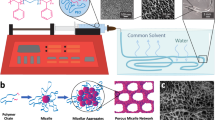
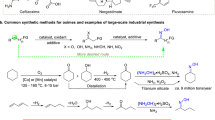
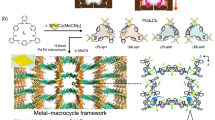
Abstract
Template method offers a promising strategy for synthesizing large pore inaccessible through traditional molecular design. However, this approach has not yet been successfully implemented in molecular assemblies based on weak non-covalent interactions (NCIs), mainly because the assemblies often deviate from original structures during template-assisted syntheses, and the resulting porous structures lack the robustness to survive upon template removal. In this work, we address these challenges through choosing small biocompatible building blocks featuring multiple hydrogen-bonded sites and extensive π conjugation, enabling self-assembly into desired structure in the presence of templates and ensure structural integration upon template removal. As a result, the transformation from densely packed hydrogen-bonded crystalline materials to macroporous structure, referred to as hydrogen-bonded organic frameworks (HOFs), becomes achievable. This strategy facilitates the fabrication of highly ordered materials in single-crystal form with high physiological stability, and enhanced mass transfer. Importantly, it greatly broadens the HOF library to small, affordable, low-toxic, and clinically applicable molecules, making HOFs promising biocompatible porous substrates for bio-related applications such as enzyme immobilization. Herein, we successfully loaded trypsin into macroporous HOFs, which function as effective cellular scaffolds and promote the differentiation of peripheral blood mononuclear cells into fibrocytes, demonstrating their promising potential for biologic applications.
Similar content being viewed by others
Data availability
The experimental data generated in this study are provided within the Article, Supplementary Information and Source Data file. All data are available from the corresponding authors upon request. Source data is available for Fig. 2 and Supplementary Figs. 2–9, 13, 15–27 in the associated source data file. Source data are provided with this paper.
References
Liu, Y. et al. Multiple hydrogen bonding-driven supramolecular architectures and their biomedical applications. Chem. Soc. Rev. 53, 1592–1623 (2024).
Li, S. et al. Advances in hierarchically porous materials: fundamentals, preparation and applications. Renew. Sustain. Energy Rev. 202, 114641 (2024).
Qian, Y., Li, B., Irfan, M., Li, D. & Jiang, H.-L. Reactive oxygen species generation for catalysis and biotherapeutic applications based on crystalline porous materials. Coord. Chem. Rev. 518, 216068 (2024).
Song, X. et al. Design rules of hydrogen-bonded organic frameworks with high chemical and thermal stabilities. J. Am. Chem. Soc. 144, 10663–10687 (2022).
Ma, Y., Li, H., Liu, J. & Zhao, D. Understanding the chemistry of mesostructured porous nanoreactors. Nat. Rev. Chem. 8, 915–931 (2024).
Yu, J., Corma, A. Li, Y. Functional porous materials chemistry. Adv. Mater. 32, e2006277 (2020).
Zhao, X. et al. Theory-guided design of hydrogen-bonded cobaltoporphyrin frameworks for highly selective electrochemical H(2)O(2) production in acid. Nat. Commun. 13, 2721 (2022).
Zhang, A. A., Wang, Z. X., Fang, Z. B., Li, J. L. & Liu, T. F. Long-range π–π stacking brings high electron delocalization for enhanced photocatalytic activity in hydrogen-bonded organic framework. Angew. Chem. Int. Ed. 63, e202412777 (2024).
Liu, B. T. et al. Construction of function-oriented core-shell nanostructures in hydrogen-bonded organic frameworks for near-infrared-responsive bacterial inhibition. Angew. Chem. Int. Ed. 60, 25701–25707 (2021).
Huang, C. et al. Hydrogen-bonded organic framework-based bioorthogonal catalysis prevents drug metabolic inactivation. Nat. Catal. 6, 729–739 (2023).
Huang, S., Chen, G. & Ouyang, G. Confining enzymes in porous organic frameworks: from synthetic strategy and characterization to healthcare applications. Chem. Soc. Rev. 51, 6824–6863 (2022).
Wei, W. et al. Stimulus-responsive hydrogen-bonded organic frameworks: Construction strategies, research progress and applications. Coord. Chem. Rev. 507, 215760 (2024).
Shen, K. et al. Ordered macro-microporous metal-organic framework single crystals. Science 359, 206–210 (2018).
Zhao, X. et al. Macro/microporous covalent organic frameworks for efficient electrocatalysis. J. Am. Chem. Soc. 141, 6623–6630 (2019).
Hong, H. et al. Ordered macro-microporous metal-organic framework single crystals and their derivatives for rechargeable aluminum-ion batteries. J. Am. Chem. Soc. 141, 14764–14771 (2019).
Liu, G. et al. An oxygen-vacancy-rich semiconductor-supported bifunctional catalyst for efficient and stable zinc-air batteries. Adv. Mater. 31, e1806761 (2019).
Kandambeth, S. et al. Self-templated chemically stable hollow spherical covalent organic framework. Nat. Commun. 6, 6786 (2015).
Lin, R.-B. & Chen, B. Hydrogen-bonded organic frameworks: chemistry and functions. Chem 8, 2114–2135 (2022).
Chen, S. et al. Multistate structures in a hydrogen-bonded polycatenation non-covalent organic framework with diverse resistive switching behaviors. Nat. Commun. 15, 298 (2024).
Lin, Z.-J., Mahammed, S. A. R., Liu, T.-F. & Cao, R. Multifunctional porous hydrogen-bonded organic frameworks: current status and future perspectives. ACS Cent. Sci. 8, 1589–1608 (2022).
Hisaki, I., Xin, C., Takahashi, K. & Nakamura, T. Designing hydrogen-bonded organic frameworks (HOFs) with permanent porosity. Angew. Chem. Int. Ed. 58, 11160–11170 (2019).
Cui, J.-W., Liu, S.-H., Tan, L.-X. & Sun, J.-K. Engineering hierarchy to porous organic cages for biomimetic catalytic applications. Acc. Mater. Res. 6, 484–498 (2025).
Halliwell, C. A. et al. Hierarchical assembly of a micro- and macroporous hydrogen-bonded organic framework with tailored single-crystal size. Angew. Chem. Int. Ed. 61, e202208677 (2022).
Tothadi, S., Koner, K., Dey, K., Addicoat, M. & Banerjee, R. Morphological evolution of two-dimensional porous hexagonal trimesic acid framework. ACS Appl. Mater. Interfaces 12, 15588–15594 (2020).
Yu, D., Zhang, H., Ren, J. & Qu, X. Hydrogen-bonded organic frameworks: new horizons in biomedical applications. Chem. Soc. Rev. 52, 7504–7523 (2023).
Zou, Y. et al. Strategy to efficient photodynamic therapy for antibacterium: donor-acceptor structure in hydrogen-bonded organic framework. Adv. Mater. 36 2406026 (2024).
Yin, Q. et al. An ultra-robust and crystalline redeemable hydrogen-bonded organic framework for synergistic chemo-photodynamic therapy. Angew. Chem. Int. Ed. 57, 7691–7696 (2018).
Wen, L. et al. Layer-by-layer assembly of metal-organic frameworks in macroporous polymer monolith and their use for enzyme immobilization. Macromol. Rapid Commun. 37, 551–557 (2016).
Zhang, X.-L. & Chen, X.-M. Supramolecular architectures and helical water chains in cocrystals of melamine and aromatic carboxylic acids. Cryst. Growth Des. 5, 617–622 (2005).
Kaur, J. & Singh, P. K. Trypsin detection strategies: a review. Crit. Rev. Anal. Chem. 52, 949–967 (2022).
Vestling, M. M., Murphy, C. M. & Fenselau, C. Recognition of trypsin autolysis products by high-performance liquid chromatography and mass spectrometry. Anal. Chem. 62, 2391–2394 (1990).
Várallyay, É, Pál, G., Patthy, A., Szilágyi, L. & Gráf, L. Two mutations in Rat trypsin confer resistance against autolysis. Biochem. Biophys. Res. Commun. 243, 56–60 (1998).
Sahin, S. & Ozmen, I. Covalent immobilization of trypsin on polyvinyl alcohol-coated magnetic nanoparticles activated with glutaraldehyde. J. Pharm. Biomed. Anal. 184, 113195 (2020).
Sun, J. et al. Stability and activity of immobilized trypsin on carboxymethyl chitosan-functionalized magnetic nanoparticles cross-linked with carbodiimide and glutaraldehyde. J. Chromatogr. B. 1054, 57–63 (2017).
Lu, J. et al. Fabrication of microporous metal-organic frameworks in uninterrupted mesoporous tunnels: hierarchical structure for efficient trypsin immobilization and stabilization. Angew. Chem. Int. Ed. 59, 6428–6434 (2020).
Tong, L. et al. Pore-environment-dependent photoresponsive oxidase-like activity in hydrogen-bonded organic frameworks. Angew. Chem. Int. Ed. 62, e202218661 (2023).
Tang, Z. et al. A biocatalytic cascade in an ultrastable mesoporous hydrogen-bonded organic framework for point-of-care biosensing. Angew. Chem. Int. Ed. 60, 23608–23613 (2021).
Aggarwal, S. & Ikram, S. Zinc oxide nanoparticles-impregnated chitosan surfaces for covalent immobilization of trypsin: stability & kinetic studies. Int J. Biol. Macromol. 207, 205–221 (2022).
White, M. J., Glenn, M. & Gomer, R. H. Trypsin potentiates human fibrocyte differentiation. PLoS ONE 8, e70795 (2013).
Yu, L., Zhang, H., Xiao, L., Fan, J. & Li, T. A bio-inorganic hybrid hemostatic gauze for effective control of fatal emergency hemorrhage in “Platinum Ten Minutes”. ACS Appl. Mater. Interfaces 14, 21814–21821 (2022).
Shah, D. & Mital, K. The role of trypsin: chymotrypsin in tissue repair. Adv. Ther. 35, 31–42 (2018).
Vernikovskii, B. V. & Stepanova, E. F. Immobilized proteases for wound cleaning. Russ. J. Gen. Chem. 82, 572–578 (2012).
Hu, D. et al. Accelerated healing of intractable biofilm-infected diabetic wounds by trypsin-loaded quaternized chitosan hydrogels that disrupt extracellular polymeric substances and eradicate bacteria. Int. J. Biol. Macromol. 278, 134677 (2024).
Xiang, Y., Jiang, Y. & Lu, L. Low-dose trypsin accelerates wound healing via protease-activated receptor 2. ACS Pharmacol. Transl. Sci. 7, 274–284 (2024).
Miller, J. M., Peirce, E. C. & White, B. H. The clinical use of trypsin. Mil. Med. 113, 270–278 (1953).
Saha, N. et al. PVP - CMC hydrogel: an excellent bioinspired and biocompatible scaffold for osseointegration. Mater. Sci. Eng. C. Mater. Biol. Appl. 95, 440–449 (2019).
Acknowledgements
The authors gratefully acknowledge the help from Mao-Chun Hong, Yong-Sheng Liu, financial support from the CAS Youth Interdisciplinary Team (grant no. JCTD-2022-12 (T.F.L)), Joint Funds for the Innovation of Science and Technology, Fujian Province (grant no. 2024Y9623 (Z.S.Y)), Science and Technology Program of Fujian Province (grant no. 2025J011174 (Z.S.Y), grant no. 2025J011175 (Z.S.Y)), Fujian Provincial Young and Middle-aged Health Leading Talent Training Program (grant no. 2023-2839 (Z.S.Y)).
Author information
Authors and Affiliations
Contributions
Q.X.L. performed on the design and results analysis of all experiments and wrote the manuscript. W.Z.C. assisted in the cell experiment. X.L.Y. performed TEM characterization. Z.S.Y. and Y.Z. advised on the design and interpretation of cell experiments. A.R.M.S. edited the manuscript. T.F.L. advised on the design and interpretation of all experiments and directed the overall project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Francesco Carraro, Antonio Fernandez, Yanfeng Wang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, QX., Cai, WZ., Ye, XL. et al. Highly ordered macroporous hydrogen-bonded organic frameworks based on small biocompatible molecules. Nat Commun (2025). https://doi.org/10.1038/s41467-025-67123-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-67123-7