self-protection functionality of the nanogenerators. Furthermore, the chemical structures, intermolecular interaction mechanisms, and interface configurations of polyanions and polycations are optimized to explore the fundamental principles of this wireless energy modulation system. This wireless energy modulation mechanism not only offers an approach to the electromagnetic protection of miniaturized precision instruments but also establishes a foundation for studying the environmental dependence of electromagnetic response behaviors in shielding materials, driving the development of intelligent and environment-adaptive electromagnetic protection materials.
Results
Design and principle of PIDs for wireless energy modulation
A series of MEG nanogenerators was designed and fabricated to investigate the effect of moisture on material properties and its role in modulating wireless electromagnetic energy mechanisms. As shown in Fig. 1a, d and Supplementary Fig. 1, PPy was deposited and coated on CF by electrochemical deposition, while PPy with a porous structure (PPPy) was obtained by etching away the Methylene blue (MB) deposited in the PPy network. CFs coated with PPy and PPPy were defined as PPy@CF and PPPy@CF, respectively. Additionally, gradient electrochemical deposition time (0, 600, 1200, and 1800 s) was employed to control the deposition amount of the PPy (PPPy) layer to explore the effect of the thickness of the polycation layer on the charge transport properties (Fig. 2b and Supplementary Table 2). The thicknesses of the deposited PPPy (tPPPy) and PPy (tPPy) layers were estimated from the increase in fiber diameter to CF (dCF) after coating by scanning electron microscopy (SEM) images, as determined by dPPPy@CF and dPPy@CF. The reduced tPPPy compared to tPPy reflects its slower deposition kinetics, which can be attributed to the larger steric hindrance introduced by MB and the molecular chain disorder caused by hybridization, resulting in an enrichment of active sites in PPPy. PIDs were obtained by coating polyanion, polystyrene sulfonic acid (PSSA), on the outside of PPy (PPPy). Polyvinyl alcohol (PVA) was mixed into PSSA to enhance its hydrophilicity and hygroscopicity. The coordination effect of Fe3+ and the Prussian blue analogs (PBAs) type metal-organic frameworks (MOFs) were utilized to enhance the intermolecular interactions of PSSA and PVA, while also modifying the molecular network structure. As a result, three types of composite polyanionic electrolyte networks were obtained, that is, hydrogen-bonded PSSA, ion-coordinated PSSA-Fe, and MOF-modified PSSA-PB. The detailed nomenclature of polyanion@PPy@CF type PIDs is presented in Supplementary Table 1.
a Schematic of the construction of polymer ionic diodes (PIDs). b Schematic demonstration of energy harvesting and electromagnetic protection of PIDs. c The electromagnetic interference (EMI) shielding mechanism of PIDs. d Schematic illustration of the molecular structures and intermolecular interactions of polyanions and polycations.
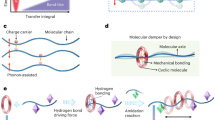
a Bragg position, d and ID/IG values of carbon fabric (CF), polypyrrole (PPy), and porous polypyrrole (PPPy). b Thickness of PPy and PPPy, dimension of PPy@CF and PPPy@CF. Data are presented as mean values ± SD (n = 50). c SEM images of PPy and PPPy with regulated deposition time. d Schematic illustration of the chemical and physical mechanism of self-protective nanogenerators.
The SEM and corresponding energy-dispersive spectrometer (EDS) elemental mapping images of PPPy-3 revealed that the counterion of the polycation is Cl−, enabling it to function as both an electronically and ionically conductive polymer (Supplementary Fig. 2). The unique polycationic structure of PPyn+Cl−n endows it with dual functionality as a proton collection layer and a Cl− release agent, significantly enhancing the potential difference across both sides of the PIDs23. The rigid structure of the PPy molecular chain dictates that the transport of ion carriers primarily relies on its molecular crystal morphology and the configuration of its ion transport channels24. As shown in the Raman spectrum (Supplementary Fig. 3), two classic characteristic peaks appear at 1350 cm−1 and 1580 cm−1, which are attributed to the breathing vibration of the A1g symmetry mode (D peaks) and the in-plane vibration of the E2g mode (G peaks), implying defect structures and regular molecular arrangements in the lattices, respectively25. The ratio of the D peak to the G peak (ID/IG) increases with extended electrochemical deposition time (Fig. 2a), likely because PPy chains tend to grow more uniformly and linearly along the carbon fiber surface during the early stages, resulting in relatively coherent molecular packing with fewer structural defects26. In contrast, at later stages, the PPy chains are more prone to forming irregular aggregation, disturbance of π-conjugation, and chain clusters due to the anions (Cl−) doping, leading to an increase in localized defects. The particle clusters observed in the SEM images further validate the accumulation of molecular chain disorder with extended deposition time, which is more pronounced in PPPy, attributed to its inherently porous structure induced by MB (Fig. 2c and Supplementary Fig. 4). Furthermore, the interlayer channels in the unit cell of graphitized PPy were investigated using the (002) interplanar spacing obtained via the Bragg positions in X-ray diffraction (XRD) patterns (Supplementary Fig. 5)27. It can be inferred that the interlayer spacing of pure PPy decreases with extended deposition time, whereas that of PPPy increases, indicating that the molecular defects introduced by MB further expand the ion transport channels (Fig. 1e and Supplementary Table 3).
Subsequently, PIDs are expected to be used for simultaneous moist energy harvesting and electromagnetic protection (Fig. 1b, c). In a humid environment, owing to the difference in hygroscopicity between the polyanion (hydrophilic) and polycation (hydrophobic) sides of PIDs, the COOH groups of PSSA undergo hydrolysis and ionization, releasing H+ ions that are driven toward the PPy side by osmotic pressure. Simultaneously, Cl− ions migrate from PPy to the polyanion, ultimately establishing a dynamic balance between surface H2O molecules absorption and ionic charge carrier migration. In this manner, by connecting the PIDs through the positive electrode (CF) and the negative electrode (Al film) to form an external circuit, a MEG nanogenerator can be successfully obtained, with the PPy side carrying a positive charge and the polyanion side carrying a negative charge (Fig. 2d). Therefore, a built-in electric field (BIEF), an internal electrostatic field formed due to the asymmetric distribution of ionic and electronic species, is established across the PID structure, directed from the PPy layer to the polyanionic layer. In parallel, an ionic double layer (IDL), defined as the interfacial accumulation of H+ and Cl− ions at the junction of the two oppositely charged layers, forms dynamically under humid conditions. This intrinsic field imparts selective ion permeability to the system, effectively hindering the migration of H+ ions from the PPy side to the polyanion side. On one hand, this diode-like rectifying effect ensures the stable harvesting of moist energy. On the other hand, the high conductivity of CF and PPy enables PIDs to function as rectennas for absorbing EMW. Ultimately, the PID-based MEG nanogenerators, with diverse physical and chemical properties in varying ambient moist environment, will demonstrate tunable charge transport and EMW response characteristics, thereby enabling the wireless electromagnetic energy modulation through moist energy, achieving intelligent electromagnetic protection.
Ambient moist energy harvesting by PIDs
The diode-like rectifying effect of PIDs was first investigated under an alternating potential (± 1 V, 0.1 Hz). All PIDs exhibited obvious reverse current cutoff properties (Fig. 3a, b and Supplementary Figs. 6–12). Under forward bias, H+ ions originating from the polyanion and Cl− ions from PPy are driven toward the polyanion/PPy interface, enabling their translocation across the junction. This process disrupts the H+/Cl− IDL, thereby promoting efficient ion transport throughout the PIDs and resulting in enhanced current flow and forward conduction characteristics28. Under reverse bias, the IDL undergoes thickening as a result of ions interfacial accumulation, analogous to the intensified depletion region in conventional p-n heterojunctions, effectively impeding ion transport across the interface29. As illustrated in Fig. 3c, the forward direct current (DC, IF) significantly exceeded the reverse DC current (IR), demonstrating the exceptional ion rectification effect of PIDs. The ratio of forward to reverse current (IF/IR) serves as the rectification ratio, providing a quantitative measure of the diode performance30. As illustrated in Fig. 3d, PIDs lacking PPy/PPPy exhibited low IF/IR values (<5), whereas those incorporating PPy/PPPy demonstrated significantly higher values, further determining the role of IDL in the rectification effect. In particular, regardless of whether the polyanion is PSSA, PSSA-Fe, or PSSA-PB, most PPPy-based PIDs demonstrate higher IF values, leading to a stronger rectification effect compared to PPy-based PIDs. This enhanced performance is likely attributed to the more abundant ion transport channels in PPPy, which promote H+ migration across the IDL and into the polycation29. Conversely, the dense and positively charged PPy networks impede H+ entry, thereby diminishing the forward conduction capability. Additionally, PIDs with thicker PPy/PPPy layers demonstrated higher IF/IR values, highlighting the beneficial role of PPy/PPPy as an effective proton-collecting layer in enhancing the performance of the ion diode.
a Rectification by the SBPP-3 at alternating voltage of ±1 V at 0.1 Hz. b Schematic illustration of the ionic rectification effect of PIDs. c DC current density of PIDs. Data are presented as mean values ± SD (n = 312). d Rectification ratio of PIDs. e VO and h IS values of PIDs at 60% RH. Data are presented as mean values ± SD (n = 60,000). f VO and g IS values of SP-0, SP-3, SPP-3, SFP-0, SFP-3, SFPP-3, SBP-0, SBP-3, and SBPP-3 at regulated RH. Data are presented as mean values ± SD (n = 60,000). i Pden and Iload values of SBPP-3 at regulated Rload, 70% RH. j Pden values of SP-0, SP-3, SPP-3, SFP-0, SFP-3, SFPP-3, SBP-0, SBP-3, and SBPP-3 at regulated Rload, 60% RH.
The electrical output performance of PIDs is evaluated by the open circuit voltage (VO) and short circuit current (IS) values31. Under indoor conditions (relative humidity (RH) = 60%, temperature = 23.5 °C), the VO of pure CF-based PIDs followed the trend of SP-0 (1.00 V) < SFP-0 (1.12 V) < SBP-0 (1.32 V), indicating that Fe3+ coordination and the incorporation of MOFs can promote the delocalization of ionic charges (Fig. 3e and Supplementary Fig. 13). The coating of PPy/PPPy on CF slightly increased the VO value. Similarly, the PSSA-Fe and PSSA-PB electrolytes can effectively enhance the IS values of CF-based PIDs, among which the IS values for SP-0, SFP-0, and SBP-0 are 0.03, 0.16, and 0.20 mA, respectively. It is worth noting that the optimization of MEG performance by PPy/PPPy is mainly reflected in the IS, and thicker electrochemical deposition is more conducive to DC output, especially PPPy, which is attributed to the abundant charge transfer channels (Fig. 3h and Supplementary Fig. 14). Over a period of 3 h, SBPP-3 demonstrated continuous discharge with an average IS value of 0.95 mA. The environmental adaptability of MEG nanogenerators based on PIDs was evaluated under varying RH conditions (20%–90%). As illustrated in Fig. 3f, g and Supplementary Figs. 16 and 17, both the VO and IS values exhibited an initial increase followed by a subsequent decrease as the humidity level rose, especially for SBPP. When the RH was 70%, the IS value of SBPP-3 peaked, achieving an average stable current output of 8.24 mA over a duration of 600 s. This phenomenon can be attributed to the increased humidity, which facilitated greater adsorption of H2O into the polyanion networks, thereby enhancing the ionization of COOH groups and the generation of H+. However, excessive H2O content may diminish the osmotic pressure difference of H+ across the hydrogel system, ultimately leading to the loss of the driving force for directional ion migration. The humidity gradient plays a crucial role in driving the directional migration of charge carriers. However, under excessively high humidity conditions, the ion distribution tends to become more uniform, which diminishes the driving force and ultimately hinders effective current output.
The moist energy harvesting performance of PIDs is assessed by measuring the output power density while connected in series with varying resistances ranging from 100 Ω to 107 Ω (see details in Supplementary Note 1)32. As illustrated in Fig. 3j and Supplementary Fig. 18, under indoor conditions, output energy density (Pden) of PIDs with three different polyanion electrolytes consistently exhibited the trend PPPy > PPy > CF, aligning with the performance observed in the ionic rectification effect. The maximum Pden of PPPy-based PIDs is observed at 103 Ω, whereas those of CF and PPy-based PIDs peaked at 104 Ω. In addition, Fe3+ coordination and MOFs demonstrated positive effects on moist energy harvesting. As a result of the synergistic optimization of polyanions and polycations, at a loading of 103 Ω, the Pend value of SPP-3, SFPP-3, and SBPP-3 in an indoor environment reached 49.12 μW·cm−2, 148.30 μW·cm−2, and 367.15 μW·cm−2, respectively. To investigate the power output performance of PIDs under tunable environmental conditions, the energy harvesting capabilities of SBPP-3 were further investigated under an RH of 70%. As shown in Fig. 3i and Supplementary Fig. 19, under a load of 103 Ω, the Pend of SBPP-3 reached 480.19 μW·cm−2, demonstrating significant potential for practical applications in energy supply. Furthermore, as summarized in Supplementary Table 4, the PIDs developed in this work exhibit superior current output performance compared to recently reported representative MEGs, including those based on biomaterials, carbon-based materials, inorganic nanomaterials, and polymer composites.
The long-term operational stability of the PIDs is evaluated by analyzing the IS values over three sequential stages: 0–1 h, 1–2 h, and 2–3 h. As shown in Supplementary Fig. 15, in the initial stage (0–1 h), the IS values show relatively large fluctuations and standard deviation. During the mid-term (1–2 h), the current output gradually stabilizes, and the error bars become significantly smaller. In the final stage (2–3 h), the current reaches a nearly constant value with minimal variation and near-zero error bars, indicating that the device tends toward steady-state current output in the latter operation phase. Furthermore, the 12-h continuous discharge behavior of an SBPP-3 film was measured on the first day and again after 7 days. As shown in Supplementary Fig. 20, the average IS value measured after 7 days retained 95.8% of its initial value on the first day, confirming the excellent long-term stability of the PIDs.
To demonstrate the adaptability of PIDs under dynamic humid conditions, SPP-1 was selected as a representative sample to evaluate its VO and IS responses under periodic exposure to human breath. As shown in Supplementary Fig. 21, when moisture was blown onto the surface of the PIDs by human breath, both the VO and IS increased significantly, particularly IS, due to enhanced moist energy harvesting and reduced internal resistance. Once the moisture was removed, VO and IS gradually returned to their original baseline values. This reversible response demonstrates the excellent environmental adaptability and practical harvester applicability of PIDs under dynamic humid conditions.
Thermodynamic and kinetic mechanisms of charge transfer in PIDs
To thoroughly analyze the energy harvesting principles of the MEG nanogenerators, the charge transfer mechanism was investigated from both thermodynamic and kinetic perspectives. From a thermodynamic perspective, the adsorption capacity of polyanion surfaces for water molecules was investigated using density functional theory, which is related to the driving force for ionic charge transportation induced by moisture gradients (see details in Supplementary Note 2)33. From a kinetic perspective, the charge conduction capability, ion diffusion behavior, and interfacial effects were analyzed using electrochemical techniques.
As shown in Fig. 4d34, the water molecule adsorption energies (ΔE) of pristine PVA and PSSA are −2.55 KJ·mol−1 and −4.39 KJ·mol−1. The ΔE of Fe3+-coordinated PVA decreases to −2.96 KJ·mol−1, while that of PBA is even lower at −4.83 KJ·mol−1, indicating that PSSA-PB exhibits a stronger spontaneity for H2O molecule adsorption. In the PSSA-Fe system, the ionized COO− groups can coordinate with Fe3+ to form a stable coordination-crosslinked polymer network, which could effectively prevent the recombination of H+ with COO−, enhancing the thermodynamic stability of H+. The Fe4[Fe(CN)6]3·(H2O)14 unit cell structure formed after PBA absorbs water can significantly enhance the H2O storage capacity of PSSA-PB, thereby facilitating the generation of H+ ions35. Furthermore, PBA nanoparticles typically possess a negative surface charge in aqueous systems36, which can promote the hydrolysis of COOH and facilitate H+ generation by attracting the electron cloud around the COOH group, thereby further enhancing the spontaneity and driving force of charge generation and transport. The differential charge density map further provides theoretical confirmation of the role of the IDL, with the red regions indicating electron accumulation and the blue regions representing electron depletion (Fig. 4f)37. At the heterojunction interface, a greater accumulation of negative charges is observed on the PVA side compared to the PPy side, which promotes the formation of an interfacial micro-electric field. The excellent thermodynamic stability of H+ and superior surface hydrophilicity ensure outstanding ionic charge transport capabilities in PSSA-PB-based PIDs.
a H+ concentration on the surface of PIDs. Data are presented as mean values ± SD (n = 5). b Rct values of PIDs. c Ionic conductivity of PIDs. d Water adsorption energy of polyvinyl alcohol (PVA), Fe3+ coordinated PVA, PBA, and PSSA. Schematic illustration of water absorption of Prussian blue analog (PBA). e D values of PIDs. f Charge density difference of the PVA/PPy heterointerface. g Interface charge distribution and transportation model of SBPP.
The primary ion carrier generation capability was evaluated by measuring the H+ concentration (cH+) on the surface of the polyanion networks. As shown in Fig. 4a, the cH+ on the surfaces of SFP-0, SFP-Fe, and SBP-0 were 9.33, 3.95, and 8.68 mM, respectively. The relatively lower cH+ observed on PSSA-Fe can be attributed to polymer crystallization, which restricts the hydrolysis of COOH groups. Notably, the c(H+) on the PSSA-PB surface of PPy/PPPy-based PIDs became lower than that of PSSA, particularly in the cases of the SBPP samples, further confirming the role of PPy/PPPy as a proton collection layer in promoting H+ migration. Electrochemical impedance spectroscopy was employed to analyze the charge transfer and ion diffusion properties (Supplementary Figs. 22 and 23)38. As shown in Fig. 4c, the ionic conductivity of SP-0, SFP-0 and SBP-0 was only 1.34 mS·cm−1, 1.39 mS·cm−1 and 1.54 mS·cm−1, respectively. After the deposition of PPy/PPPy, the PIDs exhibited enhanced ionic conductivity, particularly in the case of PPPy, which can be attributed to the multiple defect structures among the molecular chains of PPPy. For instance, in PSSA-based PIDs, the ionic conductivity progressively increased to 2.45 mS·cm−1 (600 s), 2.98 mS·cm−1 (1200 s), and 4.39 mS·cm−1 (1800 s) with the increase of PPPy deposition time. Finally, the PSSA-PB-based PIDs demonstrated the highest ionic conductivity, with SBPP-1 achieving an ionic conductivity of 4.81 mS·cm−1. Notably, the ionic conductivity of the PSSA-based PID increases gradually with longer PPPy deposition times, while no such monotonic trend is observed for the PSSA-PB and PSSA-Fe-based PIDs. This indicated that the ionic conductivity in SFP/SFPP and SBP/SBPP systems is more dependent on the molecular interactions within the polyanionic networks, while in the pure PSSA system, it benefits more directly from PPPy-induced pathway formation. Therefore, it can be inferred that the abundant micro- and nanopores in MOFs can significantly enhance the pathways for the ionic current based on H+, Cl−, etc. The ionic carrier diffusion kinetics were studied based on the H+ diffusion coefficient (DH+, see details in Supplementary Note 3)39. As shown in Fig. 4e and Supplementary Fig. 24, the increase of PPy/PPPy decreased the DH+ value of PIDs, indicating that the transport of H+ in rigid and positively charged PPy is more challenging compared to the more flexible polyanions. The DH+ value of SBPP-3 reached 6.96 × 10−12 cm−2·s−1. The diffusion of positively charged H⁺ ions (H3O+) within polyanions and PPPy nanopores is driven by a concentration gradient. In this case, based on a qualitative analysis of the Poisson and Nernst-Planck equation, when ion diffusion and electrical migration reach dynamic equilibrium, a stable electrostatic field is established within the system (see details in Supplementary Note 4)40. This field couples with the substance transport process, enabling the generation of direct current output driven by humidity gradients.
Subsequently, the DC output capability of PIDs was investigated based on the charge transfer resistance (Rct, Fig. 4b and Supplementary Fig. 25)41. The Rct values of most PPPy-based PIDs were lower than those of PPy-based PIDs, which can be attributed to the larger specific surface area of PPPy. This enhanced surface area of PPPy facilitated more efficient charge transfer at the interface between the PPy molecular structure and the polyanion gel electrolyte. Thicker PPPy deposition was also accompanied by lower Rct values, further confirming the beneficial role of enhanced surface effects in promoting interfacial charge transfer. However, in the case of pure PPy, excessive deposition resulted in the densification of molecular chains and an increase in polymer crystallinity, which hindered the charge transfer process. Moreover, the interfacial charge transfer process was governed by a combination of Faradaic and non-Faradaic processes in the PIDs. In particular, in SBPP-3, the redox process (Faradaic process) of Fex+ on the surface of PBA grains further boosted the DC output capability, reducing its Rct value to 100.1 Ω35. For non-Faradaic reactions, the interfacial capacitance (C(ω) = C’(ω) + jC”(ω)) effect of PIDs is analyzed based on a typical IDL model, of which the real (C’(ω)) and imaginary part (C”(ω)) refers to energy storage and attenuation, respectively (see details in Supplementary Note 5 and Supplementary Figs. 26–31)42. The results indicate that PPy/PPPy-based PIDs exhibit higher C’(ω) values compared to pure CF, demonstrating their enhanced double layer capacitance (Cdl)43. Particularly under high-frequency alternating electric fields, PPPy-based PIDs exhibited a more significant advantage in C’(ω) compared to C”(ω), highlighting their stable interfacial polarization kinetics at the IDL.
Based on the above analysis, as shown in Fig. 4g, a physical model illustrating the charge transfer mechanism in PIDs is proposed. First, the strong hydrophilicity of the polyanion surface facilitates the spontaneous adsorption of water molecules and promotes the ionization of COOH groups, generating H+ ions. Subsequently, under osmotic pressure driven by the moisture gradient, H+ ions migrate toward the PPy/PPPy side, forming an IDL at the heterogeneous interface, which induces a potential difference across the film. Then, the transfer of charge at the multiple interfaces of positive electrode-polycation-polyanion-negative electrode enables the stable DC output. Additionally, the porous structural PPPy and PB provide abundant charge transfer channels, enhancing charge transfer kinetics. Furthermore, PB, acting as a redox electrolyte, contributes to improved power generation performance through Faradaic charge transfer at the interface. The diverse ionic and electronic transportation properties, coupled with IDL on the heterointerface, not only ensure the stable operation of the MEG nanogenerators but also provide a foundation for investigating the modulation effects of moisture response on electromagnetic energy.
Modulation and protection of wireless electromagnetic energy
Wireless electromagnetic energy modulation and protection performance via PIDs based MEG nanogenerators were studied through EMI shielding effectiveness (EMI SE, see details in Supplementary Note 6). According to the Drude model, the total EMI SE (SET) is primarily attributed to the surface reflection (SER) and EMW absorption (SEA)44. To thoroughly investigate the electromagnetic protection properties of PIDs, the EMW characteristics of their polycationic and polyanionic layers were studied, respectively. CF, PPy@CF, and PPPy@CF exhibited effective surface reflection due to excellent electrical conductivity, whereas the polyanionic electrolyte, with its limited electronic conductivity, allowed most EMW to pass through (Fig. 5c and Supplementary Figs. 32–36). The reflection (R), absorption (A), and transmission (T) coefficients are used to directly reflect the energy transfer and conversion properties45. When external EMW was irradiated onto the surface of PPPy-3, the R, A, and T values reached 28, 58, and 14%, respectively, demonstrating the excellent electromagnetic energy absorption capability of PPPy. The electromagnetic energy transmitted through pure PSSA, PSSA-Fe, and PSSA-PB films reached 42, 63, and 69%, respectively. However, when these films were integrated into pure CF-based PIDs, the T values dropped to 8, 2, and 4% (Supplementary Figs. 37 and 38), all below 10%, which corresponds to SET values exceeding 10 dB, which is generally recognized as effective EMI shielding. As shown in Fig. 5e, f, the EMI SE values primarily increased progressively with the deposition thickness of PPy/PPPy. Similar to pure CF-based PIDs, incorporating PSSA-PB into PPy@CF-based PIDs also enhanced EMI shielding performance. However, in PPPy@CF-based PIDs, pure PSSA exhibited the best performance, with the SET value of SPP-3 reaching 24.48 dB.
a electromagnetic interference shielding effectiveness (EMI SE) values and b power coefficients of SBPP-3. c EMI SE values of polypyrrole (PPy), porous polypyrrole (PPPy), polyanions, and PIDs with carbon fabric (CF). Data are presented as mean values ± SD (n = 201). d Power coefficients of PIDs. Data are presented as mean values ± SD (n = 201). EMI SE values of PIDs with e PPy and f PPPy. Data are presented as mean values ± SD (n = 201). RCS data of g SPP, h SFPP, and i SBPP. Power flow in the j x-y, k x-z, and l y-z planes of SBPP-3 film.
Fig. 5d illustrates the energy modulation behavior of the PPy/PPPy-based PID system as represented by the power coefficient. For most PIDs, an increase in PPy/PPPy thickness resulted in a decreased T value, along with a reduced ratio of the R value to the A value, indicating that the improvement in electromagnetic protection was primarily attributed to enhanced surface reflection driven by increased conductivity. In PSSA-Fe and PSSA-PB-based PIDs, the incorporation of PPPy generally enabled greater absorption of EMW radiation compared to PPy, with SBPP-1 achieving an A value of 51%. However, the opposite trend is observed in PSSA-based PIDs. This indicated that polyanions coordinated with metal ions or featuring MOF structures can more effectively achieve synergistic effects to enhance electromagnetic energy dissipation.
The wireless electromagnetic energy modulation of PIDs showed frequency dependence (Supplementary Figs. 39–42). Take SBPP-3 as an example (Fig. 5b), its A value exhibits two distinct peaks at 7.68 GHz (0.73) and 14.24 GHz (0.49), corresponding to minimal R values (0.27 at 7.68 GHz, 0.51 at 0.49 GHz). This indicates that at these specific frequencies, the incident EMW can more effectively penetrate the surface of the polyanion layer, enter the interior of the PIDs, and achieve stronger electromagnetic energy attenuation. Correspondingly, ascribed to the simultaneous effects of matching frequency and strong absorption, the EMI protection effect of SBPP-3 at 8.00 GHz was optimal, and its SET value reached 25.20 dB, which means more than 99% of the electromagnetic radiation can be shielded (Fig. 5a). Notably, the peak matching frequency of the A values for most PIDs gradually decreased with increasing PPy/PPPy thickness, especially SBPP, which can be attributed to the enhanced dielectric response properties of PPy/PPPy. Furthermore, the peak frequency of SET aligns closely with that of SEA, indicating that the optimization of electromagnetic shielding performance in PIDs primarily arose from enhanced electromagnetic energy absorption.
To investigate the effect of moist-electromagnetic coupling under different humidity conditions, the EMI shielding performance of PIDs was evaluated at extremely low (20% RH) and high (90% RH) humidity levels. Using SBPP-3 as a representative example, the PIDs were preconditioned at 20% and 90% relative humidity for 1 h prior to measurement. As shown in Supplementary Fig. 43, the EMI shielding performance of SBPP-3 under 20% RH is comparable to that of pure PPPy-3, while its SET value increases significantly to 26.70 dB at 90% RH. This enhancement in shielding performance with increasing RH may be attributed to a stronger moist-electromagnetic coupling effect.
The in-depth reflection and propagation performance of EMW are analyzed using Radar Cross Section (RCS) simulations (see details in Supplementary Note 7)46. Under the irradiation of x-polarized plane waves propagating along the -z direction at 18 GHz, the PIDs exhibit significantly reduced RCS values (Supplementary Fig. 44). The increase of deposition thickness of PPPy has the benefit of attenuating the penetration of plane waves, which applies to PIDs based on PSSA, PSSA-Fe, and PSSA-PB, among which the RCS value of SBPP-3 reaches −35.55 dBm2 at 180° (Fig. 5g–i). A thinner PPPy layer can mitigate specular reflection, with the vertical RCS values of SBPP-1 reaching as low as −36.52 dBm2. To validate the modulation of propagating waves by the PID films, the electric field (E-field) distributions in the xoy-, xoz-, and yoz-planes are analyzed47. As shown in Supplementary Fig. 45, SBPP-3 exhibits pronounced polarization rotation under the radiation of an x-polarized incident wave, particularly in the x-to-z direction, which may influence the propagating orientation. The power flow distribution intuitively demonstrates that the incident EMW propagating along the -z direction is coupled into energy flows along the +x and -x directions (Fig. 5j–l). Ultimately, the PIDs exhibit negligible internal energy disturbances, demonstrating excellent self-protection capabilities, which can in turn ensure the stable operation.
Moist-electromagnetic coupling enabled wireless energy modulating mechanism
The modulation mechanism for the coupling of moist and electromagnetic energy has been systematically investigated. Different from traditional EMI shielding materials based on metallic-like films, the PIDs studied in this work ingeniously harness environmental moisture energy to regulate the transmission and conversion of electromagnetic energy, thereby achieving efficient electromagnetic protection. Additionally, the PIDs perform based on a synergistic transport mechanism involving both ions and electrons. The proposed wireless energy modulation process primarily operates through the following stages: moist energy harvesting, ionic charge generation and migration, the establishment of polarization centers, and multi-energy coupling responses.
The dielectric properties of polyanionic and polycationic components are studied by the complex permittivity (ɛr = ɛ’–jɛ”), of which the real and imaginary part refers to the dielectric energy storage and attenuation, respectively (Supplementary Figs. 46, 47, 49)48. Thicker PPPy exhibits higher ɛr values, whereas an increase in thickness results in a decrease in the ɛr value of PPy, highlighting the superior polarization capability of PPPy (Fig. 6a, b). The ɛr values align well with the quarter-wavelength model, where higher ɛr values correspond to thinner matching thicknesses, further confirming the impact of the dielectric layers of PPy/PPPy on frequency dependence49. As shown in Fig. 6c, the ɛr of the polyanionic electrolytes is 2 to 3 orders of magnitude smaller than the dielectric constants of CF, PPy, and PPPy. However, the polyanions demonstrated higher tanδɛ (ɛ”/ɛ’) values, indicating that the polyanions predominantly contribute to polarization loss, while CF/PPy/PPPy primarily facilitate reflection loss in electromagnetic protection (Fig. 6d and Supplementary Figs. 46, 48, 50)50.
a ɛ’ and b ɛ” values of carbon fabric (CF), polypyrrole (PPy), and porous polypyrrole (PPPy). c ɛr values of polyanions. d tanδε values of CF, PPy, PPPy, and polyelectrolyte. The inset is the transmission line equivalent circuit model. Data are presented as mean values ± SD (n = 201). Z values of e PSSA, f PSSA-Fe, and g PSSA-PB. h Schematic of impedance matching and quarter-wavelength resonance. i Spatial charge distribution in PSSA-PB and SBPP-3 at tunable time of radiation. j Schematic of energy harvesting and self-protection mechanism of moist-enabled electricity generators. k Microscopic electromagnetic protection physical model. l Dynamic changes of terminal charge. m Schematic of dielectric loss model on the polyanion/PPPy heterointerface.
Typically, when EMW radiate toward the PIDs, the impedance matching between the polyanion surface and the ambient air significantly influences the ability of the incident waves to penetrate into the interior of the PIDs. Based on transmission line theory, the interfacial impedance matching between the polyanion surface and free space was analyzed using the Z value, as expressed by the following equation51:
where Zin, Z0, c, f, d, μr refer to the input impedance of polyanions, characteristic impedance of free space (377 Ω), light in free space, frequency of incident wave, thickness of polyanion layers, and complex permeability of polyanions, respectively. Figure 6e–g plots the Z values of three kinds of polyanions depending on both frequency and film thickness. Generally, the closer the Z value is to 1, the better the impedance matching effect52. At the optimal matching frequency and thickness, the Z values of PSSA, PSSA-Fe, and PSSA-PB reached 0.42, 0.40, and 0.91, respectively, illustrating the superior impedance matching characteristics of PSSA-PB, enabling incident waves to more effectively penetrate into SBP and SBPP-type PIDs.
Afterward, the EMW will be radiated on the surface of CF, PPy, or PPPy. The sinusoidal alternating electric field induces the periodic oscillation of electronic charges within the conductive matrix, thereby generating an alternating microcurrent on the surface, as described by the following equation53:
where S, k, E0, t, and ρ0 are surface area, electrostatic constant, E-field amplitude, time, and initial phase, respectively. On one hand, the skin current induces reverse secondary EMW, hindering the propagation of EMW54. As illustrated conceptually in Fig. 6h, based on the well-established quarter-wavelength resonance principle, the reflected secondary wave can interfere destructively with the incident wave, thereby promoting the attenuation of electromagnetic energy through coherent destructive interference. On the other hand, based on Joule’s law, electron oscillation can induce ohmic losses, converting a portion of the electromagnetic energy into thermal energy27. Simultaneously, the positive and negative charge dipoles within the polyanions and polycations undergo polarization relaxation under the effect of external alternating electric fields, thereby further facilitating the absorption of electromagnetic energy. Typically, polarization loss is associated with the following energy conversion55:
where D, E, and η represent a volumetric structural coefficient, power of EMW, and the electromagnetic dissipation factor, respectively56. Based on Debye relaxation theory, Cole-Cole plots were utilized to investigate the relaxation behaviors of polyanions and polycations (see details in Supplementary Note 8)57,58. As shown in Supplementary Fig. 51, CF and PPy primarily exhibited conductive properties, whereas PPPy displayed distinct Cole-Cole semicircles with increasing thickness, highlighting the beneficial effect of porous structure on the polarization relaxation of the electronically conductive matrix. The polyanions exhibited multiple Cole-Cole semicircles, implying the presence of diverse polarization relaxation processes.
Above all, this electromagnetic energy transmission and conversion process can be coupled with the harvested moist energy, enabling the modulation of electromagnetic energy by the moist environment. Moist energy not only enables PIDs to exhibit ionic diode properties, allowing them to function as rectennas, but also promotes the formation of IDLs at the heterointerfaces. The interfacial charge plays a crucial role in regulating the storage and release of electromagnetic energy, driven by the local microcapacitance effect. To further investigate the modulation mechanism of moist energy on electromagnetic energy, a physical model of a sinusoidal periodic oscillating electric displacement field (Di) was employed for finite element analysis (FEA, see details in Supplementary Note 9)59. Taking SBPP-3 type PIDs as an example, pure PSSA-PB polyanion, SBPP-3 without moist energy harvesting, and SBPP-3 with moist energy harvesting were employed for FEA, respectively. As shown in Fig. 6i, under the action of D10GHz, PSSA-PB and the assumed SBPP-3 without internal charge aggregation were easily penetrated by the external electric field, while SBPP-3, with surface charges on the PPPy, effectively blocked electromagnetic radiation. Supplementary Videos 1-–3 illustrate the dynamic polarization process occurring within these three models over two periods. Simulation results showed that the local potential difference induced by moist energy can effectively mitigate the interference from external EMW. As shown in Fig. 6k, l, the periodic resonance of the terminal charge (Q = Q’–jQ”) on PPPy substantiates the periodic dielectric polarization of SBPP-3, where Q’ and Q” are attributed to energy storage and attenuation, respectively60. Moreover, the Q’ value after one period (Q’(1.25 T) = 3.49997) is slightly lower than its initial value (Q’(0.25 T) = 3.5), further supporting the occurrence of polarization relaxation. During this process, the interfacial positive and negative charges at the IDL can be periodically polarized under the influence of the external electric field (Fig. 6m). The phase lag between these charges and the external field would induce polarization relaxation loss, thereby enhancing electromagnetic energy absorption.
Based on the above experiments and theoretical analysis, the principle of wireless energy modulation through moist-electromagnetic coupling can be summarized as follows (Fig. 6j):
i) MEG nanogenerators drive H2O adsorption on the hydrophilic polyanion surface, COOH dissociation, and H+ gradient diffusion by harvesting the free energy difference between gaseous H2O and liquid H2O molecules.
ii) The charge carrier concentration gradient fosters the development of a potential difference between the polyanion and polycation surfaces, facilitating the conversion of moist energy into electrical energy and enabling an external DC output.
iii) Simultaneously, the carrier gradient distribution and IDL enhance the ability of PIDs to effectively capture radiated EMW by the rectenna effect, which integrates antenna and rectifier functionalities. This process also facilitates polarization relaxation of the BIEF at the heterointerface under the influence of the alternating electric field.
iv) Ultimately, moist energy can effectively modulate the propagation and attenuation mechanisms of electromagnetic energy through optimized impedance matching and dielectric loss, thereby enhancing electromagnetic protection performance.
Leveraging the synergistic moist-electromagnetic energy coupling mechanism, the wireless energy modulation nanotechnology proposed in this work holds great promise for practical applications in IoT, artificial intelligence, and robotics, etc. First, SPP-1, which does not exhibit the highest current output performance, was selected as a representative example to demonstrate the practical application of PIDs as power sources. As shown in Supplementary Fig. 52, a single SPP-1 can sustain the stable operation of an electronic clock. The device was able to maintain stable operation under various mechanical deformations, including bending, stretching, and pressing, et al., with no observable interruption in energy output. These results strongly support the mechanical robustness and deformation-tolerant functionality of the PID, demonstrating its practical potential for integration into wearable electronics and applications involving body motions. The electromagnetic self-protection capability of PIDs was validated through repeated radiation exposure from a mobile phone. As shown in Supplementary Videos 4 and 5, when the phone gradually approached the PIDs, the radiation detector triggered an alarm, indicating an increase in radiation intensity. This surge in radiation caused significant disturbances in the current signal of the pure PPPy-3 thin film. The curves on the display screen represent time on the horizontal axis and current on the vertical axis. More detailed and clearer electrical signals are shown in Supplementary Fig. 53. However, when the same radiation was applied to SBPP-3-based PIDs, their current output remained nearly unchanged, demonstrating exceptional self-protection ability enabled by the moist-electromagnetic coupling effect.
Therefore, this work not only explores an approach to wireless electromagnetic energy modulation for electromagnetic protection based on moist-electromagnetic coupling, but also systematically investigates the operational physical mechanism of coupled energy conversion, transmission, and attenuation, thereby advancing the evolution of environment-adaptive, self-protective intelligent flexible electronic devices.
Discussion
In summary, we report a wireless energy modulation method enabled by the moist-electromagnetic coupling effect with PIDs. A series of polyanions featuring tunable intermolecular interactions, including hydrogen bonding, metal ion coordination, and MOF modifications, alongside polycations with regulated porous structures, are utilized to assemble PIDs for the simultaneous moisture energy harvesting (480.19 μW·cm−2) and EMI shielding (> 99%). The results demonstrate that MOFs enhance the thermodynamic stability of polyanion polyelectrolytes adsorbed with water, while the abundant micropores within them enrich the ion transport channels, thereby improving carrier transport kinetics. The porous structure of PPPy enhances the interfacial interactions between polyanions and polycations, thereby strengthening the IDL and facilitating the diode-like rectification effect. Leveraging the diode-like rectifying effect endowed by moist energy, PIDs with the essence of ionic-electronic dual response mechanism operate as rectennas, which optimizes their impedance matching. Simultaneously, the IDL enhances polarization relaxation under incident EMW, enabling absorption-dominated electromagnetic protection. We anticipate that our wireless energy modulation mechanism will assist in broad applicability in the device and information security of IoT systems, and will contribute to enabling future self-powered, self-protective microelectronic systems.
Methods
Preparation of PPy@CF and PPPy@CF
PPy was in-situ deposited onto a conductive CF (1 × 3 cm) through a three-electrode system, in which CF, Ag/AgCl electrode, and platinum plate were used as working electrode, reference electrode, and counter electrode, respectively. An electrolyte with 0.5 M KCl and 0.2 M Py was prepared for deposition, while the area of the CF immersed therein was controlled to be 2 cm2. In the constant current mode at a current density of 10 mA·cm−2, Py was stably polymerized and deposited on the CF surface. Subsequently, the working electrode after PPy deposition was washed in deionized water and acetone to remove the remaining monomers and KCl, respectively. After drying, a PPy@CF can be obtained. The PPy@CF obtained at different electrochemical deposition times (600, 1200, and 1800 s) were named PPy-1, PPy-2, and PPy-3, respectively. PPPy@CF (PPPy-1, PPPy-2, PPPy-3) were prepared by a similar method, except that 2 mM MB was added to the electrolyte as a pore-forming agent.
Synthesis of PSSA, PSSA-Fe, and PSSA-PB polyelectrolyte
PSSA polyelectrolyte aqueous solution (10 wt%) was prepared by mixing 5.33 × g PSSA solution (30 wt%) and 0.4 × g PVA in 14.27 ml deionized water. 0.0324 × g FeCl3 was added to the above solution and stirred to obtain Fe3+ coordinated cross-linked polyelectrolyte (PSSA-Fe). The PB-modified polyelectrolyte (PSSA-PB) was obtained by uniformly dissolving 0.05 × g K3[Fe(CN)6] in the PSSA-Fe system and stirring for 6 h.
Preparation of PIDs
PIDs with polyanion@CF, polyanion@PPy@CF, and polyanion@PPPy@CF structures were prepared by impregnating polycation polyelectrolytes on CF, PPy@CF, and PPPy@CF, respectively. First, 1 ml of PSSA was added to a polytetrafluoroethylene mold (3 × 1 × 0.5 cm) and dried in an oven. The part of PPy-1 with PPy deposition was then placed on the surface of the dried PSSA. Subsequently, another 1 ml of PSSA was added to the surface of PPy-1. After drying, a PSSA@PPy-1@CF PID was obtained and defined as SP-1. All the PSSA@CF, PSSA-Fe@CF, PSSA-PB@CF, PSSA@PPy@CF, PSSA@PPPy@CF, PSSA-Fe@PPy@CF, PSSA-Fe@PPPy@CF, PSSA-PB@PPy@CF, and PSSA-PB@PPPy@CF type PIDs were prepared and defined as SP-0, SFP-0, SBP-0, SP, SPP, SFP, SFPP, SBP, and SBPP, respectively. PPy and PPPy with tunable electrochemical deposition time were utilized in PIDs. Finally, the detailed nomenclature of all PIDs is presented in Supplementary Table 1. In indoor environments, the thickness and density of all PIDs are approximately 0.8 mm and 1.1 g·cm−3.
Construction of PIDs based moist-enabled electricity generators
The polyanion electrolyte on one side of the PIDs was adhered by Al tape and served as the negative electrode of the moisture-enabled electricity generators, while the exposed CF portion served as the positive electrode.
Characterizations
The crystalline structure of CF, PPy, and PPPy was characterized by XRD (Rigaku SmartLab 9 kW, Cu-Kα radiation). A Renishaw Micro Raman spectroscopy (532 nm) was used to characterize the chemical and defect structure of CF, PPy, and PPPy. The morphology of CF, PPy, and PPPy was investigated by an SEM (Tescan VEGA3) equipped with EDS. A CHI660E electrochemical workstation was employed to study the charge carrier transportation and impedance characteristics of PIDs. The concentration of H+ on the polycation’s surface was detected by a pH tester (98109, Lianchuang). The VO, IS, and Pden values of moist-enabled electricity generators were characterized by an electrometer (Keithley 6514 system). A vector network analyzer (ENA5071C, Agilent) was used to detect the scattering parameters (S11, S12, S21, and S22) and electromagnetic parameters (ɛr = ɛ’–jɛ”, μr = μ’–jμ”). The detailed calculation method of R, A, T coefficients and SET, SER, SEA values is provided in Supplementary Note 6.
FEA of wireless electromagnetic energy modulation
The wireless energy response mechanism on the surface of PIDs is studied by RCS simulation (see details in Supplementary Note 7). The polarization relaxation mechanism of nanogenerators was simulated on a COMSOL Multiphysics software (see details in Supplementary Note 9).
Data availability
The data generated in this study are provided in the Supplementary Information. Source data are provided as a Source Data file. Source data are provided with this paper.
References
Zhu, S. et al. Integrated lithium niobate photonic millimetre-wave radar. Nat. Photonics 19, 204–211 (2025).
Sun, Y., Su, Y., Chai, Z., Jiang, L. & Heng, L. Flexible solid-liquid bi-continuous electrically and thermally conductive nanocomposite for electromagnetic interference shielding and heat dissipation. Nat. Commun. 15, 7290 (2024).
Lipton, J. et al. Scalable, highly conductive, and micropatternable MXene films for enhanced electromagnetic interference shielding. Matter 3, 546–557 (2020).
Iqbal, A. et al. Anomalous absorption of electromagnetic waves by 2D transition metal carbonitride Ti3CNTx (MXene). Science 369, 446–450 (2020).
Barbruni, G. L., Cordara, C., Carminati, M., Carrara, S. & Ghezzi, D. A Frequency-switching inductive power transfer system for wireless, miniaturised and large-scale neural interfaces. IEEE T. Biomed. Circ. S. 18, 679–690 (2024).
Faraday, M. I. Experimental researches in electricity. —fifteenth series. Philos. Trans. R. Soc. Lond. 129, 1–12 (1997).
Chen, Z. et al. Flexible, transparent and conductive metal mesh films with ultra-high FoM for stretchable heating and electromagnetic interference shielding. Nano-Micro Lett. 16, 92 (2024).
Ma, X. et al. Ultrathin wood-derived conductive carbon composite film for electromagnetic shielding and electric heating management. Adv. Funct. Mater. 33, 2213431 (2023).
Liu, M. et al. A popcorn-inspired strategy for compounding graphene@NiFe2O4 flexible films for strong electromagnetic interference shielding and absorption. Nat. Commun. 15, 5486 (2024).
Iqbal, A., Hassan, T., Naqvi, S. M., Gogotsi, Y. & Koo, C. M. MXenes for multispectral electromagnetic shielding. Nat. Rev. Electr. Eng. 1, 180–198 (2024).
Isari, A. A., Ghaffarkhah, A., Hashemi, S. A., Wuttke, S. & Arjmand, M. Structural design for EMI shielding: from underlying mechanisms to common pitfalls. Adv. Mater. 36, 2310683 (2024).
Zhao, Z. et al. Advancements in microwave absorption motivated by interdisciplinary research. Adv. Mater. 36, 2304182 (2024).
Qu, N. et al. 2D/2D coupled MOF/Fe composite metamaterials enable robust ultra-broadband microwave absorption. Nat. Commun. 15, 5642 (2024).
Kim, S. H. et al. Strain-invariant stretchable radio-frequency electronics. Nature 629, 1047–1054 (2024).
Chamanian, S. et al. Wearable battery-less wireless sensor network with electromagnetic energy harvesting system. Sens. Actuat. A-Phys. 249, 77–84 (2016).
Chen, G. et al. Mechanisms, design, and fabrication strategies for emerging electromagnetic wave-absorbing materials. Cell Rep. Phys. Sci. 5, 102097 (2024).
Lv, H. et al. Functional nanoporous graphene superlattice. Nat. Commun. 15, 1295 (2024).
Cao, W. Q., Zhang, M. & Cao, M. S. A perspective of tailoring dielectric genes for 2D materials toward advanced electromagnetic functions. Adv. Funct. Mater. 34, 2410928 (2024).
Xu, W. H. et al. A droplet-based electricity generator with high instantaneous power density. Nature 578, 392 (2020).
Ni, K. et al. Advances in asymmetric moist-electric generators with innovative heterogeneous structures. Energ. Environ. Sci. 17, 9406–9424 (2024).
Wang, H. et al. Bilayer of polyelectrolyte films for spontaneous power generation in air up to an integrated 1000 V output. Nat. Nanotechnol. 16, 811–819 (2021).
Gao, Z. et al. Hybrid electromagnetic and moisture energy harvesting enabled by ionic diode films. Nat. Commun. 16, 312 (2025).
Qu, L., Shi, G., Chen, F. & Zhang, J. Electrochemical growth of polypyrrole microcontainers. Macromolecules 36, 1063–1067 (2003).
Liu, C. et al. Ionic power generation on a scalable cellulose@polypyrrole membrane: the role of water and thermal gradients. Adv. Fiber Mater. 6, 243–251 (2024).
Wang, Y. C. et al. Graphene implanted shape memory polymers with dielectric gene dominated highly efficient microwave drive. Adv. Funct. Mater. 33, 2303560 (2023).
Pavlou, C. et al. Effective EMI shielding behaviour of thin graphene/PMMA nanolaminates in the THz range. Nat. Commun. 12, 4655 (2021).
Tang, Z. et al. Synthesis of CuCo2S4@Expanded Graphite with crystal/amorphous heterointerface and defects for electromagnetic wave absorption. Nat. Commun. 14, 5951 (2023).
Gao, D. & Lee, P. S. Rectifying ionic current with ionoelastomers. Science 367, 735–736 (2020).
Jiang, F. et al. Ion rectification based on gel polymer electrolyte ionic diode. Nat. Commun. 13, 6669 (2022).
Vogel, Y. B., Zhang, J., Darwish, N. & Ciampi, S. Switching of current rectification ratios within a single nanocrystal by facet-resolved electrical wiring. ACS Nano 12, 8071–8080 (2018).
Feng, J. C. et al. High output power moist-electric generator based on synergistic nanoarchitectonics for effective ion regulation. Nano Energy 119, 109103 (2024).
Liu, X. et al. Power generation from ambient humidity using protein nanowires. Nature 578, 550–554 (2020).
Xu, J. et al. Sustainable moisture energy. Nat. Rev. Mater. 9, 722–737 (2024).
Yang, S. et al. Green moisture-electric generator based on supramolecular hydrogel with tens of milliamp electricity toward practical applications. Nat. Commun. 15, 3329 (2024).
Chen, Y. et al. A stable and high-capacity redox targeting-based electrolyte for aqueous flow batteries. Joule 3, 2255–2267 (2019).
Lu, D. et al. DNA-dependent prussian blue nanoflowers for biosensing, catalysis and imaging. Adv. Funct. Mater. 33, 2208897 (2023).
Liu, Y. et al. Multifunctional power generators beyond moisture limitation. Adv. Funct. Mater. 34, 2407204 (2024).
Liu, M. et al. Improving Li-ion interfacial transport in hybrid solid electrolytes. Nat. Nanotechnol. 17, 959–967 (2022).
Zhu, Y. et al. Organic photovoltaic catalyst with extended exciton diffusion for high-performance solar hydrogen evolution. J. Am. Chem. Soc. 144, 12747–12755 (2022).
Bui, J. C. et al. Ion-specific phenomena limit energy recovery in forward-biased bipolar membranes. Nat. Chem. Eng. 2, 63–76 (2025).
Su, A. Y., Apostol, P., Wang, J., Vlad, A. & Dincă, M. Electrochemical capacitance traces with interlayer spacing in two-dimensional conductive metal-organic frameworks. Angew. Chem. 136, e202402526 (2024).
Wang, L. et al. Eliminating the micropore confinement effect of carbonaceous electrodes for promoting Zn-ion storage capability. Adv. Mater. 34, 2203744 (2022).
Wu, L. & Hofmann, J. P. Comparing the intrinsic HER activity of transition metal dichalcogenides: pitfalls and suggestions. ACS Energy Lett. 6, 2619–2625 (2021).
Kim, T. et al. Megahertz-wave-transmitting conducting polymer electrode for device-to-device integration. Nat. Commun. 10, 653 (2019).
Zhou, Y. et al. Absorption-reflection-transmission power coefficient guiding gradient distribution of magnetic MXene in layered composites for electromagnetic wave absorption. Nano-Micro Lett. 17, 147 (2025).
Liu, Y., Hao, Y., Li, K. & Gong, S. Wideband and polarization-independent radar cross section reduction using holographic metasurface. IEEE Antenn. Wirel. Pr. 15, 1028–1031 (2016).
Wan, X., Li, Y. B., Cai, B. G. & Cui, T. J. Simultaneous controls of surface waves and propagating waves by metasurfaces. Appl. Phys. Lett. 105, 121603 (2014).
Zhou, X. et al. Insulating electromagnetic-shielding silicone compound enables direct potting electronics. Science 385, 1205–1210 (2024).
Cheng, J. et al. Emerging materials and designs for low- and multi-band electromagnetic wave absorbers: the search for dielectric and magnetic synergy?. Adv. Funct. Mater. 32, 2200123 (2022).
Qu, N. et al. Multi-scale design of metal–organic framework metamaterials for broad-band microwave absorption. Adv. Funct. Mater. 34, 2402923 (2024).
Guo, Y., Ruan, K., Wang, G. & Gu, J. Advances and mechanisms in polymer composites toward thermal conduction and electromagnetic wave absorption. Sci. Bull. 68, 1195–1212 (2023).
Liu, X. et al. Manipulation of impedance matching toward 3D-Printed lightweight and stiff MXene-based aerogels for consecutive multiband tunable electromagnetic wave absorption. ACS Nano 17, 8420–8432 (2023).
Zhao, T. et al. Ultrathin MXene assemblies approach the intrinsic absorption limit in the 0.5-10 THz band. Nat. Photonics 17, 622–628 (2023).
Yu, Z., Mix, J. A., Sajuyigbe, S., Slattery, K. P. & Fan, J. An improved dipole-moment model based on near-field scanning for characterizing near-field coupling and far-field radiation from an IC. IEEE T. Electromagn. C. 55, 97–108 (2013).
Vinoy, K. J. & Jha, R. M. Radar Absorbing Materials: From Theory to Design and Characterization (Kluwer Academic, 1996).
Lv, H. et al. A flexible electromagnetic wave-electricity harvester. Nat. Commun. 12, 834 (2021).
Jonscher, A. K. Physical basis of dielectric loss. Nature 253, 717–719 (1975).
Cheng, S. et al. Carbon nanolayer-mounted single metal sites enable dipole polarization loss under electromagnetic field. Nat. Commun. 15, 9077 (2024).
Gao, Z. et al. Tailoring built-in electric field in a self-assembled zeolitic imidazolate framework/MXene nanocomposites for microwave absorption. Adv. Mater. 36, 2311411 (2024).
Zhang, A. X. et al. Analytical modeling of capacitances for GaN HEMTs, including parasitic components. IEEE T. Electron Dev. 61, 755–761 (2014).
Acknowledgements
We would acknowledge the financial support received from the Research Institute for Intelligent Wearable Systems (1-CD43, Receiver: B.X.) and The Hong Kong Polytechnic University (Project No.: 4-YWEQ, Receiver: B.X.) for the work reported here.
Author information
Authors and Affiliations
Contributions
Z.G. leaded the conceptualization, investigation, data curation, and writing–original draft; B.X. leaded the conceptualization, leaded the methodology, supervised the research work, held the funding acquisition, leaded the review & editing; Y.G., J.W., and Y.W. contributed to materials preparation; X.L., F.C., and S.D. contributed to materials characterization; C.M.K. contributed to simulation and visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chang Kyu Jeong, Sundaramoorthy Palanisamy, Renyun Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, Z., Gao, Y., Liu, X. et al. Moist-electromagnetic coupling enabled by ionic-electronic polymer diodes for wireless energy modulation. Nat Commun 16, 10073 (2025). https://doi.org/10.1038/s41467-025-65034-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-65034-1