Abstract
This phase II study (ALTER-H006; NCT05111366) evaluates adjuvant benmelstobart plus anlotinib in patients with high-risk recurrence (including ≥4 tumors, portal vein tumor thrombus [Vp1/2], or hepatic vein tumor thrombus [Vv1/2]) after hepatocellular carcinoma (HCC) resection. Primary endpoint is 1-year recurrence-free survival (RFS) rate. Secondary endpoints include overall survival (OS), 1-year OS rate, RFS, and safety. Median follow-up is 12.6 months. Among 37 patients enrolled, 1-year RFS rate is 59.7%, and median RFS is 15.6 months. Subgroups with Vp1/2 and Vv1/2 show median RFS of 18.2 months and not reached (NR), respectively. The longest recurrence-free duration is 25.9 months. Median OS is NR (1-year OS rate, 91.7%). Grade ≥3 treatment-related adverse events occur in 45.9% of patients, most commonly hypertension. No treatment-related deaths occur. Here, we show that adjuvant benmelstobart plus anlotinib is a feasible treatment option for HCC patients with high-risk recurrence after HCC resection, warranting confirmation in large-scale randomized clinical trials.
Similar content being viewed by others
Introduction
Over 40% of hepatocellular carcinoma (HCC) patients develop early recurrence within two years of resection, with a dismal 1-year post-recurrence survival of only 55.9%1,2,3. Particularly high-risk patients with macrovascular invasion or multifocal disease relapse earlier (1-year recurrence-free survival [RFS], 27.7%) and were associated with worse prognosis (1-year overall survival [OS] rate, 54.5%)4, while such a population has been largely excluded from adjuvant trials. As such, there is an urgent clinical need to establish effective adjuvant therapies for these particularly high-risk cases.
Preclinical studies showed that simultaneous targeting of tumor vessels and immunity is a viable strategy to normalize aberrant vascular-immune crosstalk and enhance antitumor immune response5,6. This synergistic effect has been confirmed through the pivotal phase III IMbrave050 trial, in which atezolizumab plus bevacizumab effectively delayed early recurrence in high-risk HCC patients (tumor diameter >5 cm, >3 tumors, microvascular invasion, Vp1/2 portal vein tumor thrombus [PVTT], or tumor differentiation of grade 3 or 4) following resection7,8. Notably, over a 2-year follow-up, patients outside the “up-to-7” criteria had longer RFS than those within the criteria9. Beyond the IMbrave050 study population, particularly high-risk patients with ≥4 tumors or macrovascular invasion could also derive the benefit of delaying early recurrence from the combination of anti-angiogenic agent and ICI, as demonstrated in the camrelizumab plus apatinib study (1- and 2-year RFS rates of 48.9% and 41.0%, respectively)10. In parallel, the feasibility of this approach for particularly high-risk populations is further emphasized by ongoing phase III trials in the domestic and abroad (NCT03847428, NCT04682210, and NCT04639180).
Benmelstobart is a humanized monoclonal antibody targeting programmed death ligand-1 (PD-L1)11. Anlotinib, an oral, small-molecule multi-targeted tyrosine kinase inhibitor (TKI), has demonstrated potent anti-tumor activity across various solid tumors, including primary HCC12,13. A preclinical study revealed synergistic effects of ICI and anlotinib in HCC models14; and neoadjuvant benmelstobart plus anlotinib clinically achieved a 100% R0 resection rate and a 23.1% pathological complete response in high-risk resectable HCC15.
Here, we initiated a phase II clinical study (ALTER-H006) to evaluate the efficacy and safety of adjuvant benmelstobart plus anlotinib for particularly high-risk HCC patients following resection.
Results
Patient characteristics
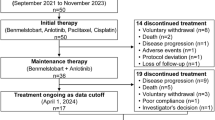
Between January 2022 and April 2024, 53 patients were screened, of whom 37 were eligible for enrollment and included in the efficacy and safety analysis (Fig. 1). As of the cut-off date (October 25, 2024), 9 (24.3%) patients remained on study treatment, 9 (24.3%) patients completed study treatment, and 19 (51.4%) patients discontinued study treatment due to disease recurrence (n = 15), loss to follow-up (n = 2), and intolerable toxicity (n = 2).
The median age was 56.5 (range, 33–75) years. Most patients were male (94.6%). Among the 37 patients, 18 (48.6%) had four or more tumors, 13 (35.1%) had Vp1/2 PVTT, 4 (10.8%) had Vv1/2 HVTT, and 2 (5.4%) had both four or more tumor nodules and Vp1/2 PVTT. The baseline demographics and disease characteristics are listed in Table 1.
Efficacy
Thirty-seven patients were included in the efficacy analysis. As of the data cut-off, the median follow-up was 12.6 (range, 4.2–30.6) months. The median number of cycles of both benmelstobart and anlotinib was 10 (range, 2–18) cycles. This study met the prespecified primary endpoint with a 1-year RFS rate of 59.7% (95% CI, 38.9%–75.4%) (Fig. 2A). A total of 15 (40.5%) patients had recurrence or died. The median RFS was 15.6 (95% CI, 7.9–not evaluable [NE]) months. The proportion of patients experiencing intrahepatic and extrahepatic recurrence was 93.3% and 6.7%, respectively. A total of 2 deaths were recorded, and the median OS was NR. The 1-year OS rate was 91.7% (95% CI, 70.2%–97.9%) (Fig. 2B).
The China Liver Cancer (CNLC) IIIa subgroup exhibited a numerically longer RFS of 18.2 months compared to 12.6 months in the CNLC IIb subgroup (Fig. 3)16. The RFS in the Vp1/2 subgroup, Vv1/2 subgroup, patients with ≥4 tumor nodules, and those with both ≥4 tumor nodules and Vp1/2 were 18.2 months, NR, 12.6 months, and NR, respectively. Representative cases from these subgroups further support the observed efficacy (Fig. S1).
NE not evaluable, ECOG PS Eastern Cooperative Oncology Group performance status, BCLC Barcelona Clinic LiverCancer, CNLC China Liver Cancer, HBV hepatitis B virus, RFS recurrence-free survival, HR hazard ratio. The China Liver Cancer Staging (CNLC) system recommends classifying HCC patients with stage IIb, which is defined by the presence of four or more nodules, and stage IIIa, which is characterized by blood vessel invasion16. Source data are provided as a Source Data file.
Safety
The safety analysis encompassed data from 37 patients. Treatment-related adverse events (TRAEs) occurred in 34 patients (91.9%). Grade 3 or higher TRAEs were observed in 17 (45.9%) patients, with the most common being hypertension (32.4%; Table 2). TRAEs led to anlotinib discontinuation and dose reductions in 2 (5.4%) and 7 (18.9%) patients, respectively. No deaths due to TRAEs occurred during the entire study treatment.
Discussion
ALTER-H006 study demonstrated a 1-year RFS rate of 59.7%, which met its prespecified primary efficacy endpoint. Besides, the particularly high-risk population (those with ≥4 tumors, Vp1/2, or Vv1/2) following HCC resection also achieved a promising OS (1-year OS rate, 91.7%) and RFS (15.6 months), as well as a well-tolerated safety profile. Overall, benmelstobart plus anlotinib may be considered a feasible adjuvant therapy in this challenging clinical setting.
For the population at particularly high risk of post-resection recurrence (those with ≥4 tumors, Vp1/2, or Vv1/2), targeted monotherapy often yields suboptimal efficacy, with a 1-year RFS rate of only 36.1% reported for apatinib monotherapy17. In contrast, adding an ICI to anti-angiogenic therapy has demonstrated improved outcomes in this particularly high-risk cohort, as evidenced by the 1-year RFS rate increasing to 48.9% with camrelizumab plus apatinib10. The enhanced efficacy observed in this patient population with heavy tumor burdens may result from the sensitivity of residual disease to ICI plus anti-angiogenic therapy, as similarly reflected by the early separation of the RFS Kaplan-Meier curves in the IMbrave050 trial8. In this context, our combination study further achieved a numerically higher 1-year RFS rate of 59.7%, possibly attributed to the broader, multitargeted activity of anlotinib differing from the VEGFR2-specific inhibition of apatinib18,19.
Based on the effectiveness of ICI combined with anti-angiogenic therapy as adjuvant treatment, identifying the patient population that benefits most becomes a key issue. The enrollment of a low-risk patient population (92% with a single lesion, median diameter 3.5 cm, with only 32% showing microvascular invasion) was one of the reasons for the failure of the phase III STORM study with sorafenib20. Similarly, the IMbrave050 trial also yielded negative results due to the inclusion of an overall low-risk population (median tumor size in patients who underwent ablation, 2.5 cm; 6.6% with Vp1/2 and 1.0% with ≥4 tumors in resection patients)21. By contrast, our study focused on particularly high-risk patients (e.g., those with ≥4 tumors, Vp1/2, or Vv1/2) and observed the promising efficacy in this population. This finding aligns with the insight from the IMbrave050 subgroup analysis, where patients outside the up-to-7 criteria showed significant RFS improvements (hazard ratio, 0.84; 95% CI, 0.62–1.13) with adjuvant therapy over active surveillance21. Such observations suggest that patients with more high-risk factors derive greater benefit from adjuvant ICI plus an anti-angiogenic agent. However, direct comparisons between ALTER-H006 and other studies are not possible, as the high-risk populations in most studies overlap with the relatively lower-risk population in IMbrave050 and largely exclude patients with the clinically highest recurrence risk, such as those with ≥4 tumors, Vp1/2, or Vv1/212,22,23,24. Overall, ALTER-H006, with complete enrollment of particularly high-risk patients, achieved encouraging results and offered valuable evidence for disease management in this population. Future randomized controlled trials targeting specific populations are necessary to confirm the superior efficacy of ICI plus anti-angiogenic therapy as adjuvant treatment in particularly high-risk patients.
Most key subgroups, especially PVTT patients who harbor substantial residual disease following resection25, derived the pronounced benefits in RFS (median, 18.2 months). Notably, in the ALTER-H006, one patient remained recurrence-free after 18 cycles of treatment with an RFS of 25.9 months, indicating that the synergistic effect of PD-L1 inhibition and TKI in the context of a favorable physical condition (ECOG PS, 0) and well-preserved liver function (Child-Pugh score, 6) may offer long-term benefit in preventing recurrence. Although another case with poorer physical condition (ECOG PS, 1) and pre-resection extensive vascular involvement ultimately experienced recurrence, he achieved an RFS of 12.6 months and relapsed with only oligometastatic disease. Additionally, even a high-risk case with HVTT (Vv2) attained 24.6 months of RFS, underscoring the potential for benmelstobart plus anlotinib across heterogeneous tumor biological profiles. Overall, under the feasibility of adjuvant benmelstobart plus anlotinib, it is crucial to emphasize rigorous postoperative monitoring and a multidisciplinary approach to tailor a management plan based on tumor burden, extent of metastasis, functional status, and other relevant factors, especially considering the impact of tumor heterogeneity on treatment outcomes26,27.
The toxicity profiles of our cohort aligned with previous studies on benmelstobart and anlotinib, without new safety signals identified28,29. The incidence of grade 3 or higher TRAEs (45.9%) was slightly higher than IMbrave050 (35%), possibly due to differences in baseline patient characteristics8. The most common grade 3 or higher TRAE was hypertension, a known class effect of anlotinib30, which was generally manageable by dose modifications and supportive care. No fatal bleeding occurred during the observation period, possibly attributed to initiating anlotinib 4–8 weeks post-surgery, which alleviated delayed wound healing associated with anti-angiogenesis targeted therapy31. Dose discontinuation occurred infrequently in the ALTER-H006, with a comparable incidence to the historical study (5.4% vs. 19.0%)8. In summary, along with the preliminary promising efficacy data, adjuvant benmelstobart plus anlotinib also showed favorable tolerability in patients with HCC at a particularly high risk of recurrence, presenting a potential treatment option to prevent tumor recurrence after surgical resection. Nevertheless, further optimization of immune-targeted combination therapies is necessary, particularly in determining the optimal timing and duration of treatment.
This study has several limitations. First, the single-arm design and small sample size might introduce data bias. Second, patient enrollment from under-represented minority groups, such as those with hepatic vein tumor thrombus, was limited, resulting in insufficient statistical power to identify the precise patient populations most likely to benefit from adjuvant benmelstobart plus anlotinib. Third, further follow-up is required, and additional analyses will be conducted as more mature data become available. Fourth, no biomarker analysis was performed to determine which patients benefited more from this treatment.
In conclusion, adjuvant benmelstobart plus anlotinib showed preliminary efficacy and acceptable safety in ALTER-H006, potentially representing a promising option for adjuvant treatment for patients at high risk of recurrence following resection.
Methods
Study design and patients
ALTER-H006 is a multi-center, single-arm, prospective, phase II study conducted in China. This study was prospectively registered in ClinicalTrials.gov (NCT05111366). Eligible patients were aged 18–75, had histologically or cytologically confirmed HCC, and had undergone R0 resection (grossly and microscopically negative margins), achieving full recovery (well-preserved liver function and primary healing) without recurrence or metastasis on imaging within 4–8 weeks post-resection. Eligible patients must meet any of the following criteria: four or more tumors, portal vein tumor thrombus Vp1/2, hepatic vein tumor thrombus (HVTT) Vv1/232,33. Other eligibility criteria included a Child-Pugh A grade of liver function without hepatic encephalopathy, an expected life expectancy of at least 3 months, Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1.
Key exclusion criteria included recurrent HCC occurring between the most recent resection and enrollment; previous resection for liver cancer within six months before the most recent resection (patients with resection ≥6 months before the most recent resection were eligible); extrahepatic metastasis; and previous treatment of vascular endothelial growth factor (receptor) (VEGF[R]) inhibitors (e.g., anlotinib, sorafenib, lenvatinib) or immune checkpoint inhibitors (e.g., programmed death-1 [PD-1], PD-L1, cytotoxic t-lymphocyte-associated protein 4 [CTLA-4] inhibitors). A comprehensive list of the inclusion and exclusion criteria is available in Method S1.
Procedures
Patients were enrolled in the study and received anlotinib (12 mg, orally, days 1–14) and benmelstobart (1200 mg, intravenously, day 1) in 21-day cycles. The treatment continued until development of unacceptable toxicities, the completion of 18 cycles, the recurrence of disease or extrahepatic metastasis, withdrawal of informed consent, or other conditions requiring termination as deemed by investigators, whichever occurred first. Delays in the administration of benmelstobart and anlotinib were permitted during this study. Anlotinib doses could be reduced sequentially from 12 mg to 10 mg and then to 8 mg according to the protocol-defined dose modification criteria, after which discontinuation was required (toxicities leading to dose reductions of anlotinib are shown in Method S2). Anlotinib could be re-escalated to 10 mg once daily if the investigator identified possible disease progression; each patient was allowed only one dose escalation.
Endpoints and assessments
The primary endpoint of this study was the 1-year RFS rate, defined as the proportion of patients with no relapse or death from any cause from enrollment to the completion of one year of treatment. Secondary endpoints were OS, defined as the time from entry to death from any cause; 1-year OS rate, defined as the proportion of patients who remain alive from enrollment to the completion of one year of treatment; RFS, defined as the time from enrollment to disease recurrence or death from any cause, whichever occurred first; and safety.
Tumor responses were assessed by the investigators using computed tomography (CT) or magnetic resonance imaging (MRI) according to the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 at baseline and every 6 weeks for the first 6 weeks, and every 12 weeks thereafter. Intrahepatic recurrence was defined as the appearance of one or more intrahepatic lesions with the longest diameter of at least 10 mm and a typical vascular pattern of HCC on dynamic imaging (i.e., arterial hypervascularity and venous or delayed phase washout)34. Lesions larger than 10 mm that did not show a typical vascular pattern could be diagnosed as HCC by evidence of a growth interval of at least 1 cm in subsequent scans. Extrahepatic recurrence was defined as per RECIST.
AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.035, throughout the therapy period and up to 30 days after the last dose of the study drug or until the initiation of new anticancer therapy, whichever occurred first.
Statistics and reproducibility
Considering the 1-year RFS rate of 36% achieved by previous adjuvant therapies for patients with resected high-risk HCC17,36, we anticipated that adjuvant anlotinib plus benmelstobart would increase the rate to 56%. The study was planned for a 12-month recruitment period, followed by a 24-month follow-up. We set a one-sided type I error rate of 2.5% and a type II error rate of 20% (80% statistical power) to ensure statistical validity. The total sample size was determined to be 37 patients based on a 10% dropout rate, using PASS software version 15.0. No stopping rules based on safety were defined.
Demographic characteristics and efficacy were analyzed in the full analysis set (FAS), defined as all enrolled patients who received at least one dose of study treatment. The safety analysis set (SAS) included all patients with safety data who had received at least one dose of the study therapy.
The demographic and clinical characteristics of the patients, safety outcomes, and tumor responses were summarized descriptively. The median RFS, median OS, and associated two-sided 95% confidence intervals (CIs) were calculated using the Kaplan–Meier method. Patients who were alive or lost to follow-up were censored at the time of last contact to estimate OS. For RFS, patients who had neither experienced disease recurrence nor died by the time of analysis were censored at the date of their last assessment for HCC occurrence; patients without any post-baseline efficacy assessment were censored at the date of enrollment. Investigators or designated representatives record all data for each patient through eCRFs using the Electronic Data Capture (EDC) system. No data were excluded from the analyses. All statistical analyses were performed using SAS version 9.4 or higher.
Ethics statement
The study protocol was reviewed and approved by the institutional ethics committees of the primary center with Hunan Provincial People’s Hospital (NO. 2021–25.1) and the ethics committees of each participating center, including Hubei Cancer Hospital (NO. LCKY2021028), Jiangxi Cancer Hospital (NO. 2021ky223), and Fujian Provincial Hospital (NO. 2021-021-02). ALTER-H006 was conducted in accordance with the Declaration of Helsinki, the international standards of good clinical practice, and applicable local laws and regulations. All patients gave written informed consent. No compensation was provided to participants. Travel expenses associated with research activities were reimbursed, and any procedures conducted solely for research purposes were covered by the study. Both male and female patients were eligible for enrollment, and sex was self-reported. Data were reported disaggregated by sex.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The protocol of the study is available in the Supplementary Information. Source Data are provided with this paper. All data generated in this study are included in the Supplementary Information and Source Data files. Clinical data were not publicly available due to involving patient privacy, but can be accessed from the corresponding author, beginning 12 months after article publication; individual de-identified patient data will be shared for clinical study analyses. Responses to external data requests will be provided within three months, depending on the nature of the request. Source data are provided with this paper.
References
Poon, R. T. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology 54, 757–759 (2011).
Imamura, H. et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 38, 200–207 (2003).
Yao, L.-Q. et al. Clinical features of recurrence after hepatic resection for early-stage hepatocellular carcinoma and long-term survival outcomes of patients with recurrence: a multi-institutional analysis. Ann. Surg. Oncol. 29, 4291–4303 (2022).
Huang, D. Q. et al. Characteristics and outcomes of hepatocellular carcinoma patients with macrovascular invasion following surgical resection: a meta-analysis of 40 studies and 8,218 patients. Hepatobiliary Surg. Nutr. 11, 848 (2022).
Liu, Z.-L., Chen, H.-H., Zheng, L.-L., Sun, L.-P. & Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 8, 198 (2023).
Yi, M. et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 18, 1–12 (2019).
Lee, W. S., Yang, H., Chon, H. J. & Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 52, 1475–1485 (2020).
Qin, S. et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet 402, 1835–1847 (2023).
Yopp, A. et al. LBA39 Updated efficacy and safety data from IMbrave050: Phase III study of adjuvant atezolizumab (atezo)+ bevacizumab (bev) vs active surveillance in patients (pts) with resected or ablated high-risk hepatocellular carcinoma (HCC). Ann. Oncol. 35, S1230 (2024).
Yang, X. et al. 944P Adjuvant camrelizumab combined with apatinib treatment after resection of hepatocellular carcinoma in CNLC II and III stage: a single-center prospective phase II trial. Ann. Oncol. 32, S824 (2021).
Akinleye, A. & Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 12, 92 (2019).
Wang, J. et al. Efficacy and safety of anlotinib as an adjuvant therapy in hepatocellular carcinoma patients with a high risk of postoperative recurrence. Chin. J. Cancer Res. 35, 399 (2023).
Chen, X.-Q. et al. Effectiveness and safety of anlotinib with or without PD-1 blockades in the treatment of patients with advanced primary hepatocellular carcinoma: a retrospective, real-world study in China. Drug Des. Dev. Ther. 16, 1483–1493 (2023).
Song, F. et al. Anlotinib potentiates anti-PD1 immunotherapy via transferrin receptor-dependent CD8+ T-cell infiltration in hepatocellular carcinoma. Clin. Transl. Med. 14, e1738 (2024).
Huang, Z. et al. 181P TQB2450 (PD-L1 blockade) in combination with anlotinib as a perioperative treatment for patients with hepatocellular carcinoma at high risk of recurrence: Primary results from a prospective, single-arm, phase Ib study. Ann. Oncol. 34, S1545 (2023).
Zhou, J. et al. Guidelines for the diagnosis and treatment of primary liver cancer (2022 Edition). Liver Cancer 12, 405–444 (2023).
Sun, H.-C. et al. Adjuvant apatinib treatment after resection of hepatocellular carcinoma with portal vein tumor thrombosis: a phase II trial. Ann. Transl. Med. 8, 1301 (2020).
Shen, G. et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J. Hematol. Oncol. 11, 1–11 (2018).
Zhou, K. et al. Apatinib, a selective VEGFR2 inhibitor, improves the delivery of chemotherapeutic agents to tumors by normalizing tumor vessels in LoVo colon cancer xenograft mice. Acta Pharmacol. Sin. 40, 556–562 (2019).
Bruix, J. et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 16, 1344–1354 (2015).
Yopp, A. et al. LBA39 Updated efficacy and safety data from IMbrave050: phase III study of adjuvant atezolizumab (atezo) + bevacizumab (bev) vs active surveillance in patients (pts) with resected or ablated high-risk hepatocellular carcinoma (HCC). Ann. Oncol. 35, S1230 (2024).
Wang, K. et al. Adjuvant sintilimab in resected high-risk hepatocellular carcinoma: a randomized, controlled, phase 2 trial. Nat. Med. 30, 708–715 (2024).
Chen, J. et al. 945P Adjuvant lenvatinib in combination with TACE for hepatocellular carcinoma patients with high risk of postoperative relapse (LANCE): Updated results from a multi-center prospective cohort study. Ann. Oncol. 32, S824–S825 (2021).
Ma, T. et al. 239P Adjuvant TACE combined with tislelizumab in patients with resected hepatocellular carcinoma at high risk of recurrence: an open-label, multicenter, phase II trial. Ann. Oncol. 35, S1494 (2024).
Sakamoto, K. & Nagano, H. Surgical treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus. Hepatol. Res. 47, 957–962 (2017).
Shah, S. A. et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 141, 330–339 (2007).
Siddique, O. et al. The importance of a multidisciplinary approach to hepatocellular carcinoma. J. Multidiscip. Healthc. 10, 95–100 (2017).
Xue, J. et al. TQB2450 in patients with advanced malignant tumors: results from a phase I dose-escalation and expansion study. Ther. Adv. Med. Oncol. 16, 17588359231220516 (2024).
Zhou, J. et al. Phase Ib study of anlotinib combined with TQB2450 in pretreated advanced biliary tract cancer and biomarker analysis. Hepatology 77, 65–76 (2023).
Lv, B., Chen, J. & Liu, X.-L. Anlotinib-induced hypertension: current concepts and future prospects. Curr. Pharm. Des. 28, 216–224 (2022).
Bodnar, R. J. Anti-angiogenic drugs: involvement in cutaneous side effects and wound-healing complications. Adv. wound care 3, 635–646 (2014).
Chan, A. W. et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 69, 1284–1293 (2018).
Xie, D.-Y. et al. A review of 2022 Chinese clinical guidelines on the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg. Nutr. 12, 216 (2023).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Shah, S. Common terminology criteria for adverse events. Natl Cancer Inst.: USA 784, 785 (2022).
Sun, H.-C. et al. Effect of postoperative apatinib treatment after resection of hepatocellular carcinoma with portal vein invasion: a phase II study. J. Clin. Oncol. 38, 514 (2020).
Acknowledgments
We gratefully thank the patients and their families for participating in this study. This work was supported by the Key R&D funding of Hunan Province (2023SK2060, grant to X.M.); High-level Talent Support Program of Hunan Cancer Hospital (20250731-1036, grant to X.M.); Natural Science Foundation of Hunan Province (2022JJ70015, grant to X.D.); Natural Science Foundation of Hunan Province (2023JJ60021, grant to X.M.); and Tai Tianqing Pharmaceutical Group Co., LTD. The study sponsor collaborated with the investigators on the design and conduct of the trial; data collection; data management, data analysis, and data interpretation; and preparation, review of the manuscript.
Author information
Authors and Affiliations
Contributions
Concept and design: X.D., D.W., and X.M.; Acquisition, analysis, or interpretation of data: X.D., D.W., C.Z., Y.T., W.D., B.C., J.S., G.X, Y.B., and X.M; Drafting of the manuscript: X.D. and D.W.; Critical review of the manuscript for important intellectual content: X.M., X.D., and D.W.; Statistical analysis: X.D. and D.W.; Obtained funding: X.M.; Administrative, technical, or material support: X.D., D.W., C.Z., Y.T., W.D., B.C., J.S., G.X, Y.B., and X.M; Supervision: X.D., D.W., C.Z., Y.T., W.D., B.C., J.S., G.X, Y.B., and X.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Duan, X., Wu, D., Zhou, C. et al. Adjuvant benmelstobart plus anlotinib in patients with high-risk recurrence after resection of hepatocellular carcinoma: a phase II study (ALTER-H006). Nat Commun 16, 11464 (2025). https://doi.org/10.1038/s41467-025-66342-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-66342-2