Abstract
Fe-N5 single-atom catalysts (SACs) hold great promise for water decontamination, however, the fundamental relationship between their high coordination shell environment and catalytic performance in Fenton-like reactions remains poorly understood. Here, we precisely regulate the high coordination shell defects of a model SAC with well-defined axial Fe-N5 configurations to elucidate the impact of remote interactions on peroxymonosulfate (PMS) activation. Experimental and theoretical studies confirm that remote modulation of Fe-N5 sites through high coordination shell defects profoundly enhance Fenton-like catalytic activity, enabling FeN5-SD2 to achieve a turnover frequency (TOF) value of 0.338 min⁻1, surpassing state-of-the-art SACs. Our findings reveal a critical volcano-type correlation between defect content and catalytic efficiency, where coordinated modulation of Fe d-band center positioning and PMS adsorption energetics governs reaction dynamics. Only the FeN5-SD2 configuration with an optimal level of defects density and moderate adsorption energy enables sufficient O-O bond elongation in PMS to lower the energy barrier for selective singlet oxygen (1O2) evolution. This study unveils the mechanistic role of higher coordination shell defects in regulating Fe-N5 active sites and introduces a well-defined model to investigate the structure–property correlations of higher coordination shells in SACs for Fenton-like reactions.
Similar content being viewed by others
Introduction
Fenton-like chemistry leveraging solid oxyanions, particularly peroxymonosulfate (PMS) activation, represent a promising avenue as alternatives to liquid-phase hydrogen peroxide (H2O2)-based reactions due to their enhanced efficacy and versatility in degrading recalcitrant organic contaminants1. While homogeneous first-row transition metal ions such as Co2+, Fe2+, Cu2+, and Mn2+ have shown promising activation capabilities for oxyanions, they suffer from drawbacks including poor recyclability and sludge buildup. In contrast, heterogeneous activators offer potential solutions to these challenges. However, the intrinsic heterogeneity of nanoparticles (NPs) lowers atom utilization efficiency and makes it difficult to identify true active sites and reaction pathways in Fenton-like reactions, compared to homogeneous catalysts2,3.
Single-atom catalysts (SACs) represent a pivotal advancement, offering enhanced atomic utilization efficiency and adjustable electronic structure, thereby bridging the divide between homogeneous and heterogeneous catalysis4,5,6. Within this category, atomically dispersed metals supported on nitrogen-doped carbon frameworks (M-N-C) stand out due to their remarkable catalytic efficacy in activating PMS, in which the planar four-coordinated configuration of metal-N4 (M-N4) moieties has been widely recognized as the active sites7,8. Despite this, a lack of comprehensive understanding regarding the true active sites and the intricate structure-property relationships has resulted in an opaque and largely empirical understanding of the catalytic activation mechanisms. Using Fe-N4 catalysts as an exemplar, various pathways, including radical (sulfate radical (SO4•−) and hydroxyl radical (•OH)), non-radical (singlet oxygenation), and direct electron-transfer processes (ETP), have all been proposed as potential routes for PMS activation9. This seeming contradiction highlights the need for a more quantitative grasp and precise assessment of reactive sites and reaction mechanisms, which are vital for advancing the rational design of SACs and optimizing PMS activation efficiency.
In general, the catalytic efficacy of SACs in PMS activation hinges profoundly upon the coordination intricacies and electronic configurations of the catalytically active metal centers. This principle reflects the behavior of natural metalloenzymes10, where catalytic activity is finely tuned by a well-aligned framework. The central metal site, metal–ligand clusters in the primary coordination sphere, and the binding pocket in the secondary sphere work together through non-covalent interactions to optimize intermediate adsorption11,12. Modifying the coordination number or introducing heteroatoms (e.g., O, S, or P) into the M–N4 framework has been widely used to regulate the first coordination shell, which is directly bonded to the metal center. These strategies have led to the development of asymmetric coordination structures13,14. For instance, the newly formed active sites (e.g., Co-N3O1, Fe-N3S1, or Fe-N3P1) with tailored charge/spin states and optimized electron density near the Fermi level. These features adjust the binding energies of reactive intermediates, controlling both the activation efficiency and selectivity in PMS activation15. Additionally, the introduction of out-of-plane coordination at the central metal sites in the axial direction, known as M-N5 species, further alters the electronic structure of the conventional M-N4 configurations. This modification significantly influences the perpendicular adsorption behavior of PMS molecules and thereby tuning the overall catalytic performance16. Beyond the first-shell coordination, the environment of the second and higher coordination shells, which are not directly bonded to the metal sites, also plays a critical role in shaping the electronic structure of the active site and influencing catalytic efficiency. Engineered coordination defects in higher coordination shells significantly affect the Fe active sites through a remote modulation effect. This “remote modulation” refers to catalytic changes induced by structural defects or heteroatoms located more than ~4 Å from the Fe center17,18,19, beyond the primary (directly bonded) and secondary (bonded to first-shell atoms) coordination spheres. This realm remains relatively unexplored and has seldom been addressed in studies concerning PMS activation, possibly attributed to uncertainties in the local structure of active sites stemming from uncontrolled pyrolysis synthesis. To overcome this limitation, precise engineering of the high coordination shell environment is essential for uncovering the structure–property relationships of SACs beyond conventional M-N4 centers. By targeting Fe-N5 configurations and drawing inspiration from the spatial precision of natural metalloenzymes, this work aims to advance the design of SACs for more efficient PMS activation.
In this work, to unravel the intricate fundamental relationship between the high coordination shell environment and the catalytic performance of SACs in Fenton-like reactions, we designed a model catalytic architecture by integrating fully conjugated polyphthalocyanine frameworks wrapped on nitrogen-doped carbon nanotubes (NCNTs). This strategy integrates axial Fe-N5 configurations in the primary coordination shell, complemented by planar structural defect in higher coordination shells (FeN5-SDx). The remote modulation of Fe-N5 sites via high coordination shell defects significantly optimizes the electronic properties of Fe centers, thereby enhancing their efficacy in selectively activating PMS. Consequently, the FeN5-SD2 catalyst demonstrated exceptional performance in PMS activation, achieving a turnover frequency (TOF) value of 0.338 min⁻1 for bisphenol A (BPA) degradation. In-depth theoretical calculations have demonstrated that the higher coordination shells defects modifying the d-band center of the isolated Fe sites, coupled with a defect content-dependent volcano-like trend in PMS adsorption and activation. Additionally, it promotes charge migration at the reaction interface and notably elongates the O − O bond. Consequently, this modification reduces the energy barrier for generating crucial reaction intermediates, thereby facilitating the selective production of singlet oxygen (1O2). This study broadens the design of SACs beyond the conventional M-N4 motif by tuning multiple coordination shells simultaneously. It reveals fundamental structure–property relationships for PMS activation and offering insights into the rational design of next-generation catalysts for water purification.
Results
Synthesis and structure characterization
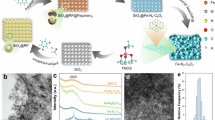
This synthesis strategy fundamentally differs from conventional M-N-C catalyst preparation by eliminating high-temperature pyrolysis while maintaining well-defined active site structure (Fig. 1a). The polyphthalocyanine framework inherently facilitates transition metal incorporation within its macrocyclic cavity. Simultaneously, nitrogen-rich NCNTs promote axial Fe–N coordination. Together, these features stabilize highly ordered Fe-N5 centers as the dominant catalytic sites. To engineer the higher coordination shells around Fe centers, which typically include atoms beyond the primary and secondary shells, we introduced coordination defects (Fig. 1b). This was achieved by co-polymerizing a controlled amount of 1,2-dicyanobenzene (DCB) with benzene-1,2,4,5-tetracarbonitrile (BTC). Incorporating DCB systematically disrupts the polymer matrix, creating tailored defects in the higher coordination shells. These defects lie beyond 5 Å from the Fe center, as confirmed by DFT calculations (Supplementary Figs. 1-2), and are denoted as FeN5-SDx. Scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HR-TEM) analyses confirm that FeN5-SD2 retains an interconnected three-dimensional network of nanofibers with no observable particulate aggregates on surfaces (Fig. 1c). The roughened texture observed along the curved NCNTs originates from a conformal polymer overlayer16. X-ray diffraction (XRD) patterns of FeN5-SDx (Supplementary Fig. 3) display two characteristic peaks at ~26° and ~43°, indexed to the (002) and (100) crystallographic planes of graphitic carbon in NCNTs20. The absence of diffraction signals for metallic iron or iron oxides confirms that Fe species are atomically dispersed. Furthermore, Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM) images directly visualizes isolated Fe single-atoms as discrete bright contrast features (red circles, Fig. 1d), with no evidence of clustering. Complementarily, energy-dispersive X-ray spectroscopy (EDS) elemental mapping reveals homogeneous spatial distributions of C, N, and Fe across the NCNT architecture (Supplementary Fig. 4), further corroborating the uniform anchoring of single-atom Fe sites along the nanotube surfaces without agglomeration.
a Schematic of the preparation strategy for FeN5-SDx using 1,2-dicyanobenzene (DCB), benzene-1,2,4,5-tetracarbonitrile (BTC) and nitrogen-doped carbon nanotubes (NCNTs) as precursors. Crystal structures visualized using VESTA software49. b Schematic illustration of the coordination structure of FeN5-SD2. Crystal structures visualized using VESTA software49. c SEM and HRTEM image of FeN5-SD2. d AC-HAADF-STEM of FeN5-SD2. e FTIR and f Raman spectra of BTC, FeN5, FeN5-SD1, FeN5-SD2, FeN5-SD3 and NCNTs. Source data are provided as a Source Data file.
Fourier transform infrared (FTIR) spectra of all FeN5-SDx samples exhibit the characteristic absorption bands of NCNTs, along with additional peaks assigned to polyphthalocyanine vibrations (Fig. 1e). The progressive disappearance of the -C ≡ N stretching vibration at 2240 cm−1, characteristic of the BTC monomer, is accompanied by the emergence of new peaks corresponding to C = N (1500 and 1135 cm−1) and C-N (1310 cm−1) stretching modes. These changes indicate successful polymerization into a polyphthalocyanine-like framework. A strong absorption at 1396 cm⁻¹ is attributed to C = C stretching within the conjugated macrocycle, while a peak at 731 cm⁻¹ arises from C-H vibrations, further confirming the formation of extended polyphthalocyanine structure21. With increasing defect concentration, the intensity of the C = N and C-N bands moderately decrease, suggesting partial disruption of the pyrrole units within the phthalocyanine ring. In contrast, the C = C band remains stable, implying that the benzene moieties are preserved. These observations are supported by Raman spectra (Fig. 1f), where in addition to the D and G bands of NCNTs, distinct A1g, B1g, and B2g modes related to C–C and C–N–C vibrations in the phthalocyanine ring are observed22. The gradual attenuation of these peaks implies localized structural perturbations rather than a complete collapse of the polyphthalocyanine network. To further examine the coordination environment, solid-state 13C nuclear magnetic resonance (NMR) spectroscopy was conducted using NCNT-free FeN4-SDx samples. As shown in Supplementary Fig. 5, a broad resonance at 100–130 ppm is assigned to aromatic carbons in benzene rings, while a distinct signal at ~171 ppm corresponds to C = N groups in the pyrrole ring21,22,23. The progressive decrease in the 171 ppm signal from FeN4 to FeN4-SD3 reflects a gradual loss of pyrrolic C = N groups, consistent with the formation of high coordination shell defects. Meanwhile, the signals from benzene carbons remain largely unchanged, confirming that structural disruption is confined to the pyrrole moieties. X-ray photoelectron spectroscopy (XPS) C 1 s spectra (Supplementary Fig. 6) show a decrease in C = N and C–N components with increasing defect concentration, supporting the partial loss of coordinated nitrogen. Brunauer–Emmett–Teller (BET) analysis (Supplementary Table 1) further confirms a progressive increase in surface area and pore volume with defect introduction, while preserving the overall covalent framework.
Local geometry and coordination environment
The chemical states of Fe in FeN5-SDx catalysts were systematically investigated through high-resolution XPS measurements. Analysis of the Fe 2p spectra revealed distinct peaks corresponding to Fe2+ and Fe3+ at 709.2 eV and 711.9 eV, respectively, in the 2p3/2 orbital, and at 722.4 eV and 724.0 eV, respectively, in the 2p1/2 band (Fig. 2a). Crucially, the absence of a characteristic zero-valent iron (Fe0) signal at 706.7 eV confirms the atomic dispersion of Fe species, eliminating the possibility of metallic clusters or nanoparticles. A notable positive binding energy shift of 0.74–1.30 eV was observed for both Fe2+ and Fe3+ species in FeN5-SDx relative to FeN5, indicative of strengthened electronic interaction between Fe nuclei and outer-shell electrons. This phenomenon, consistent with defect-induced modulation in higher coordination shells16, suggests altered orbital configurations and energy levels at Fe active sites.
Complementary insights into the coordination geometry and electronic structure were obtained through Fe K-edge X-ray absorption fine-structure (XAFS) studies. The X-ray absorption near-edge structure (XANES) spectra revealed absorption edges for FeN5 and FeN5-SD2 positioned between Fe foil and Fe2O3 references, confirming an intermediate valence state between 0 and +3 (Fig. 2b). The distinct rightward shift of the FeN5-SD2 edge relative to FeN5 demonstrates successful valence state elevation through defect engineering. Notably, both samples lack the pre-edge shoulder characteristic of square-planar FeN4 configurations in FePc, confirming reconstruction of the Fe coordination environment24. Fourier transform (FT) analysis of EXAFS spectra identified a dominant peak at 1.53 Å corresponding to Fe-N coordination (Fig. 2c), with no detectable Fe-Fe scattering signal at 2.2 Å, corroborating XRD and AC-HAADF-STEM observations. Quantitative fitting analysis (Supplementary Fig. 7 and Supplementary Table 2) revealed an average Fe-N coordination number of 5 in FeN5-SD2 with an elongated bond length of 2.01 Å compared to FePc (1.93 Å). This bond lengthening is attributed to additional axial N coordination, confirming successful structural modification of Fe-N5 basal planes. Wavelet transform (WT) analysis further validated the monatomic configuration, exhibiting a singular intensity maximum at 3.6 Å−1 (Fig. 2d) corresponding exclusively to Fe−N interactions. This observation contrasts with reference materials such as Fe2O3, and FePc, where no intensity peaks corresponding to Fe−Fe and Fe−O interactions were detected. Collectively, these characterizations provide conclusive evidence for the precise engineering of defect-modulated Fe-N5 architectures with tailored electronic and coordination structures.
Remote defect enhances Fe-N5 SACs for Fenton-like reactions
The role of coordination defects modulation in Fe-N5 SACs for enhanced PMS activation was rigorously evaluated to achieve efficient BPA removal. Prior to comparative analysis of catalytic performances across FeN5-SDx variants, systematic optimization of operational parameters (Supplementary Fig. 8a-d) was conducted. The FeN5-SD2/PMS system showed the optimal performance at a catalyst dosage of 0.03 g L−1 and a PMS concentration of 0.05 mM. As shown in Fig. 3a, all defect-engineered FeN5-SDx catalysts outperformed pristine FeN5, confirming that coordination defects enhance the intrinsic activity of FeN5 SACs. FeN5-SD2 achieved complete BPA removal within 20 minutes, with a pseudo-first-order rate constant (kobs) of 0.403 min−1. This value is significantly higher than those of FeN5 (0.200 min−1), FeN5-SD1 (0.243 min−1), and FeN5-SD3 (0.268 min−1) (Fig. 3b), indicating an optimal defect density. Moderate levels accelerate reaction kinetics, while excessive defects apparently compromise activity. We performed kinetic normalization using both Fe-molar normalized rate constant (Kper-mol Fe) and specific surface area-normalized rate constant (Kper-area) to rule out other influencing factors (Fig. 3b). Inductively coupled plasma emission spectrometer (ICP-OES) data quantified metal contents across the FeN5-SDx catalysts (Supplementary Table 3). Among these catalysts, FeN5-SD2 exhibited the highest Kper-mol Fe (3.40 × 104 min−1·M−1), outperforming FeN5 (1.30 × 104 min−1·M−1), FeN5-SD1 (1.75 × 104 min−1·M−1) and FeN5-SD3 (2.96 × 104 min−1·M−1). Similarly, Kper-area values confirmed the highest surface-normalized rate for FeN5-SD2 (0.0072 min−1/(m2·g−1)) (Supplementary Table 4). These metrics rule out Fe content and surface area as dominant performance factors. Thus, the improved performance primarily stems from electronic and geometric modulation induced by high coordination shell defects.
a The impact of diverse catalyst systems on the degradation of bisphenol A (BPA). Error bars indicate standard deviation derived from three parallel measurements. b Normalized rate constant of BPA degradation by per mole Fe, per surface area. c Comparison of TOF values for pollutant degradation in SAC/PMS systems featuring coordination environment regulation. The numbers indicated in the figure correspond to the respective references. The effects of d pH and e inorganic anions and HA on the removal of BPA in FeN5-SD2/PMS system. Error bars indicate standard deviation derived from three parallel measurements. f Different pollutants removal in FeN5-SD2/PMS system. Routine conditions: [pollutants] = 80 μΜ, [catalyst] = 0.03 g L−1, [PMS] = 0.05 mM, temperature = 25 °C, without pH adjustment. Source data are provided as a Source Data file.
The TOF was employed to quantitatively evaluate the intrinsic reactivity against previously reported SAC-based PMS activation systems. As shown in Fig. 3c and Supplementary Table 5, FeN5-SD2 catalyst exhibits a significantly high TOF of 0.338 min⁻1. This performance surpasses other recently reported coordination-regulated SACs in PMS systems2,15,25,26,27,28,29,30,31,32,33,34. Control experiments confirmed that neither FeN5-SD2 alone (BPA removal <10%) nor PMS alone (BPA removal <3%) made a significant contribution to contaminant elimination (Fig. 3a). This result excludes substantial adsorption or non-catalytic oxidative pathways. The pH tolerance and recyclability of FeN5-SD2 were systematically assessed to evaluate its operational robustness. The FeN5-SD2/PMS system showed negligible pH dependence across a wide pH range (2–11) (Fig. 3d and Supplementary Fig. 9), achieving >98% BPA removal under all tested conditions. Cycling tests showed excellent stability, with over 95% pollutant removal maintained across four consecutive runs (Supplementary Fig. 10). To further evaluate catalyst durability, post-reaction XRD and XPS analyses were conducted. XRD patterns revealed no significant structural changes after cycling (Supplementary Fig. 11), while XPS C 1 s spectra showed no notable increase in the C–O peak, indicating strong resistance to carbon oxidation (Supplementary Fig. 12). These results confirm that the FeN5-SDx catalysts retain their structural integrity under reaction conditions. In addition, inductively coupled plasma mass spectrometry (ICP-MS) analysis detected minimal iron leaching (85.67 μg L−1), well below the Class III surface water regulatory limit (0.3 mg L−1)26. Control experiments further demonstrated that dissolved Fe ions contributed less than 15% removal even at concentrations up to 1 mg L−1 (Supplementary Fig. 13), ruling out a homogeneous Fenton-like mechanism.
The system further displayed remarkable resistance to common environmental interferents, including inorganic anions (Cl−, SO42−, HCO3−) and humic acid (HA) (Fig. 3e and Supplementary Fig. 14). Field applicability tests in real water matrices (tap water, river water) demonstrated sustained efficacy, achieving > 97% BPA removal within 20 min (Supplementary Fig. 15), highlighting operational reliability in complex matrices. The FeN5-SD2/PMS system demonstrated distinct Fenton-like catalytic behavior across structurally diverse organic pollutants (Fig. 3f). Target analytes spanned multiple classes: dyes (rhodamine B, RhB; methyl orange, MO), chlorinated phenolics (2,4-dichlorophenol, 2,4-DCP; 4-chlorophenol, 4-CP), pharmaceutical contaminants (sulfamethoxazole, SMX), and other contaminants (atrazine, ATZ; benzoic acid, BA). Notably, substrates functionalized with electron-donating groups demonstrated enhanced degradation efficiencies, contrasting sharply with the recalcitrance of electron-withdrawing group-bearing species. Specifically, ATZ and BA, recognized as radical-specific probes35, exhibited minimal degradation. This marked substrate selectivity strongly implies the prevalence of non-radical oxidation pathways over conventional radical-mediated mechanisms in the FeN5-SD2/PMS system.
Roles of ROS during Fenton-like chemistry
Reactive species generation includes radical pathways, such as superoxide radicals (O2•–), •OH, and SO4•−, as well as non-radical pathways like 1O2, high-valence metal oxidation, and ETP. To identify the primary active species in the FeN5-SDx/PMS system and understand how high coordination environments modulate their production, systematic scavenging assays were conducted.
Quenching experiments were first performed to determine the reactive oxygen species (ROS) involved (Fig. 4a). Tert-butanol (TBA) and methanol (MeOH), employed as scavengers for •OH and SO4•−, respectively (Supplementary Fig. 16), had negligible effects on BPA degradation across all FeN5/PMS and FeN5-SDx/PMS systems, indicating minimal contributions from these radicals. In contrast, p-benzoquinone (p-BQ) partially inhibited BPA degradation (Supplementary Fig. 17), suggesting the involvement of O2•–. However, given its limited redox potential (−0.33 V vs. NHE), O2•– is unlikely to drive BPA degradation directly, implying its role as an intermediate in ROS generation36,37. These findings suggest that non-radical pathways primarily govern BPA degradation.
a Comparison of degradation kinetics under various quenching conditions. Error bars indicate standard deviation derived from three parallel measurements. b Decomposition rate of PMS in different systems. c Effect of premixing in FeN5-SD2/PMS system on bisphenol A (BPA) removal. Error bars indicate standard deviation derived from three parallel measurements. d EPR spectra of 1O2 captured by TEMP. e 9,10-diphenylanthracene (DPA) signals in different systems. Darker shades represent earlier time points (0 min) and lighter shades represent later time points (20 min). f Trends in the kobs and 1O2 contribution for various catalysts. g The contribution of ROS in different system. Source data are provided as a Source Data file.
To further explore this, additional quenching experiments using 2,2,6,6-tetramethylpiperidine (TEMP) as a 1O2 scavenger revealed a near-complete inhibition of BPA degradation across all systems (Supplementary Fig. 18). Similarly, β-carotene (βC) and furfuryl alcohol (FFA), both known 1O2 quenchers38, exhibited lower PMS decomposition efficiencies compared to other scavengers and demonstrated comparable inhibitory effects (Supplementary Figs. 19–20)39. These results strongly support 1O2 as the dominant reactive species in BPA degradation within FeN5/PMS and FeN5-SDx/PMS systems. To examine the role of high-valence iron-oxo species, dimethyl sulfoxide (DMSO) was introduced as a selective trapping agent40. Despite its presence, both FeN5/PMS and FeN5-SDx/PMS systems maintained efficient degradation performance (Supplementary Fig. 21), suggesting minimal involvement of high-valence iron-oxo species in BPA oxidation. Moreover, PMS decomposition remained unaffected by the presence of BPA, with the FeN5-SD2/PMS system exhibiting a significantly higher PMS decomposition rate than FeN5, FeN5-SD1, and FeN5-SD3 (Fig. 4b, Supplementary Fig. 22), correlating with its superior pollutant degradation efficiency. Notably, pre-mixing the catalyst with PMS before adding BPA led to a marked suppression of BPA removal (Fig. 4c, Supplementary Figs. 23–24), contradicting the expected characteristics of a direct electron-transfer pathway7. To further investigate this aspect, graphite electrodes coated with FeN5-SDx were employed in galvanic oxidation processes (GOP)41. In this setup, PMS and BPA were exposed to separate half-cells connected by a salt bridge to isolate electron transfer as the only possible oxidation pathway (Supplementary Fig. 25a). However, no current was detected, and BPA remained undegraded after 12 h (Supplementary Fig. 25b–e). These findings confirm that none of the FeN5-SDx/PMS systems facilitate a direct electron-transfer mechanism.
The quenching experiment results were corroborated by electron paramagnetic resonance (EPR) analysis (Fig. 4d). The addition of TEMP led to an enhanced triplet EPR signal characteristic of 1O2 in all FeN5-SDx/PMS systems. The signal intensity followed the order FeN5-SD2 > FeN5-SD3 > FeN5-SD1 > FeN5, aligning with the observed kobs values. In contrast, using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) to detect •OH and SO4•− yielded negligible signals in the FeN5-SD2 system (Supplementary Fig. 26), confirming the absence of these radicals. The TEMP-1O2 signal intensity decreased upon the addition of p-BQ (Supplementary Fig. 27), indicating that O2•– serves as an intermediate in 1O2 formation. Control experiments under O2, air, and N2 atmospheres revealed negligible differences in degradation efficiency (Supplementary Fig. 28), indicating that dissolved oxygen (DO) is not essential to the reaction. This suggests that 1O2 generation proceeds via direct activation of PMS at FeN5 sites, independent of external oxygen input.
Further evidence for the critical role of 1O2 was obtained through solvent exchange experiments using deuterium oxide (D2O), which extends the lifetime of 1O242. As shown in Fig. 4d and Supplementary Fig. 29, the TEMP-1O2 signal intensity increased, and BPA degradation accelerated in D2O relative to H2O, reinforcing the significance of 1O2 in the oxidation process. The production of 1O2 was further validated through the oxidation of 9,10-diphenylanthracene (DPA)43, where the characteristic absorption peak at 378 nm progressively diminished over time in all FeN5-SDx/PMS systems (Fig. 4e), with the most pronounced effect observed in FeN5-SD2.
Kinetic analysis based on pseudo-first-order kinetics44 revealed a volcano-type correlation between defect content and 1O2 generation. Notably, the proportion of 1O2 production closely matched the BPA removal efficiency across the FeN5-SDx/PMS systems (Fig. 4f, g). These findings collectively demonstrate that although variations in high coordination environments within Fe-N5 structures do not alter the fundamental reaction mechanism, they significantly enhance 1O2 production, thereby improving catalytic performance for water purification.
Mechanistic understanding of the coordination structure modulation in FeN5 SACs
To discern the active sites in FeN5-SD2 for PMS activation, an experimental study was conducted using potassium thiocyanate (KSCN) poisoning. SCN− has a high affinity for Fe and poisons the single-atom Fe sites during the Fenton-like reaction45. The introduction of KSCN led to a significant decrease in BPA degradation, with the kobs value dropping from 0.403 to 0.025 min−1 (Supplementary Fig. 30). This result unequivocally confirms the dominant role of single-atom Fe sites in PMS activation within the FeN5-SD2 system.
To further elucidate the intrinsic catalytic mechanism, density functional theory (DFT) calculations were conducted to investigate the impact of higher coordination shell modulation in Fe-N5 SACs on PMS activation. The total density of states (DOS) for FeN5, FeN5-SD1, FeN5-SD2, and FeN5-SD3 revealed a high concentration of electronic activity at the Fe center (Fig. 5a), attributed to nonzero Fe-3d occupation near the Fermi level. Notably, the overlap of Fe d-orbitals with the DOS near the Fermi level signifies enhanced electron transfer capability upon Fe incorporation. In both FeN5 and FeN5-SDx, the population of antibonding states is governed by the Fe d-orbitals. A d-band closer to the Fermi level suggests lower occupancy of these states, which promotes PMS adsorption27,46. The d-band center of FeN5-SDx is upshifted relative to FeN5, shifting closer to the Fermi level in the order of FeN5 (−2.385 eV) < FeN5-SD1 ( − 2.295 eV) < FeN5-SD2 ( − 2.251 eV) < FeN5-SD3 ( − 2.108 eV). Thus, structural defects in the higher coordination shell induce a positive shift in the d-band center, optimizing electronic occupancy and enhancing PMS adsorption.
a Density of states of FeN5, FeN5-SD1, FeN5-SD2 and FeN5-SD3. b Correlation among the oxidation capacity, coupling strength to PMS, and d-band structure of FeN5, FeN5-SD1, FeN5-SD2 and FeN5-SD3. c Electron density difference diagrams and Bader charges for PMS adsorbed on FeN5, FeN5-SD1, FeN5-SD2 and FeN5-SD3, with blue and yellow indicating electron depletion and accumulation, respectively. Crystal structures visualized using VESTA software49. d Calculated potential energy diagrams for various bond-cleavage pathways during PMS decomposition on FeN5-SD2. e Calculated potential energy diagrams for the 1O2 formation pathway. f Schematic diagram of 1O2 generation process. g Length of the O − O bonds after adsorption of PMS adsorbed on different catalysts. Source data are provided as a Source Data file.
To clarify PMS adsorption preferences, the adsorption configurations and energies for three oxygen atoms on FeN5, FeN5-SD1, FeN5-SD2 and FeN5-SD3 were calculated. In all cases, the terminal oxygen bonded to sulfur exhibited the strongest adsorption, identifying it as the preferred site for PMS activation (Supplementary Figs. 31–34). The optimized structures also show a gradual decrease in adsorption energy (Eads) with increasing defect density, consistent with the DOS results (Fig. 5b). Specifically, FeN5 exhibits a weaker adsorption energy (−1.72 eV), whereas FeN5-SD3 displays a stronger PMS binding affinity (−1.91 eV), suggesting that higher coordination shell defects enhance the interaction between Fe sites and PMS molecules. However, the observed kobs follows a volcano trend, with FeN5-SD2 exhibiting the highest Fenton-like activity due to its moderate adsorption energy. The lower reactivity on FeN5-SD3 results from excessive binding strength, which inhibits catalytic turnover by saturating active sites47. These findings highlight that controlled tuning of higher coordination shells can strategically shift the d-band center, optimizing electronic occupancy to enhance PMS adsorption and accelerate Fenton reaction kinetics.
Charge density analysis and Bader charge calculations further elucidate the electron transfer dynamics between Fe-N5 sites and PMS. Charge density difference maps reveal robust electron transfer between FeN5/FeN5-SDx and PMS (Fig. 5c, Supplementary Fig. 35a–d), with an evident charge redistribution between the Fe atom and adjacent N atoms, indicating preferential electron flow from Fe to PMS. Notably, FeN5-SD2 exhibits the highest electron transfer (0.811 e), surpassing FeN5 (0.795 e), FeN5-SD1 (0.798 e), and FeN5-SD3 (0.807 e). Bader charge analysis confirms that tuning higher coordination shells significantly modulates Fe-centered charge density, thereby enhancing electron transfer efficiency. Electrochemical impedance spectroscopy (EIS) and chronoamperometry further support the improved charge transfer interactions in FeN5-SDx. In Supplementary Fig. 36a, FeN5-SD2 exhibits the smallest semicircle diameter, while current responses at the FeN5-SD2 electrode increase significantly upon PMS injection (Supplementary Fig. 36b), confirming that moderate defects in higher coordination shells enhance PMS activation.
To further understand PMS activation in FeN5-SDx, we calculated the energy barriers along the 1O2 evolution pathway (Fig. 5d-e). We first compared the energy barriers for cleaving three key PMS bonds (O-O, S-O, and O-H) on FeN5 and FeN5-SDx. As shown in Fig. 5d and Supplementary Figs. 37-41, the O–O bond consistently has the lowest barrier, indicating it is the easiest to break and thus the preferred initiation step. Optimized free energy diagrams depict the sequential steps of 1O2 generation (Fig. 5f and Supplementary Figs. 42-45): PMS → *PMS (I) → *OH + *SO4 (II) → *OH + H2SO4 (III) → *OH + *OH (IV) → *O (V) → *O + *O (VI) → 1O2. In this pathway, PMS undergoes O–O bond cleavage, forming into *OH and *SO4 fragments. The*OH adsorbing onto FeN5, while *SO4 exothermically convert to H2SO4. Subsequently, *OH transforms into *O, which recombines into 1O2. The highest energy barrier occurs at step I (*PMS → II *OH + *SO4), suggesting it as the rate-determining step (RDS) in 1O2 generation. Introducing moderate defects in the higher coordination shell lowers this barrier from 0.85 to 0.73 eV, thereby facilitating 1O2 formation during PMS activation. Furthermore, O–O bond length (lO−O) analysis (Fig. 5g) reveals FeN5-SD2 exhibits the longest bond (1.474 Å), indicating that moderate defects extend the O–O bond, lowering its cleavage energy barrier and enhancing 1O2 evolution kinetics.
Theoretical insights confirm that defect site modulation in the higher coordination shell of Fe-N5 SACs optimizes Fe 3 d orbital electron distribution. This modulation facilitates PMS adsorption and explains the observed volcano trend in catalytic activity. Furthermore, it enhances charge migration at the reaction interface and extends the O–O bond length, thereby reducing the energy barrier for O–O bond cleavage. As a result, key reaction intermediates form more efficiently, selectively promoting 1O2 production. These findings provide fundamental theoretical validation of enhanced PMS activation through precise defect coordination engineering.
Possible removal pathways of BPA and toxicity analysis
The proposed BPA degradation pathways in the FeN5-SD2/PMS system were deduced by combining liquid chromatography-mass spectrometry (LC-MS) results (Supplementary Fig. 46) with gas chromatography-mass spectrometry (GC-MS) spectrometry data (Supplementary Figs. 47-48 and Supplementary Table 6). As shown in Supplementary Fig. 49, two primary pathways were identified based on the detected intermediates. In Pathway 1, BPA undergoes hydroxylation to form hydroxylated intermediates (e.g., P1), which are further oxidized to P2-P4, containing ketone and carboxyl groups, accompanied by aromatic ring cleavage. This pathway is likely driven by the predominant presence of 1O2 in the reaction medium, which exhibits high reactivity towards hydroxylated intermediates. In Pathway 2, β-scission of the quaternary carbon connecting the two phenyl rings leads to the formation of isopropenylphenol (P6), which subsequently converts into 2-phenyl-1-propene (P7) and p-hydroxyacetophenone (P8). These intermediates are further oxidized to form open-chain compounds, including hexa-1,5-diene-3,4-diol (P9), phenol (P10), and p-benzoquinone (P11). Ultimately, the aromatic structures are mineralized into CO2 and H2O through sequential oxidation steps.
To assess the real-world applicability of the catalytic system, we first predicted the toxicity of BPA and its intermediates using the Ecological Structure-Activity Relationships (ECOSAR) and Toxicity Estimation Software Tool (T.E.S.T.) systems (Fig. 6a-b, Supplementary Fig. 50, and Supplementary Table 7). The half-lethal concentration (LC50) values for BPA in Fathead minnow and Daphnia magna were within the “toxic” range. However, most degradation intermediates exhibited reduced toxicity. For instance, the 96 h LC50 value for BPA in Fathead minnow was 3.24 mg L−1, while intermediates like P8, P10, and P11 showed significantly higher LC50 values of 35.51 mg L−1, 38.69 mg L−1, and 31.96 mg L−1, respectively, indicating a reduced toxicity classification (Fig. 6a). Similarly, the 48-h LC50 value for BPA in Daphnia magna was 1.58 mg L−1, while most intermediates showed a marked increase in LC50 (Fig. 6b). As illustrated in Supplementary Fig. 50, BPA is classified as a “developmental toxicant”, while P8 and P9 are classified as “developmental non-toxicants”. Supplementary Table 7 shows that both acute and chronic toxicity values of the oxidized products decreased as degradation progressed. Notably, advanced oxidation products such as P9, P10, and P11 were predicted to be “not harmful”, underscoring the detoxification capacity of the FeN5-SDx system.
a Fathead minnow LC50-96 h and b Daphnia magna LC50-48 h of bisphenol A (BPA) and its degradation intermediates. Flow cytometry analysis of E. coli cells during BPA degradation in FeN5-SD2/PMS system at different reaction time: c 0 min, d 40 min. The four sections in flow cytometry images represent as below: Q1 dead cells; Q2 late apoptotic cells; Q3 early apoptotic cells; Q4 live cells. e Schematic illustration of wastewater treatment process. f PVDF/FeN5-SD2 membrane. g Photograph of the wastewater treatment experimental equipment. h Water flux of different membranes during the continuous-flow process. Insets show the averaged water flux values. i Removal efficiency of different systems. j BPA removal efficiency and Fe leaching of PVDF/FeN5-SD2 membrane system in different water matrices. Source data are provided as a Source Data file.
Beyond computational predictions, experimental toxicity validation was performed using zebrafish and microbial viability assays. Zebrafish exposed to BPA solution exhibited early mortality and behavioral abnormalities over a 12 h period. In contrast, those cultured in the post-reaction BPA solution from the FeN5-SD2/PMS system showed no abnormalities or fatalities (Supplementary Fig. 51), providing strong evidence for the biocompatibility of the treated water. Finally, flow cytometry analysis was conducted to evaluate the cytotoxic effects of treated and untreated solutions on microbial populations (Fig. 6c-d). After 40 minutes of treatment, the proportion of live cells increased from 35.1% to 70.6%, while dead cells decreased from 3.92% to 0.73%. These results highlight the robust detoxification capability of the FeN5-SD2/PMS system.
Application prospect of FeN5-SD2
The exceptional catalytic performance of the FeN5-SD2/PMS system has led to its widespread adoption in practical applications. Through synergistic integration of advanced oxidation processes (AOPs) with polyvinylidene fluoride (PVDF) membrane confinement technology, we engineered a catalytic filtration platform that effectively restricts organic pollutant diffusion while conferring autonomous self-cleaning functionality. Fabrication involved vacuum-assisted deposition of FeN5-SD2 suspensions onto PVDF substrates, producing membranes with elasticity and malleability suitable for continuous-flow wastewater treatment configurations (Fig. 6e-g). Comparative hydraulic performance evaluation of pristine PVDF and PVDF/FeN5-SD2 membranes was conducted through systematic analysis of mass transfer dynamics and solute rejection characteristics for BPA contaminated solution (Fig. 6h). The functionalized membrane exhibited stable performance over 20 consecutive cycles (10 h), maintaining a water flux of 910.1 L·m-2·h−1 with 5.2% solute rejection. This flux approaches that of unmodified PVDF (946.6 L·m−2·h−1, 3.0% rejection), while offering improved contaminant interception.
Continuous-flow evaluations demonstrated broad-spectrum contaminant elimination, sustaining >95% BPA removal efficiency over 10 h operational duration. The system exhibited universal remediation capability for recalcitrant pollutants including 4-CP, phenol (PE), and SMX, achieving near-quantitative removal (Fig. 6i). Validation across diverse aqueous matrices (TW: tap water; SW: surface water; Fig. 6j) confirmed consistent performance in complex ionic environments, with iron leaching levels (80 μg·L−1) remaining 75% below China’s Class III surface water standard (0.3 mg·L−1), verifying catalyst stability. The confluence of high contaminant removal efficiency, matrix tolerance, and exceptional metal retention positions the PVDF/FeN5-SD2 system as a technologically viable solution for advanced water remediation applications.
Discussion
The strategic engineering of primary and higher coordination spheres represents an innovative design paradigm for tailoring SACs in Fenton-like chemistry. In this study, we engineered a model catalyst with a meticulously defined axial Fe-N5 active center and defect sites in higher coordination shells, employing a pyrolysis-free methodology. The FeN5-SDx configuration demonstrates advantages in both PMS activation and selective 1O2 generation, maintaining robust stability with a TOF value of 0.338 min⁻1 within FeN5-SD2/PMS, outperforming FeN5/PMS system and other reported SAC-based systems. Mechanistic investigations reveal that the efficiency of PMS activation and the selective generation of ROS are highly dependent on the extent of defects in the higher coordination shells of SACs. Controlled tuning of higher coordination shells can strategically shift the Fe d-band center, optimizing electronic occupancy to enhance PMS adsorption. Only FeN5-SD2 with an optimal level of defects and moderate adsorption energy can effectively elongate the O–O bond of PMS, thereby reducing the cleavage energy barrier and accelerating the kinetics of 1O2 generation. Following immobilization on a PVDF membrane, FeN5-SD2 was integrated into a continuous flow system, achieving sustained ( > 95% BPA removal over 10 h) and broad-spectrum elimination of recalcitrant contaminants (4-CP, PE, SMX) across diverse aqueous matrices. This study offers profound insights into the relationships between local atomic environments and properties of Fe-N5 SACs concerning PMS activation in water purification processes.
Methods
Chemicals and reagents
1,2-Dicyanobenzene (DCB), 1,2,4,5-tetracyanobenzene (BTC), ethylene glycol, N, N-dimethylformamide (DMF), 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), nitrogen-doped carbon nanotubes (NCNTs), and β-carotene (βC) were obtained from Shanghai Macklin Biochemical Co., Ltd. Ferric chloride (FeCl3), potassium thiocyanate (KSCN), bisphenol A (BPA), and nitroblue tetrazolium chloride (NBT) were supplied by Aladdin Industrial Corporation. Potassium monopersulfate triple salt (PMS, 2KHSO5·KHSO4·K2SO4), 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), and 2,2,6,6-tetramethylpiperidinyloxyl (TEMP) were purchased from Sigma-Aldrich Chemical Co., Ltd. Potassium iodide (KI), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and p-benzoquinone (p-BQ) were obtained from J&K Chemical Co., Ltd. Tert-butyl alcohol (TBA), methanol (MeOH), ethanol (EtOH), hydrochloric acid (HCl), furfuryl alcohol (FFA), and dimethyl sulfoxide (DMSO) were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals were of analytical grade or higher and were used without further purification. Throughout the study, deionized water was used for all experiments.
Synthetic strategy for FeN5-SDx
Synthesis of FeN5-SD2 was achieved employing a one-step protocol. In detail, a mixture comprising BTC (0.7 mmol), DCB (0.7 mmol), and FeCl3 (0.085 mmol) was dispersed in a solvent system comprising ethylene glycol and DMF, followed by ultrasonication for 30 minutes. Subsequently, DBU (0.15 mmol) was introduced as a catalyst, initiating the reaction under a nitrogen atmosphere for 30 minutes. Introduction of 100 mg of NCNTs to the solution ensued, which was then stirred for 24 h. The resultant mixture underwent heating at 180 °C for 72 h, followed by gradual cooling to room temperature. The precipitated solid was harvested via centrifugation and consecutively washed with 1 M hydrochloric acid, anhydrous ethanol, and deionized water. The ensuing product, designated as FeN5-SD2, was vacuum dried at 70 °C for 48 h.
For comparative analysis, the molar weight ratio of DCB to the combined weight of BTC and DCB was adjusted to 0%, 25% and 75%, resulting in the production of samples denoted as FeN5, FeN5-SD1 and FeN5-SD3, respectively.
Characterizations
X-ray diffraction (XRD) patterns were recorded on a PANalytical Empyrean powder diffractometer using Cu Kα radiation (λ = 0.1541 nm). Fourier transform infrared (FTIR) spectra were acquired with a Thermo Scientific Nicolet iS20 spectrometer using KBr pellets. X-ray photoelectron spectroscopy (XPS) was performed on a PerkinElmer PHI 5000 C system equipped with a monochromatized Al Kα source (200 W). Sample morphology was examined by field emission scanning electron microscopy (FE-SEM, HITACHI Regulus 8100), and high-resolution transmission electron microscopy (HRTEM) images were obtained using a FEI TalosF200S microscope. The Fe content of the samples was quantified via inductively coupled plasma optical emission spectroscopy (ICP-OES, Agilent 720ES), while iron leaching concentrations were measured with an Agilent 7700 ICP-MS. High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images were recorded on a FEI Titan Themis 60-300 TEM/STEM. Fe K-edge X-ray absorption fine structure (XAFS) spectra were collected at beamline BL44B2 of the SPring-8 synchrotron (Japan), where the storage ring operated at 8.0 GeV with a maximum current of 250 mA. Data were acquired in transmission mode using a Si (111) double-crystal monochromator and ionization chambers under ambient conditions. Electron paramagnetic resonance (EPR) measurements were conducted on a Bruker A300 spectrometer.
Evaluation of catalyst decontamination performance in batch experiments
The catalytic performance was evaluated through BPA degradation experiments to assess the catalyst’s ability to activate PMS. The standard experimental procedure was conducted as follows: In a thermostatic magnetic stirring system (25 °C, 0.7 × g), 0.03 g L−1 catalyst was uniformly dispersed in 80 μM BPA solution, followed by the injection of 0.05 mM PMS to initiate the catalytic reaction. Samples were collected at predetermined intervals (with immediate addition of 0.5 mL methanol to quench the reaction) and filtered through 0.22 μm aqueous membranes for phase separation. The residual BPA concentration in supernatant was quantified using high-performance liquid chromatography (HPLC). All experiments were performed with 2-3 parallel tests, with data presented as mean ± standard deviation. Error bars were derived from parallel experimental results. The initial pH was adjusted using 1 M HCl or 1 M NaOH, with pH 4.0 used as the default unless specified otherwise. For reusability tests, the catalyst was recovered after each cycle by filtration and rinsing with deionized water.
Continuous-flow experiment for water treatment
In the practical implementation of membrane technology, a 5 mg sample of FeN5-SD2 underwent ultrasonic dispersion in deionized water. It was subsequently deposited onto a polyvinylidene fluoride (PVDF) membrane via vacuum-assisted filtration, resulting in the fabrication of a PVDF/ FeN5-SD2 composite membrane. After loading the membrane into the reactor, a composite solution comprising 80 μM of BPA and 0.10 mM PMS was delivered to the membrane reactor using a peristaltic pump. Samples were collected at the reactor outlet and subjected to filtration for the precise determination of BPA concentrations.
Quantification of organic contaminants
The concentrations of organic dyes, rhodamine B (RhB) and methyl orange (MO), were measured using a UV–vis spectrophotometer at 552 nm and 465 nm, respectively. Contaminant concentrations were further analyzed by high-performance liquid chromatography (HPLC) using a Waters e2695 system equipped with a UV detector and a C18 column (4.6 mm × 150 mm, 5 μm). Detection wavelengths and mobile phases were optimized for each compound: BPA at 225 nm, 2,4-DCP at 284 nm, and ATZ at 224 nm using methanol/water (70:30, v/v); SMX at 265 nm with methanol/water (50:50, v/v); 4-CP and BA at 280 nm and 227 nm, respectively, with methanol/water (60:40, v/v). The degradation efficiency (η) of contaminants was calculated as:
where C0 and Ct represent the initial and time-dependent contaminant concentrations, respectively. Total organic carbon (TOC) was determined using a Shimadzu TOC-L Analyzer.
For further identification of degradation products, liquid chromatography–mass spectrometry (LC/MS) was performed with an Agilent 1290 system coupled to a QQQ (Agilent 6460) tandem mass spectrometer.
Post-reaction BPA solutions (50 ppm) were acidified to pH 3.0 with HCl, mixed with 1 g NaCl, and transferred to a separatory funnel. Organic extraction was performed with dichloromethane (5 mL × 2), and the combined extracts were dried over Na2SO4, concentrated, and re-dissolved in 1 mL of acetone.
Degradation products were analyzed using GC/MS (Shimadzu QP2020 NX) equipped with a DB-17MS quartz capillary column (20 × 0.18 mm, 0.18 μm) and electron impact (EI) detector at 70 eV. Helium was used as the carrier gas at 1 mL/min. Injection volume was 1 μL in splitless mode. The oven program was: 40 °C for 3 min, ramp at 15 °C/min to 320 °C, hold for 6 min. Injector, interface, and ion source temperatures were 300, 300, and 230 °C, respectively.
PMS concentration and active species identification
The concentration of PMS was measured via the ABTS method. Briefly, 0.1 mL of sample was mixed with 0.5 mL of 2 mM ABTS solution, 1 mL acetate buffer (pH 4), and 20 μL of 1.5 mM KI solution, then diluted to 3 mL with water. Absorbance at 415 nm (ε = 34 000 M–1 cm–1) was used to quantify PMS based on the generation of ABTS•+.
The active species composition in the catalytic system was elucidated through radical scavenger experiments and non-radical probe analysis. MeOH and TBA were employed as scavengers for •OH and SO4•− radicals, respectively, with a molar ratio of 1000:1 relative to PMS. Specific concentration parameters for other scavengers are provided in the figure captions. To further validate reactive species identification, EPR spectroscopy was performed using a Bruker Model A300 spectrometer under ambient conditions. The following trapping agents were utilized: DMPO for detecting •OH and SO4•− radicals, methanol-dissolved DMPO as a specific probe for O2•−, and TEMP for singlet oxygen (1O2) identification. Systematic analysis of characteristic radical signal intensity variations provided mechanistic insights into the catalytic reaction. 9,10-diphenylanthracene (DPA) oxidation was employed to estimate the production of 1O2 in the systems. A 100 μL DPA solution (1 g L−1, in DMSO) was mixed with 1.8 mL deionized water. Subsequently, 1.6 mL of the time-dependent sample solution and 0.8 mL methanol (as a quencher) were added. The decomposition of DPA was monitored using a UV-vis spectrophotometer, with the decomposition rate determined by the change in absorbance at 378 nm.
Electrochemical measurements
Electrochemical impedance spectroscopy (EIS) and chronoamperometry were performed in a conventional three-electrode cell, with a Pt plate as counter electrode, Ag/AgCl as reference, and indium-tin oxide (ITO) glass as working electrode. The working electrode was prepared by dispersing 20 mg of catalyst in 300 μL isopropanol with 50 μL Nafion, sonicating, and coating onto pretreated ITO glass. After air drying, uncoated areas were masked with epoxy.
Galvanic oxidation processes (GOP) were carried out using PMS and BPA in separate half-cells (50 mL) connected via a KCl/agar salt bridge. The catalyst was immobilized on a graphite electrode for the GOP experiments.
DFT computational methods
All DFT calculations were performed utilizing the Vienna Ab-initio Simulation Package (VASP). Perdew-Burke-Ernzerhof (PBE) functional within the generalized gradient approximation (GGA) method was employed to account for exchange-correlation effects. The projected augmented wave (PAW) method was utilized to incorporate core-valence interactions, while spin polarization was integrated into all computations. Plane wave expansions were truncated at an energy cutoff of 400 eV. Structural optimization was conducted with energy and force convergence thresholds set at 1.0×10-4 eV and 0.05 eV Å−1, respectively. Brillouin zone sampling was accomplished with a 2 × 2× 1 grid centered at the gamma (Γ) point. Grimme’s DFT-D3 methodology was employed to describe dispersion interactions.
The Gibbs free energy changes (ΔG) of the reaction are calculated using the following formula:
where ΔE is the electronic energy difference directly obtained from DFT calculations, ΔZPE is the zero-point energy difference, T is the room temperature (298.15 K) and ΔS is the entropy change.
Calculation details of the turnover frequency
The turnover frequency (TOF) of each metal site, which represents the intrinsic activity of the catalyst could be calculated as follows:
where Δn (pollutant) is the moles of pollutants converted, Mmetal is metal atomic weight, m0 is catalyst mass, ωmetal is metal mass fraction, and t is reaction time.
Toxicity assessment
Three healthy adult zebrafish (one female and two males) were placed together in a tank containing nutrient-rich medium for 12 h to induce spawning and maximize embryo yield. Fertilized embryos were subsequently rinsed thoroughly with deionized water to remove surface contaminants. Embryos at the eight-cell stage were identified under a microscope and collected for downstream experiments.
Exposure solutions were prepared according to the experimental design. Embryos in the experimental group were treated with the post-reaction solution from the FeN5–SD2 catalytic system. The blank group received only the nutrient medium, while the control group was exposed to a BPA solution. Embryos were distributed into 24-well culture plates, with ten embryos per group and one embryo per well. Each well contained 1 mL of nutrient medium mixed with 1 mL of the designated exposure solution. Embryo development was monitored microscopically, and exposure solutions were refreshed every 24 h from the start of treatment until hatching.
Toxicity changes during BPA degradation in the FeN5-SD2/PMS system were assessed using LIVE/DEAD staining of Escherichia coli (E. coli) followed by flow cytometry. The E. coli strain was obtained from the American Type Culture Collection (ATCC), and beef extract was purchased from Beijing Coolabor Biotechnology Co., Ltd. Briefly, 10 μL of E. coli was inoculated into 10 mL of nutrient medium (3 g/L beef extract, 10 g/L tryptone, 5 g/L NaCl, pH 7.2) and incubated on a rotary shaker at 30 °C for 10 h. Cells were harvested by centrifugation at 402 × g for 5 min and washed three times with phosphate-buffered saline (PBS, 0.5 M, pH 7.4).
Next, 1 mL of the prepared bacterial suspension was added to 10 mL of the reaction solution collected at different time points (0 and 40 min). After incubation for 9 h, the mixtures were centrifuged at 402 × g for 5 min and washed three times with PBS. The cells were then stained with 50 μL propidium iodide (PI) and 50 μL SYTOX in the dark at 25 °C for 15 min. Cytotoxicity changes over time were subsequently analyzed by flow cytometry.
Data availability
The data generated in this study are provided within the article and the Supplementary Information/Source Data file. Additional data are available from the corresponding authors upon request. Source data are provided with this paper48.
References
Alvarez, P. J. J., Chan, C. K., Elimelech, M., Halas, N. J. & Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotechnol. 13, 634–641 (2018).
Zhang, L. S. et al. Carbon nitride supported high-loading fe single-atom catalyst for activation of peroxymonosulfate to generate 1O2 with 100 % selectivity. Angew. Chem. Int. Ed. 60, 21751–21755 (2021).
Mi, X. et al. Almost 100% peroxymonosulfate conversion to singlet oxygen on single-atom CoN2+2 sites. Angew. Chem. 133, 4638–4643 (2021).
Shang, Y., Xu, X., Gao, B., Wang, S. & Duan, X. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 50, 5281–5322 (2021).
Singh, B. et al. Single-atom (iron-based) catalysts: Synthesis and applications. Chem. Rev. 121, 13620–13697 (2021).
Wu, X. & Kim, J. H. Outlook on single atom catalysts for persulfate-based advanced oxidation. ACS EST Eng. 2, 1776–1796 (2022).
Pan, J. et al. Improving peroxymonosulfate activation by copper ion-saturated adsorbent-based single atom catalysts for the degradation of organic contaminants: electron-transfer mechanism and the key role of Cu single atoms. J. Mater. Chem. A 9, 11604–11613 (2021).
Zeng, T. et al. Atomically dispersed Fe–N4 site as a conductive bridge enables efficient and stable activation of peroxymonosulfate: Active site renewal, anti-oxidative capacity, and pathway alternation mechanism. Environ. Sci. Technol. 57, 20929–20940 (2023).
Gao, Y., Liu, Y. Z., Wang, W. L. & Wu, Q. Y. Recent advances in the single-atom catalysts for persulfate activation and pollutant oxidation: A review. J. Clean. Prod. 397, 136576 (2023).
Huang, L., Chen, J., Gan, L., Wang, J. & Dong, S. Single-atom nanozymes. Sci. Adv. 5, 5490 (2019).
Gotico, P. et al. Second-sphere biomimetic multipoint hydrogen-bonding patterns to boost CO2 reduction of iron porphyrins. Angew. Chem. Int. Ed. 58, 4504–4509 (2019).
Jiao, L. et al. When nanozymes meet single-atom catalysis. Angew. Chem. Int. Ed. 59, 2565–2576 (2020).
Wang, M. et al. Atomically dispersed Fe–heteroatom (N, S) bridge sites anchored on carbon nanosheets for promoting oxygen reduction reaction. ACS Energy Lett. 6, 379–386 (2021).
Tang, C. et al. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J. Am. Chem. Soc. 143, 7819–7827 (2021).
Wang, Z. et al. Cobalt single atoms anchored on oxygen-doped tubular carbon nitride for efficient peroxymonosulfate activation: Simultaneous coordination structure and morphology modulation. Angew. Chem. 134, e202202338 (2022).
Li, X., Chen, T., Yang, B. & Xiang, Z. Fundamental understanding of electronic structure in FeN4 site on electrocatalytic activity via dz2-orbital-driven charge tuning for acidic oxygen reduction. Angew. Chem. Int. Ed. 62, e202215441 (2023).
Song, W. et al. Review of carbon support coordination environments for single metal atom electrocatalysts (SACS). Adv. Mater. 36, 2301477 (2024).
Yang, H. et al. Manganese vacancy-confined single-atom Ag in cryptomelane nanorods for efficient Wacker oxidation of styrene derivatives. Chem. Sci. 12, 6099–6106 (2021).
Sun, T. et al. Design of local atomic environments in single-atom electrocatalysts for renewable energy conversions. Adv. Mater. 33, 2003075 (2021).
Praats, R. et al. Electrocatalytic oxygen reduction reaction on iron phthalocyanine-modified carbide-derived carbon/carbon nanotube composite electrocatalysts. Electrochim. Acta 334, 135575 (2020).
Li, X. & Xiang, Z. Identifying the impact of the covalent-bonded carbon matrix to FeN4 sites for acidic oxygen reduction. Nat. Commun. 13, 57 (2022).
Zhang, K. et al. Molecular modulation of sequestered copper sites for efficient electroreduction of carbon dioxide to methane. Adv. Funct. Mater. 33, 2214062 (2023).
Wu, H. et al. -Conjugated polymeric phthalocyanine for the oxidative coupling of amines. Chem. Commun. 56, 3637–3640 (2020).
Zhong, R. et al. Atomic Fe-N4 sites on electrospun hierarchical porous carbon nanofibers as an efficient electrocatalyst for oxygen reduction reaction. Chin. Chem. Lett. 31, 1588–1592 (2020).
Wu, Z. et al. Facilely tuning the first-shell coordination microenvironment in iron single-atom for Fenton-like chemistry toward highly efficient wastewater purification. Environ. Sci. Technol. 57, 14046–14057 (2023).
Liu, C. et al. The “4 + 1” strategy fabrication of iron single-atom catalysts with selective high-valent iron-oxo species generation. Pro. Natl. Acad. Sci. 121, e2322283121 (2024).
Wu, Q. Y., Yang, Z. W., Wang, Z. W. & Wang, W. L. Oxygen doping of cobalt-single-atom coordination enhances peroxymonosulfate activation and high-valent cobalt–oxo species formation. Pro. Natl. Acad. Sci. 120, e2219923120 (2023).
Cheng, C. et al. Generation of FeIV=O and its contribution to Fenton-like reactions on a single-atom iron−N−C catalyst. Angew. Chem. 135, e202218510 (2023).
Xie, M. et al. Single-atom Co-N5 catalytic sites on carbon nanotubes as peroxymonosulfate activator for sulfamerazine degradation via enhanced electron transfer pathway. Sep. Purif. Technol. 304, 122398 (2023).
Zhu, C. et al. Two-step pyrolysis to anchor ultrahigh-density single-atom FeN5 sites on carbon nitride for efficient Fenton-like catalysis near 0 °C. Appl. Catal. B 319, 121900 (2022).
Yang, T., Fan, S., Li, Y. & Zhou, Q. Fe-N/C single-atom catalysts with high density of Fe-Nx sites toward peroxymonosulfate activation for high-efficient oxidation of bisphenol A: Electron-transfer mechanism. Chem. Eng. J. 419, 129590 (2021).
Chen, T. et al. Boosting peroxymonosulfate activation by porous single-atom catalysts with FeN4O1 configuration for efficient organic pollutants degradation. Chem. Eng. J. 450, 138469 (2022).
Yin, K. et al. Microenvironment modulation of cobalt single-atom catalysts for boosting both radical oxidation and electron-transfer process in Fenton-like system. Appl. Catal. B 329, 122558 (2023).
Yin, K. et al. High-loading of well dispersed single-atom catalysts derived from Fe-rich marine algae for boosting Fenton-like reaction: Role identification of iron center and catalytic mechanisms. Appl. Catal. B 336, 122951 (2023).
He, J., Wan, Y. & Zhou, W. ZIF-8 derived Fe‒N coordination moieties anchored carbon nanocubes for efficient peroxymonosulfate activation via non-radical pathways: Role of FeNx sites. J. Hazard. Mater. 405, 124199 (2021).
Wu, L. et al. Oxygen vacancy-induced nonradical degradation of organics: Critical trigger of oxygen (O2) in the Fe–Co LDH/peroxymonosulfate system. Environ. Sci. Technol. 55, 15400–15411 (2021).
Yan, Y. et al. Ru doped graphitic carbon nitride mediated peroxymonosulfate activation for diclofenac degradation via singlet oxygen. Chem. Eng. J. 430, 133174 (2022).
Zeng, T. et al. Covalent triazine frameworks with defective accumulation sites: Exceptionally modulated electronic structure for solar-driven oxidative activation of peroxymonosulfate. Environ. Sci. Technol. 56, 9474–9485 (2022).
Yun, E. T., Lee, J. H., Kim, J., Park, H. D. & Lee, J. Identifying the nonradical mechanism in the peroxymonosulfate activation process: Singlet oxygenation versus mediated electron transfer. Environ. Sci. Technol. 52, 7032–7042 (2018).
Jin, S. et al. Unveiling the fundamental understanding of two dimensional π-conjugated FeN4+4 sites for boosting peroxymonosulfate activation. J. Mater. Chem. A 12, 11310–11321 (2024).
Dong, Y. et al. Mechanically treated Mn2O3 triggers peracetic acid activation for superior non-radical oxidation of micropollutants: Identification of reactive complexes. Water Res 255, 121486 (2024).
Liu, B. et al. Insights into the oxidation of organic contaminants by Co(II) activated peracetic acid: The overlooked role of high-valent cobalt-oxo species. Water Res 201, 117313 (2021).
Wang, Y., Lin, Y., He, S., Wu, S. & Yang, C. Singlet oxygen: Properties, generation, detection, and environmental applications. J. Hazard. Mater. 461, 132538 (2024).
Zeng, T. et al. Fluorinated defect-engineered acetylenic polymers with separated active centers for switching the photosensitized activation pathway of peroxymonosulfate. ACS Catal. 14, 1405–1418 (2024).
Yang, X. et al. Unveiling the axial hydroxyl ligand on Fe-N4-C electrocatalysts and its impact on the pH-dependent oxygen reduction activities and poisoning kinetics. Adv. Sci. 7, 2000176 (2020).
Liu, H. Z. et al. Tailoring d-band center of high-valent metal-oxo species for pollutant removal via complete polymerization. Nat. Commun. 15, 2327 (2024).
Li, X. et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis. J. Am. Chem. Soc. 140, 12469–12475 (2018).
Jin, S. et al. Remote Tuning of Single-Atom Fe-N5 Sites via High-Coordination Defects for Enhanced Fenton-Like Water Decontamination. Figshare https://doi.org/10.6084/m9.figshare.30112222 (2025).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (22276172 to T.Z., 22322602 to H.Y.Z., and 22278374 to Z.Q.H.), the Zhejiang Provincial Natural Science Foundation of China (LR23B070001 to H.Y.Z.), and Central Guiding Local Science and Technology Development Fund Projects (No.2025ZY01044 to T.Z. and H.Y.Z.).
Author information
Authors and Affiliations
Contributions
T.Z. conceived and planned the experiments, and S.J.J., W.X.T. and Y.W. conducted the synthesis, characterization, and catalytic performance tests. S.J.J. performed the theoretical studies. S.J.J., Z.Q.H., H.Y.Z. and W.X.T. conducted in collecting data and analyzing various characterizations. Y.L.H., T.Z., S.J.J., Y.Q.C. and S.S. wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hui Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, S., Tan, W., Huang, Y. et al. Remote tuning of single-atom Fe-N5 sites via high-coordination defects for enhanced Fenton-like water decontamination. Nat Commun 16, 10455 (2025). https://doi.org/10.1038/s41467-025-65425-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-65425-4