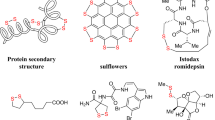
Abstract
Low-valent sulfur-containing compounds are abundant among natural and synthetic products but remain underutilized as starting materials in desulfurative transformations. Herein, we present thiols, disulfides, thioethers, and thioacetals as precursors in a direct desulfurative electrochemical process for the formation of alkylboronic esters, including late-stage functionalization of pharmaceutically relevant scaffolds and natural products. The electrochemical protocol is simple, user-friendly and scalable, successfully producing gram quantities of borylated product.
Similar content being viewed by others
Introduction
Alkylboronic acids and esters are of great importance in chemical science. In the context of medicinal chemistry, the introduction of boronic acids can improve activity and/or pharmacokinetic properties1,2,3,4,5, whereas the reversible dynamic covalent bonds of boronic esters have enabled the synthesis of self-healing macromolecular materials6. Furthermore, the compound classes are widely used in organic synthesis as reagents and coupling partners. For example, allylic boronic acids and esters are well established as reagents for allylboration of aldehydes7, Prins cyclization8, and Suzuki-Miyaura coupling9. In the context of benzylic boronic species, pinacol esters have been used as alkyl donors for Pd-catalyzed cross-couplings10,11,12,13, Cu-catalyzed C-O and C-N couplings of Chan-Lam-Evans type10,14, Minisci-type reactions15,16, and various C-C bond formations17,18,19, as well as starting materials for oxidative conversion into alcohols and ketones (Fig. 1A)10,20. Furthermore, the related class of benzylic tetrafluoroborates have been used in a variety of bond-forming reactions under electrochemical conditions21. There are a variety of synthetic routes to organoboron compounds22, including hydroboration of olefins23,24,25 and addition of organometallic nucleophiles to boron electrophiles26. Furthermore, borylative cross-couplings of alkyl and aryl (pseudo)halides using transition metal catalysis is a common strategy27,28,29,30,31,32,33,34,35,36, as well as borylation via C-H activation by means of photoredox catalysis37,38,39,40,41,42, and photoelectrocatalysis42,43, using diboron species as coupling partners. Borylative electrochemical routes with such diboron species have also been disclosed starting from aryl and alkyl halides44,45,46,47,48,49,50, redox active N-hydroxyphthalimide esters51,52 and amine derivaties53,54,55,56. In a few instances, boranes were used as cross-coupling partners for borylation of alkyl halides with Ti-catalysis, and for deoxygenative borylation of alcohols and their oxidized analogs under electrochemical conditions57,58. However, thiols and thiol derivatives remain scarce as starting materials in borylative transformations, despite their abundance in natural and synthetic products from the agrochemical, fragrance, and pharmaceutical industries59,60,61,62. While oxidized or charged species such as sulfoxides, sulfones, and sulfonium salts are used as starting materials in transition metal catalyzed protocols22,63, neutral, low-valent analogs are easily counted. A few reports on desulfurative borylation of thioethers have been disclosed, displaying full selectivity for borylation of aryl side-chains (Fig. 1B)64,65, analogous to that reported under photochemical oxidative conditions66. In addition, photoredox catalysis has been used to form alkylboronic acids and esters from cysteine or cysteine-derived thioethers using superstoichiometric amounts of phosphine reagents (Fig. 1C)67,68. Encouraged by our previous successful use of benzylic thiol derivatives as alkyl donors under electroreductive conditions69, we set out to explore desulfurative borylation using this class of compounds under electrochemical conditions (Fig. 1D).
Results
Optimization of reaction conditions
Inspired by the work of Lin and co-workers57, we set out to explore the use of pinacol borane (HBpin) as coupling partner for the targeted desulfurative borylation of model compound benzyl phenyl sulfide (1a). Evaluation of the reaction parameters (Table 1) revealed that inexpensive graphite electrodes in an undivided cell with three equivalents of HBpin in THF with tetrabutylammonium borohydride (NBu4BH4) as supporting electrolyte furnished the boronic ester (2a) in quantitative yield after passing 2 F at 10 mA under inert and anhydrous conditions (Table 1, entry 1). A switch from HBpin to bis(catecholato)diboron (B2cat2) resulted in considerably lower yields of 2a subsequent to transesterification (Table 1, entry 2). The use of acetonitrile (MeCN) as solvent resulted in a lower yield of 2a even with increased amounts of HBpin (Table 1, entries 3 and 4). Non-anhydrous conditions failed to furnish 2a (Table 1, entry 5) and exchanging the N2 atmosphere for air resulted in a lower yield (Table 1, entry 6), highlighting the importance of dry and inert conditions. Furthermore, it was found that the amount of HBpin could be reduced from 3 to 1.5 equivalents with maintained product yield (Table 1, entry 7) and these conditions were henceforth used. No reaction occurred in the absence of electricity (Table 1, entry 8), confirming that the transformation is electrochemically triggered.
Substrate scope
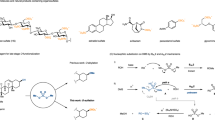
With optimized conditions at hand, the generality of the borylation protocol was assessed (Fig. 2A). Gratifyingly, a variety of benzylic thioethers were successfully transformed into the corresponding pinacolboronic esters in good yields, including substrates with reductively labile functionalities such as aryl halides (2b-d) and a benzylic trifluoromethyl group (2e). Furthermore, substituents such as Bpin (2g), methoxy groups (2h and 2i), acetal (2j), and methyl thioether (2k) were tolerated, the latter with full selectivity for benzylic C-S bond cleavage. Similarly, a methyl ester was tolerated under the reaction conditions, forming product 2f in a moderate yield. Moreover, the protocol is compatible with various heterocycles, including furan (2l), thiophene (2m), morpholine (2n), and N-methyl indole (2o). The alkene-containing boronic ester 2p formed in moderate yield, and the di-borylated product (2q) was smoothly synthesized from the corresponding di-thioether upon the use of twice the amount of pinacolborane and 2.5 times the charge.
Furthermore, thioethers with multiple substituents on the phenyl ring formed the target boronic esters in moderate yields (2v and 2w). The tertiary and secondary pinacolboronic esters 2r-2u were successfully formed under standard conditions, with generally higher yields for the less sterically hindered starting materials. Interestingly, preferential borylation of a tertiary over a secondary thioether was observed for the formation of product 2y from a derivative of the antihistamine terfenadine, indicating that the selectivity may rely on a combination of steric and electronic effects. The phenyl thioether based on the muscarinic antagonist diphenidol formed the corresponding borate ester 2x in moderate yield, whereas the phenyl thioethers based on the core structure of rosuvastatin, used for treatment of cardiovascular disease, the phosphodiesterase inhibitor roflumilast, and acetylated salicin formed the targeted borylation products in good yields (2z, 2aa, and 2ab, respectively). Furthermore, a selection of thioacetals was assessed under slightly modified standard conditions (Fig. 2B). Gratifyingly, these conditions successfully furnished the corresponding mono-borylated products in fair yields (2a, 2ac-2ae), including a derivative of fenofibrate, a lipid-modifying agent used in the treatment of hypertriglyceridemia and mixed dyslipidemia. Due to the use of thioacetals as protecting groups of aldehydes and ketones as well as starting materials for C-C bond formation in the Corey-Seebach reaction70,71, this borylation strategy may find its use in coupled sequences for the synthesis of complex organic compounds ahead. In addition, allylic thioethers were amenable to desulfurative borylation (Fig. 2C), furnishing allylic boronic esters derived from myrtenol, geraniol, and perillyl alcohol in good yields (2af-2ah). Here, the formation of regioisomers was observed with clear preference for primary, less sterically hindered sites. Notably, the borylation procedure proved scalable, furnishing 1 gram of product 2a from 1a, demonstrating the utility of the procedure for practical larger batch preparations (Fig. 2D). Finally, a selection of compounds with differently substituted aryl groups were assessed to probe the protocol’s tolerance towards the aryl sidechain of the thioether (1a-1f). Interestingly, lower yields of the benchmark product 2a were obtained in all cases (Fig. 2E), demonstrating the unsubstituted phenyl group to be preferential.
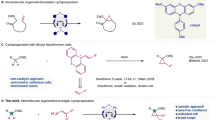
To probe whether the S-aryl group was crucial for borylation to occur, two symmetric disulfides were assessed as starting materials (Fig. 3A), gratifyingly furnishing the targeted products 2a and 2l in 77% and 35% yield. By monitoring the reaction starting from disulfide 4a over time via sampling and off-line HPLC analysis, it was found that the transformation proceeds via initial reductive cleavage of 4a to form the corresponding thiol 5a that, in turn, is transformed into the borylated product 2a (Fig. 3B). To probe whether such direct -SH bond cleavage could present synthetic benefits, a small selection of thiols was assessed. Gratifyingly, a significant increase in yield was obtained for 2l using thiol as starting material instead of the corresponding disulfide (Fig. 3C). Furthermore, a selection of p-substituted thiols resulted in the corresponding boronic ester products in good yields (2ai-2ak), as did the 2-thiophene thiol (2m). However, halide-substituted thiols were found to undergo reductive dehalogenation in addition to desulfurative borylation, in contrast to the reactivity of their thioether counterparts that maintained their halides throughout the reaction (Fig. 1A). This reactivity difference clearly demonstrates that the S-phenyl sidechain has a beneficial effect on selectivity, a feature that may be understood in light of the anodic shift in reduction potential that it induces in the starting material69,72. This phenyl sidechain can be be easily installed onto the thiol using a variety of synthetic procedures73,74,75, including transition metal catalysis76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100.
A Borylation of disulfides. B Reaction profile for electrolytic borylation of 4a. C Borylation of thiols. D Radical trap experiments. E Cyclic voltammetry study of compound 1a and HBpin. F Proposed mechanism for borylation of thioethers and thiols. Yields are isolated unless otherwise noted. aqNMR yield. b4F.
Mechanistic studies
To probe the mechanism of the borylation reaction, a radical trap experiment was carried out using a thioether equipped with an alkene sidechain (1ak). When subjected to standard conditions, 1ak did not convert to the 5-exo-trig product 2al (Fig. 3D). Instead, the linear product 2am was obtained in 77%, indicating that the transformation does not proceed via a C-centered radical with a lifetime sufficient for cyclization. Analogously, thioether 1al did not undergo borylative ring-opening but formed the cyclopropyl-containing borate ester 2ao in 79% yield, further supporting the hypothesis of short lifetimes for intermediate benzylic radicals. These combined results are in line with our previous findings69, in which benzylic phenyl thioethers were demonstrated to undergo reductive C-S bond cleavage to form carbanions via a rapid radical-polar crossover under electroreductive conditions, with the aryl mercaptan sidechain eventually forming the corresponding disulfide upon anodic oxidation. Furthermore, the formation of carbanionic intermediates is consistent with the observed mixture of regioisomers for allylic substrates, resulting from carbanion delocalization. This delocalization favors positioning of the carbanion in primary position, effectively resulting in borylation at the sterically more accessible site (Fig. 2C). Based on these findings, we propose that the desulfurative borylation proceeds via a (semi-)paired electrolytic mechanism (Fig. 3F). Starting from thiols or thioethers, an initial cathodic SET sets off a mesolytic C-S bond cleavage to furnish a benzylic open-shell species. This intermediate rapidly undergoes a reductive radical-polar crossover via a second SET to furnish a carbanion, which reacts with the reductively stable pinacolborane electrophile to form an anionic borohydride intermediate, with an associated energy barrier of around 14 kcal/mol for the C-B coupling step as determined by DFT calculations. Finally, we propose that the resulting borohydride intermediate undergoes subsequent oxidation at the anode to furnish the neutral boronic ester product along with hydrogen gas. Such electrochemical oxidation of borohydrides is in line with our previous studies101, although alternative chemical routes to the formation of the neutral borylated product cannot be ruled out57,102,103,104.
Conclusions
In this work, an efficient desulfurative electrochemical protocol for the synthesis of alkylboronic esters from thiols, disulfides, thioacetals, and thioethers is presented. Mechanistically, the borylation proceeds via carbanionic intermediates with pinacolborane as unconventional electrophilic coupling partner. The (semi-)paired electrolytic transformation tolerates a wide range of functional groups and was successfully applied to pharmaceutically relevant scaffolds and natural products. Complete selectivity for activation of the C(sp3)-S bond in aryl alkyl thioethers and acetals was observed, orthogonal to that of transition metal catalyzed protocols. With its operational simplicity and scalable nature, this electrochemical method presents an attractive avenue to synthetically useful alkylboronic esters.
Methods
General procedure for borylation of thioethers and thioacetals
To an oven-dried 10 mL-ElectraSyn vial equipped with a magnetic stir bar (dimensions 15 mm × 6 mm), graphite electrodes, the starting material (1.0 equiv., 0.50 mmol), and NBu4BH4 (1.0 equiv., 0.50 mmol, 130 mg) were added. The mixture was evacuated and back-flushed with nitrogen three times before anhydrous stabilizer-free THF (5 mL) was added, followed by HBpin (0.75 mmol for thioethers and 1.5 mmol for thioacetals). The reaction was carried out by applying 10 mA (~10 mA/cm2) at room temperature for the indicated charge (2 F for thioethers and 4 F for thioacetals) with a stir rate of 750 rpm. After electrolysis, the solvent was removed under reduced pressure and the crude reaction mixture was dissolved in EtOAc, washed with an aqueous solution of NH4Cl (30 mL), and extracted with EtOAc (15 mL × 3). The combined organic phases were dried over sodium sulfate and purified by column chromatography on oven-dried silica gel to provide the desired product.
General procedure for borylation of disulfides and thiols
To an oven-dried 10 mL-ElectraSyn vial equipped with a magnetic stir bar (dimensions 15 mm×6 mm), a magnesium anode and a tin cathode, the starting material (1.0 equiv., 0.50 mmol) and NBu4Br (1.0 equiv., 0.50 mmol, 161 mg) were added. The mixture was evacuated and back-flushed with nitrogen three times before anhydrous stabilizer-free THF (5 mL) was added, followed by HBpin (3.0 mmol for disulfides and 1.5 mmol for thiols). The reaction was carried out by applying 10 mA ( ~ 10 mA/cm2) at room temperature for the indicated charge (8 F for disulfides and 3 F for thiols) with a stir rate of 750 rpm. After electrolysis, the solvent was removed under reduced pressure and the crude reaction mixture was dissolved in EtOAc, washed with an aqueous solution of NH4Cl (30 mL), and extracted with EtOAc (15 mL x 3). The combined organic phases were dried over sodium sulfate and purified by column chromatography on oven-dried silica gel to provide the desired product.
Data availability
The data generated in this paper are provided within the article and its Supplementary Information file. Data supporting the findings of this manuscript are also available from the corresponding author upon request.
References
Plescia, J. & Moitessier, N. Design and discovery of boronic acid drugs. Eur. J. Med. Chem. 195, 112270 (2020).
Silva, M. P., Saraiva, L., Pinto, M. & Sousa, M. E. Boronic acids and their derivatives in medicinal chemistry: Synthesis and biological applications. Molecules 25, 4323 (2020).
RI, C.-C. et al. Boron-containing compounds for prevention, diagnosis, and treatment of human metabolic disorders. Biol. Trace Elem. Res. 201, 2222–2239 (2023).
Beenen, M. A., An, C. & Ellman, J. A. Asymmetric copper-catalyzed synthesis of α-amino boronate esters from N-tert-butanesulfinyl aldimines. J. Am. Chem. Soc. 130, 6910–6911 (2008).
Albers, H. M. H. G. et al. Discovery and optimization of boronic acid based inhibitors of autotaxin. J. Med. Chem. 53, 4958–4967 (2010).
Cho, S., Hwang, S. Y., Oh, D. X. & Park, J. Recent progress in self-healing polymers and hydrogels based on reversible dynamic B–O bonds: boronic/boronate esters, borax, and benzoxaborole. J. Mater. Chem. A 9, 14630–14655 (2021).
Dennis, F. M., Robertson, C. C. & Partridge, B. M. Nickel-catalysed allylboration of aldehydes. Synthesis 52, 1903–1914 (2020).
Veeraraghavan Ramachandran, P. & Gagare, P. D. One-pot allyl-/crotylboration-Prins cyclization of aldehydes. Tetrahedron Lett. 52, 4378–4381 (2011).
Ardolino, M. J. & Morken, J. P. Construction of 1,5-enynes by stereospecific Pd-catalyzed allyl–propargyl cross-couplings. J. Am. Chem. Soc. 134, 8770–8773 (2012).
Bastick, K. A. C. & Watson, A. J. B. Pd-catalyzed homologation of arylboronic acids as a platform for the diversity-oriented synthesis of benzylic C–X bonds. Synlett 34, 2097–2102 (2023).
Chandra Rao Vasireddy, P. & Smoliakova, I. P. Reactions of cyclopalladated complexes with boronic acids. Inorg. Chim. Acta 542, 121114 (2022).
Qiu, Z. et al. Regioselective α-benzylation of 3-iodoazetidine via Suzuki cross-coupling. Tetrahedron Lett. 60, 1321–1324 (2019).
Xiao, J. et al. C(sp2)–C(sp3) Suzuki–Miyaura cross-coupling using gem-bis(boronates). J. Org. Chem. 90, 14316–14321 (2025).
Sueki, S. & Kuninobu, Y. Copper-catalyzed N- and O-alkylation of amines and phenols using alkylborane reagents. Org. Lett. 15, 1544–1547 (2013).
Zhou, Y. et al. A general minisci-type alkylation with organoboron derivatives assisted by catechol. Eur. J. Org. Chem. 27, e202301216 (2024).
Yao, L. et al. Visible-light-induced chemoselective reactions of quinoxalin-2(1H)-ones with alkylboronic acids under air/N2 atmosphere. Chin. Chem. Lett. 32, 4033–4037 (2021).
Gevorgyan, A., Hopmann, K. H. & Bayer, A. Exploration of new biomass-derived solvents: Application to carboxylation reactions. ChemSusChem 13, 2080–2088 (2020).
Gierszal, S. G. & Barker, T. J. Cu-catalyzed cross-coupling of benzylboronic esters and epoxides. Tetrahedron Lett. 82, 153369 (2021).
Hayes, J. C., Hollerbach, M. R. & Barker, T. J. Nucleophilic addition of benzylboronates to activated ketones. Tetrahedron Lett. 61, 151505 (2020).
Grayson, J. D. & Partridge, B. M. Mild Cu-catalyzed oxidation of benzylic boronic esters to ketones. ACS Catal. 9, 4296–4301 (2019).
Yin, C., Tang, S., Mei, J., Hu, X. & Zhang, H. Electrochemical synthesis and transformation of organoboron compounds. Org. Chem. Front. 10, 3361–3377 (2023).
Wang, M. & Shi, Z. Methodologies and strategies for selective borylation of C–Het and C–C bonds. Chem. Rev. 120, 7348–7398 (2020).
Wen, Y., Deng, C., Xie, J. & Kang, X. Recent synthesis developments of organoboron compounds via metal-free catalytic borylation of alkynes and alkenes. Molecules 24, 101 (2019).
Obligacion, J. V. & Chirik, P. J. Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration. Nat. Rev. Chem. 2, 15–34 (2018).
Geier, S. J. et al. The transition metal-catalysed hydroboration reaction. Chem. Soc. Rev. 51, 8877–8922 (2022).
Brown, H. C. & Cole, T. E. Organoboranes. 31. A simple preparation of boronic esters from organolithium reagents and selected trialkoxyboranes. Organometallics 2, 1316–1319 (1983).
Chow, W. K. et al. A decade advancement of transition metal-catalyzed borylation of aryl halides and sulfonates. RSC Adv. 3, 12518–12539 (2013).
Yang, C.-T. et al. Alkylboronic esters from copper-catalyzed borylation of primary and secondary alkyl halides and pseudohalides. Angew. Chem. Int. Ed. 51, 528–532 (2012).
Yi, J. et al. Alkylboronic esters from palladium- and nickel-catalyzed borylation of primary and secondary alkyl bromides. Adv. Synth. Catal. 354, 1685–1691 (2012).
Ito, H. & Kubota, K. Copper(I)-catalyzed boryl substitution of unactivated alkyl halides. Org. Lett. 14, 890–893 (2012).
Bose, S. K. et al. Highly efficient synthesis of alkylboronate esters via Cu(II)-catalyzed borylation of unactivated alkyl bromides and chlorides in air. ACS Catal. 6, 8332–8335 (2016).
Atack, T. C., Lecker, R. M. & Cook, S. P. Iron-catalyzed borylation of alkyl electrophiles. J. Am. Chem. Soc. 136, 9521–9523 (2014).
Atack, T. C. & Cook, S. P. Manganese-catalyzed borylation of unactivated alkyl chlorides. J. Am. Chem. Soc. 138, 6139–6142 (2016).
Bej, A., Srimani, D. & Sarkar, A. Palladium nanoparticle catalysis: Borylation of aryl and benzyl halides and one-pot biaryl synthesis via sequential borylation-Suzuki–Miyaura coupling. Green. Chem. 14, 661–667 (2012).
Joshi-Pangu, A. et al. Palladium-catalyzed borylation of primary alkyl bromides. J. Org. Chem. 77, 6629–6633 (2012).
Bose, S. K., Fucke, K., Liu, L., Steel, P. G. & Marder, T. B. Zinc-catalyzed borylation of primary, secondary and tertiary alkyl halides with alkoxy diboron reagents at room temperature. Angew. Chem. Int. Ed. 53, 1799–1803 (2014).
Shu, C., Noble, A. & Aggarwal, V. K. Metal-free photoinduced C(sp3)–H borylation of alkanes. Nature 586, 714–719 (2020).
Wang, M., Huang, Y. & Hu, P. Terminal C(sp3)–H borylation through intermolecular radical sampling. Science 383, 537–544 (2024).
Zhang, L., Wu, Z.-Q. & Jiao, L. Photoinduced radical borylation of alkyl bromides catalyzed by 4-phenylpyridine. Angew. Chem. Int. Ed. 59, 2095–2099 (2020).
Li, M. et al. Photoinduced metal-free borylation of aryl halides catalysed by an in situ formed donor–acceptor complex. Chem. Sci. 13, 4909–4914 (2022).
Li, B. et al. Catalyst-free C(sp2)-H borylation through aryl radical generation from thiophenium salts via electron donor–acceptor complex formation. Org. Lett. 24, 7434–7439 (2022).
Zhong, P.-F. et al. Photoelectrochemical oxidative C(sp3)−H borylation of unactivated hydrocarbons. Nat. Commun. 14, 6530 (2023).
Cao, Y., Huang, C. & Lu, Q. Photoelectrochemically driven iron-catalysed C(sp3)−H borylation of alkanes. Nat. Synth. 3, 537–544 (2024).
Wang, B. et al. Electrochemical borylation of alkyl halides: Fast, scalable access to alkyl boronic esters. J. Am. Chem. Soc. 143, 12985–12991 (2021).
Godeau, J., Pintaric, C., Olivero, S. & Duñach, E. Electrochemical preparation of pinacol allylboronic esters. Electrochim. Acta 54, 5116–5119 (2009).
Laza, C., Pintaric, C., Olivero, S. & Dunach, E. Electrochemical reduction of polyhalogenated aryl derivatives in the presence of pinacolborane: Electrosynthesis of functionalised arylboronic esters. Electrochim. Acta 50, 4897–4901 (2005).
Pintaric, C., Laza, C., Olivero, S. & Duñach, E. Electrosynthesis of benzylboronic acids and esters. Tetrahedron Lett. 45, 8031–8033 (2004).
Laza, C. & Duñach, E. New electrosynthesis of arylboronic esters from aromatic halides and pinacolborane. Adv. Synth. Catal. 345, 580–583 (2003).
Laza, C., Duñach, E., Serein-Spirau, F., Moreau, J. J. E. & Vellutini, L. Novel synthesis of arylboronic acids by electroreduction of aromatic halides in the presence of trialkyl borates. N. J. Chem. 26, 373–375 (2002).
Lai, Y., Halder, A., Kim, J., Hicks, T. J. & Milner, P. J. Electroreductive radical borylation of unactivated (hetero)aryl chlorides without light by using cumulene-based redox mediators. Angew. Chem. Int. Ed. 62, e202310246 (2023).
Wang, J. et al. Cu-catalyzed decarboxylative borylation. ACS Catal. 8, 9537–9542 (2018).
Barton, L. M., Chen, L., Blackmond, D. G. & Baran, P. S. Electrochemical borylation of carboxylic acids. Proc. Natl. Acad. Sci. 118, e2109408118 (2021).
Du, L., Zhang, B., Ji, S., Cai, H. & Zhang, H. Electrochemical borylation of nitroarenes. Sci. China Chem. 66, 534–539 (2023).
Wang, R., Chen, F., Jiang, L. & Yi, W. Electrochemical thiolation and borylation of arylazo sulfones with thiols and B2pin2. Adv. Synth. Catal. 363, 1904–1911 (2021).
Du, L., Sun, L. & Zhang, H. Photochemical and electrochemical C–N borylation of arylhydrazines. Chem. Commun. 58, 1716–1719 (2022).
Kong, X., Lin, L., Chen, Q. & Xu, B. Radical generation from electroreduction of aryl and benzyl ammonium salts: synthesis of organoboronates. Org. Chem. Front. 8, 702–707 (2021).
Guan, W., Chang, Y. & Lin, S. Electrochemically driven deoxygenative borylation of alcohols and carbonyl compounds. J. Am. Chem. Soc. 145, 16966–16972 (2023).
Wang, X., Cui, P., Xia, C. & Wu, L. Catalytic boration of alkyl halides with borane without hydrodehalogenation enabled by titanium catalyst. Angew. Chem. Int. Ed. 60, 12298–12303 (2021).
Hai, Y., Wei, M.-Y., Wang, C.-Y., Gu, Y.-C. & Shao, C.-L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar. Life Sci. Technol. 3, 488–518 (2021).
Ming, W., Cuihong, W. & Xuefeng, J. Recent progress in the sulfur-containing perfume & flavors construction. Chin. J. Org. Chem. 39, 2139 (2019).
Goeke, A. Sulfur-containing odorants in fragrance chemistry. Sulfur Rep. 23, 243–278 (2002).
Wang, N., Saidhareddy, P. & Jiang, X. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 37, 246–275 (2020).
Minami, H., Otsuka, S., Nogi, K. & Yorimitsu, H. Palladium-catalyzed borylation of aryl sulfoniums with diborons. ACS Catal. 8, 579–583 (2018).
Bhanuchandra, M. et al. Palladium-catalyzed ipso-borylation of aryl sulfides with diborons. Org. Lett. 18, 2966–2969 (2016).
Uetake, Y., Niwa, T. & Hosoya, T. Rhodium-catalyzed ipso-borylation of alkylthioarenes via C–S bond cleavage. Org. Lett. 18, 2758–2761 (2016).
Li, X., Wan, Z., Hu, X. & Zhang, H. Photoinduced aerobic C–S borylation of aryl sulfides. Org. Chem. Front. 9, 3034–3038 (2022).
Fu, X.-P. et al. Stereoretentive post-translational protein editing. ACS Cent. Sci. 9, 405–416 (2023).
Jing, R., Powell, W. C., Fisch, K. J. & Walczak, M. A. Desulfurative borylation of small molecules, peptides, and proteins. J. Am. Chem. Soc. 145, 22354–22360 (2023).
Kuzmin, J. et al. Electroreductive desulfurative transformations with thioethers as alkyl radical precursors**. Angew. Chem. Int. Ed. 62, e202304272 (2023).
Greene, T. W. & Wutz, P. G. M. Protective Groups in Organic Synthesis. Wiley-Interscience, New York, 329-344, 724-727 (1999)
Haroon, M. et al. The Corey-Seebach reagent in the 21st century: A review. Molecules 28, 4367 (2023).
Panferova, L. I. & Dilman, A. D. Light-mediated sulfur–boron exchange. Org. Lett. 23, 3919–3922 (2021).
Nikitin, M. et al. Brønsted acid-facilitated thioetherification cross-coupling reactions with nickel and visible light. ACS Catal. 15, 1467–1476 (2025).
Yu, F., Mao, R., Yu, M., Gu, X. & Wang, Y. Generation of aryl radicals from aryl halides: Rongalite-promoted transition-metal-free arylation. J. Org. Chem. 84, 9946–9956 (2019).
Cano, R., Ramón, D. J. & Yus, M. Transition-metal-free O-, S-, and N-arylation of alcohols, thiols, amides, amines, and related heterocycles. J. Org. Chem. 76, 654–660 (2011).
Chen, Y.-J. & Chen, H.-H. 1,1,1-Tris(hydroxymethyl)ethane as a new, efficient, and versatile tripod ligand for copper-catalyzed cross-coupling reactions of aryl iodides with amides, thiols, and phenols. Org. Lett. 8, 5609–5612 (2006).
Verma, A. K., Singh, J. & Chaudhary, R. A general and efficient CuI/BtH catalyzed coupling of aryl halides with thiols. Tetrahedron Lett. 48, 7199–7202 (2007).
Fernández-Rodríguez, M. A., Shen, Q. & Hartwig, J. F. A general and long-lived catalyst for the palladium-catalyzed coupling of aryl halides with thiols. J. Am. Chem. Soc. 128, 2180–2181 (2006).
Itoh, T. & Mase, T. A general palladium-catalyzed coupling of aryl bromides/triflates and thiols. Org. Lett. 6, 4587–4590 (2004).
Lin, Y., Cai, M., Fang, Z. & Zhao, H. A highly efficient heterogeneous copper-catalyzed Chan-Lam coupling between thiols and arylboronic acids leading to diaryl sulfides under mild conditions. Tetrahedron 72, 3335–3343 (2016).
Zhu, D., Xu, L., Wu, F. & Wan, B. A mild and efficient copper-catalyzed coupling of aryl iodides and thiols using an oxime–phosphine oxide ligand. Tetrahedron Lett. 47, 5781–5784 (2006).
Guo, F.-J. et al. C-S cross-coupling of aryl halides with alkyl thiols catalyzed by in-situ generated nickel(II) N-heterocyclic carbene complexes. Catal. Commun. 96, 11–14 (2017).
Jaimes–Romano, E. et al. C–S couplings catalyzed by Ni(II) complexes of the type [(NHC)Ni(Cp)(Br)]. J. Catal. 426, 247–256 (2023).
Xu, H.-J., Zhao, Y.-Q., Feng, T. & Feng, Y.-S. Chan–Lam-Type S-arylation of thiols with boronic acids at room temperature. J. Org. Chem. 77, 2878–2884 (2012).
Herradura, P. S., Pendola, K. A. & Guy, R. K. Copper-mediated cross-coupling of aryl boronic acids and alkyl thiols. Org. Lett. 2, 2019–2022 (2000).
Rout, L., Sen, T. K. & Punniyamurthy, T. Efficient CuO-nanoparticle-catalyzed C-S cross-coupling of thiols with iodobenzene. Angew. Chem. Int. Ed. 46, 5583–5586 (2007).
Zong, C., Liu, J., Chen, S., Zeng, R. & Zou, J. Efficient C-S cross-coupling of thiols with aryl iodides catalyzed by Cu(OAc)2·H2O and 2,2′-biimidazole. Chin. J. Chem. 32, 212–218 (2014).
Guan, P. et al. Efficient nickel/N-heterocyclic carbene catalyzed C–S cross-coupling. Tetrahedron Lett. 53, 5987–5992 (2012).
Thomas, A. M., Sherin, D. R., Asha, S., Manojkumar, T. K. & Anilkumar, G. Exploration of the mechanism and scope of the CuI/DABCO catalysed CS coupling reaction. Polyhedron 176, 114269 (2020).
Okauchi, T., Kuramoto, K. & Kitamura, M. Facile preparation of aryl sulfides using palladium catalysis under mild conditions. Synlett 2010, 2891–2894 (2010).
Reddy, V. P., Swapna, K., Kumar, A. V. & Rao, K. R. Indium-catalyzed C−S cross-coupling of aryl halides with thiols. J. Org. Chem. 74, 3189–3191 (2009).
Wu, J.-R., Lin, C.-H. & Lee, C.-F. Iron-catalyzed thioetherification of thiols with aryl iodides. Chem. Commun. 4450–4452. https://doi.org/10.1039/B907362K (2009).
Reddy, V. P., Kumar, A. V., Swapna, K. & Rao, K. R. Nano indium oxide as a recyclable catalyst for C−S cross-coupling of thiols with aryl halides under ligand free conditions. Org. Lett. 11, 1697–1700 (2009).
Jones, K. D., Power, D. J., Bierer, D., Gericke, K. M. & Stewart, S. G. Nickel phosphite/phosphine-catalyzed C–S cross-coupling of aryl chlorides and thiols. Org. Lett. 20, 208–211 (2018).
Katla, R., Katla, R., Goulart, T. B., Rosa, D. S. & Rosa, G. R. Pd/RHA and Pd/BPA as eco-friendly heterogeneous catalysts: Microwave-assisted C–S cross-coupling reaction in DMF. Synlett 33, 1637–1644 (2022).
Mispelaere-Canivet, C., Spindler, J.-F., Perrio, S. & Beslin, P. Pd2(dba)3/Xantphos-catalyzed cross-coupling of thiols and aryl bromides/triflates. Tetrahedron 61, 5253–5259 (2005).
Akkilagunta, V. K., Reddy, V. P. & Rao, K. R. Recyclable iron/graphite catalyst for C-S cross coupling of thiols with aryl halides under ligand-free conditions. Synlett 2010, 1260–1264 (2010).
She, J., Jiang, Z. & Wang, Y. Simple, efficient and recyclable catalytic system for performing copper-catalyzed C–S coupling of thiols with aryl iodides in PEG and PEG–H2O. Tetrahedron Lett. 50, 593–596 (2009).
Kim, K., Shin, Y. & Chae, J. Solvent-selective synthesis of diaryl disulfides and arylthio acetic acids using thioglycolic acid and copper catalysts. Synlett 35, 826–831 (2023).
Eichman, C. C. & Stambuli, J. P. Zinc-mediated palladium-catalyzed formation of carbon−sulfur bonds. J. Org. Chem. 74, 4005–4008 (2009).
Kuzmin, J. et al. Borohydride oxidation as counter reaction in reductive electrosynthesis. Angew. Chem. Int. Ed. 64, e202501653 (2025).
Pintaric, C., Olivero, S., Gimbert, Y., Chavant, P. Y. & Duñach, E. An Opportunity for Mg-catalyzed grignard-type reactions: Direct coupling of benzylic halides with pinacolborane with 10 mol % of magnesium. J. Am. Chem. Soc. 132, 11825–11827 (2010).
Clary, J. W. et al. Hydride as a leaving group in the reaction of pinacolborane with halides under ambient grignard and barbier conditions. one-pot synthesis of alkyl, aryl, heteroaryl, vinyl, and allyl pinacolboronic esters. J. Org. Chem. 76, 9602–9610 (2011).
Molander, G. A. & Ellis, N. M. Highly stereoselective synthesis of cis-alkenyl pinacolboronates and potassium cis-alkenyltrifluoroborates via a hydroboration/ protodeboronation approach. J. Org. Chem. 73, 6841–6844 (2008).
Acknowledgements
We thank Dr. Guillermo Ahumada (G. A.) and M.Sc. Nils Schwarz (N. S.) for fruitful discussions, and N. S. for assistance with the synthesis of the thioether precursors for products 2 h, 2j, 2 m, 2n, and 2r. Financial support to the corresponding author from the Swedish Research Council (grant no. 2021-05551), the European Research Council (grant no. 101164660), the Wenner-Gren foundation, Magnus Bergvalls stiftelse, Stiftelsen Lars Hiertas Minne, and KTH Royal Institute of Technology is gratefully acknowledged.
Funding
Open access funding provided by Royal Institute of Technology.
Author information
Authors and Affiliations
Contributions
Julius Kuzmin (J.K.): Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—original draft, Writing—review & editing. Cristiana Margarita (C.M.): Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—review & editing. Johannes Winter (J.W.): Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—review & editing. Helena Lundberg (H.L.): Conceptualization, Data curation, Formal analysis, Project administration, Visualization, Writing—original draft, Writing—review & editing, Funding acquisition, Resources, Supervision
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuzmin, J., Margarita, C., Winter, J. et al. Electrochemical desulfurative borylation of thiols, disulfides, thioethers and thioacetals. Nat Commun 17, 632 (2026). https://doi.org/10.1038/s41467-025-67363-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-67363-7