Abstract
Inter/transgenerational epigenetic inheritance is a crucial and controversial theory that could reshape the concept of genetics. To investigate this theory directly, we invent a system for targeted reprogramming of epigenetic memory in mouse sperm. Using this system, we erase DNA methylation at the differentially methylated region of the H19 gene (H19-DMR) in sperm, which causes Silver-Russell syndrome-like phenotypes in F1 offspring. Although DNA methylation is fully lost in the sperm, it is partially restored during pre-implantation development, suggesting the existence of epigenetic memory that instructs de novo DNA methylation. Importantly, targeted removal of histone modifications in zygotes reveals that tri-methylation at lysine 9 of histone H3 (H3K9me3), which is deposited shortly after fertilization, is required for the subsequent de novo DNA methylation at the H19-DMR. Thus, our study provides a robust germline editing tool, which reveals partial intergenerational inheritance and no transgenerational inheritance at the model locus. Furthermore, we identify H3K9me3 as a mediator for DNA methylation recovery also acting at imprinted loci.
Similar content being viewed by others
Introduction
Phenotypic inheritance is believed to be achieved via the transmission of genomic DNA. Paradoxically, ancestral environmental exposures, such as abnormal nutritional conditions, toxins, and stress, can lead to disease and phenotypic changes in offspring1,2,3,4,5,6,7,8,9, providing circumstantial evidence suggesting a non-Mendelian inheritance phenomenon, where epigenetic memory is passed to the next generation through the germline. While these data are still under discussion because of difficulties in showing the absence of genetic mutations, DNA methylation, histone modifications, and RNA may contribute to the primary molecular mechanisms underlying epigenetic inheritance1,2,10,11,12,13,14. For example, paternal prediabetes induced by feeding a high-fat diet increases the susceptibility of offspring to diabetes3, while Sun et al. reported that paternal cold exposure enhances brown adipose tissue activity in offspring4. In these cases, altered DNA methylation was detected in both sperm and offspring; however, it does not necessarily follow that germline DNA methylation changes are passed to the next generation, as it is equally possible that epimutations are first reset after fertilization and then reestablished during development by yet undetermined factors.
To provide more direct evidence for inter/transgenerational epigenetic inheritance, it is necessary to show that epimutations induced in the germline can be transmitted to the next and later generations. In this study, we define intergenerational inheritance as the transmission of epigenetic alterations originating in the gametes to the immediate offspring (F1 generation). By contrast, transgenerational inheritance refers to the sustained presence of these modifications in subsequent generations (e.g., F2 and beyond) without continued exposure to the initial trigger. Recently, Takahashi et al. showed that insertion followed by removal of a CpG-free DNA segment at CpG island promoter regions causes DNA methylation and silencing of the surrounding loci, which are maintained over multiple generations15. Curiously, the induced DNA methylation was lost during germ cell development, but then reestablished during embryonic development by unknown mechanisms15; however, the methods used in the study could not fully rule out the influence of a small genetic change required for epimutation induction16. Moreover, other concerns, including off-target mutations during insertion of CpG-free DNA by CRISPR-Cas9 genome editing and colony selection, have been raised16. Epigenome editing using a ‘dead’ Cas9 (dCas9)17,18, which does not introduce genetic changes and can rewrite the epigenetic information at a target locus, may be a direct method that can help solve this problem. Transmission of rewritten epigenetic information in the parental germline to the next generation, according to phenotype emergence, would directly demonstrate inter/transgenerational epigenetic inheritance.
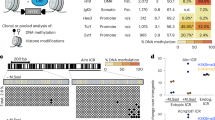
Genomic imprinting is a process wherein a gene is differentially expressed depending on whether it has been inherited from the father or mother19,20. An epimutation comprising DNA demethylation of the sperm-derived paternal allele in a differentially methylated region (DMR) of the imprinted gene, H19, (H19-DMR) is believed to cause Silver-Russell syndrome, which is characterized by intrauterine growth retardation due to Igf2 downregulation21. H19-DMR22 is located between the Igf2 and H19 imprinted genes23,24 and regulates their expression. In somatic cells of healthy individuals, H19-DMR CpG dinucleotides, including CTCF-binding sites, are normally methylated on the paternal allele and unmethylated on the maternal allele (Fig. 1a)22; this allele-specific CpG methylation upregulates paternal allele expression of Igf2 and downregulates that of H19. By contrast, in patients with Silver-Russell syndrome, demethylation of the paternal allele H19-DMR is expected to decrease the expression of Igf2 and increase that of H19 through epigenetic regulation of CTCF binding25,26, resulting in fetal intrauterine growth retardation. During establishment of genomic imprinting in the male germline, H19-DMR CpGs are progressively hypermethylated through an endogenous de novo methylation mechanism until the pro-spermatogonia stage27, while this region is unmethylated in oocytes (Fig. 1b). If the epimutation in H19-DMR occurring sperm is inherited, intrauterine growth retardation will be observed in offspring.
a In mice and humans, under normal conditions, Igf2 is expressed from the sperm-derived paternal allele (P), while H19 is expressed from the oocyte-derived maternal allele (M). In patients with Silver-Russell syndrome, DNA demethylation in H19-DMR results in biallelic expression of H19 and repression of Igf2, leading to fetal intrauterine growth retardation. Closed circles indicate methylated CpGs and open circles indicate unmethylated CpGs. b Schematic representation of germline-specific epigenome-edited mice. In WT male mice, H19-DMR sites are progressively hypermethylated until the pro-spermatogonia stage by an endogenous de novo methylation mechanism (left), while this region is demethylated during spermatogenesis in germline-specific epigenome-edited mice (right). This system can reveal whether an epimutation introduced in sperm is transmitted to the next F1 generation, leading to phenotypic changes.
In this study, we developed a germline-specific DNA methylation editing system and applied it to a genomic imprinting disorder disease model28,29, to investigate inter/transgenerational inheritance of epimutations in sperm (Fig. 1b). To demonstrate this, we induced CpG demethylation in H19-DMR by germline-specific epigenome editing and investigated whether the epimutation introduced in sperm was transmitted to the next generation and led to phenotypic changes. Furthermore, we developed a targeted histone editing technique that can be used to reveal the roles of histone modifications in DNA methylation reestablishment.
Results
Germline-specific epigenome-edited mice show complete erasure of DNA methylation at H19-DMR in sperm
To induce CpG demethylation of H19-DMR, we constructed a vector that expresses epigenome editing factors driven by the Stra8 premeiotic-specific marker gene promoter (Fig. 2a)30. In this system, dCas9 with a SunTag recruits the scFv-superfolder green fluorescent protein (sfGFP)-TET1CD fusion protein31,32, which contains the catalytic domain (CD) of the ten-eleven translocation (TET) 1 hydroxylase, to the H19-DMR target locus, leading to targeted demethylation during spermatogenesis. By injecting the linearized vector into fertilized eggs, three transgenic (TG) strains were established from founder mice. A previous study showed that a transgene driven by the Stra8 promoter is activated only in the postnatal testis, and not in the ovary or prenatal gonad33. RT-qPCR and immunohistochemistry analyses confirmed that epigenome editing factors were specifically expressed in adult testis, from spermatogonia to spermatocytes (Fig. 2b, cand Supplementary Fig. 1). As paternal imprinting in H19-DMR is established by the pro-spermatogonia stage27, germline-specific epigenome editing is thought to occur after paternal imprinting establishment. H19-DMR CpG methylation levels were examined in sperm and MII oocytes from TG mice by bisulfite sequencing (Fig. 2d) and combined bisulfite restriction analysis (COBRA)34 (Supplementary Fig. 2), and we found robust CpG demethylation in sperm from all TG strains. By contrast, little difference in CpG methylation levels between TG and wild-type (WT) sperm was detected on analysis of six potential off-target regions (Supplementary Fig. 3). As expected, H19-DMR was unmethylated in both TG and WT mother-derived oocytes (Fig. 2d). These data indicate that we successfully established germline-specific epigenome-edited TG mice (StrH19EpiTG), and strain 445-7 was used in subsequent experiments.
a Design of the spermatogenesis-specific epigenome editing vector (pStrPlatTET-gRNA2-H19DMRx9). dCas9-SunTag recruits five copies of the catalytic domain (CD) of TET1 to H19-DMR via 9 guide RNAs (gRNAs). b Immunohistochemistry to detect GFP clarified that epigenome editing factors were expressed in spermatogonia and spermatocytes. Scale bar: 100 μm. c The epigenome editing factor dCas9 was specifically expressed in the testis. Values represent mean ± s.d. (n = 3 technical replicates). d Bisulfite sequencing analysis of H19-DMR in sperm and MII oocytes derived from TG (strain 445-7) and wild-type (WT) mice. The map shows locations of CTCF-binding sites (m1–m4), gRNAs, and PCR amplified regions used in this study. Closed circles indicate methylated CpGs and open circles indicate unmethylated CpGs.
Target-specific H19-DMR demethylation is partially inherited by the next generation and causes phenotypic changes
We next compared the offspring of TG mice with those of WT control mice. Offspring derived from TG mice were obtained by crossing heterozygous TG and WT mice (Fig. 3a), and those derived from TG fathers showed intrauterine growth retardation at birth (Fig. 3b, c). To exclude the possibility that the presence of the transgene might influence phenotypes, we also explored the phenotypes in offspring with and without TG alleles (TG and non-TG, respectively). Non-TG offspring derived from TG fathers exhibited a reduced birth weight (Fig. 3c), which is thought to be associated with inherited hypomethylation of H19-DMR from sperm. As expected, CpG methylation levels at the H19-DMR (CTCF binding sites m1–m4) in offspring of TG fathers were lower than those in offspring with WT fathers (Fig. 3d). Along with lowered methylation, offspring of TG fathers showed altered gene expression patterns, with increased H19 and decreased Igf2 levels (Fig. 3e). Non-TG offspring derived from TG fathers exhibited H19-DMR demethylation and altered gene expression at comparable levels to those in TG offspring (Fig. 3d, e). In addition to intrauterine growth retardation, other Silver-Russell syndrome-like phenotypes35, including postnatal growth retardation, reduced food intake, and body asymmetry, were observed (Supplementary Fig. 4). By contrast, offspring derived from TG mothers did not differ from WT offspring in terms of birth weight or CpG methylation levels (Fig. 3b–d), consistent with the fact that H19-DMR is demethylated in oocytes (Fig. 2d). Thus, the developed system proved that induced loss of DNA methylation at the DMR in sperm was partially transmitted to the F1 offspring, which exhibited resulting phenotypes.
a Schematic representation of F1 offspring derived from TG fathers, TG mothers, and WT mice. TG fathers/mothers produce offspring with and without TG alleles (TG and non-TG, respectively). b Appearance of newborn mice derived from TG and WT parents. Scale bars: 1 cm. c Birth weights of offspring mice with TG and WT parents. d CpG methylation analysis (COBRA) of H19-DMR (m1–m4) in newborn (P0) mice with TG and WT parents. eIgf2 and H19 mRNA expression in newborn mice with TG and WT fathers (TG, n = 8; non-TG, n = 4; WT, n = 10). Box plots show the median (center line), the 25th and 75th percentiles (box edges), and the minimum and maximum values (whiskers). P-values were obtained using one-way ANOVA with Tukey’s post-hoc HSD test (c–e) and Welch’s t test (two-tailed) (e). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: not significant.
We next investigated whether DNA methylation erasure in sperm was inherited transgenerationally (Supplementary Fig. 5a), and found that H19-DMR DNA hypomethylation was not inherited in sperm from F1 non-TG (Supplementary Fig. 5b, c) and their derivative F2 offspring (Supplementary Fig. 5d). These results indicate DMR DNA hypomethylation in F0 sperm is normalized during F1 germline cell development and not transgenerationally inherited by F2 offspring.
H19-DMR methylation is partially recovered from the 5’ end of the DMR during embryogenesis
Based on our experimental data, we surmised that DNA methylation was partially recovered in newborn mice, although it was completely erased in the sperm (Figs. 2d, 3d); we termed this phenomenon “intergenerational DNA methylation recovery”. The degree of DNA methylation recovery varied among individuals, and there was a strong positive correlation between birth weight and DNA methylation levels at the H19-DMR m3 and m4 sites and promoter regions (Supplementary Fig. 6). By contrast, DNA methylation levels at the 5’ end of the DMR (sites m1 and m2) were restored to almost WT levels (Supplementary Fig. 6), and there was no correlation between birth weight and DNA methylation levels at sites m1 and m2 (Supplementary Fig. 6). Further, time-course DNA methylation analysis across F1 development demonstrated that most DNA methylation at the 5’ end of the DMR (sites m1 and m2) was recovered before implantation, while that at 3’ end of the DMR (sites m3 and m4) was recovered after E7.5 (Fig. 4a). Furthermore, bisulfite sequencing analysis in sperm, early 1-cell zygotes (5 h post-fertilization), and morula stage embryos derived from TG fathers revealed that DNA methylation recovery at m1 and m2 sites had not yet occurred in zygotes, but had occurred by the morula stage (Fig. 4b). Allele-specific bisulfite sequencing analysis confirmed that the paternal allele underwent DNA methylation recovery (Fig. 4c). These data suggest the existence of an undetermined factor that mediates DNA methylation recovery in early preimplantation embryos.
a CpG methylation analysis (COBRA) of H19-DMR (CTCF-binding sites m1–m4) in sperm (TG, n = 3; WT, n = 3), morula (TG, n = 3; WT, n = 3; 80 pooled embryos per sample), E7.5 (TG, n = 5; WT, n = 7), E11.5 (TG, n = 9; WT, n = 8), and P0 newborn mice (TG, n = 12; WT, n = 9). Values represent mean ± s.d. P-values were obtained using Welch’s t test (two-tailed). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. b CpG methylation profiles (bisulfite amplicon sequencing) of H19-DMR in sperm, 1-cell zygotes, and morula derived from TG fathers. c Allele-specific bisulfite sequencing analysis of morula embryos derived from a TG father. For bisulfite reaction experiments, pooled samples from zygotes (n = 959) and morula (n = 80) were used, as well as 500 ng samples of sperm genomic DNA.
H3K9me3 is required for DNA methylation recovery
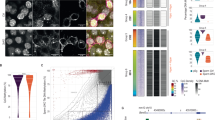
H3K9me3 is a repressive histone mark associated with heterochromatin and gene repression36, and known to co-occur with DNA methylation at the DMRs of imprinted genes37. Further, genome-wide H3K9me3 loss leads to a reduction of DNA methylation at DMRs in preimplantation embryos38. Nevertheless, although these studies suggest that H3K9me3 is required for DNA methylation maintenance, it is unknown whether H3K9me3 guides de novo DNA methylation in early embryos. According to public ChIP-seq data from gametes39,40, H3K9me3 is rarely enriched around the H19-DMR in either sperm or oocytes (Fig. 5a). To determine when H3K9me3 is deposited at the H19-DMR after fertilization, we applied an ultra-sensitive chromatin immunoprecipitation sequencing (ChIP-Seq) method, termed carrier DNA-assisted ChIP-Seq (CATCH-Seq)41,42, Interestingly, H3K9me3 CATCH-seq analysis in early 1-cell zygotes (5 h post-fertilization) and morula embryos revealed that H3K9me3 could already be detected at the paternal allele of H19-DMR in early zygotes, particularly at the 5’ end of the DMR (sites m1 and m2) (Fig. 5a). H3K9me3 was further accumulated by the morula stage (Fig. 5a).
a Visualization of CATCH-seq analysis of H3K9me3 around H19-DMR using Integrative Genomics viewer (IGV). P, paternal allele; M, maternal allele. Sperm and oocyte data are from Yamaguchi et al.39 and Wang et al.40, respectively. b H3K9 demethylation by histone editing using dCas9-SunTag-Kdm4d. c, d CATCH-seq analysis of H3K9me3 around H19-DMR (c) and other imprinted regions (d) in morula embryos derived from WT and TG fathers, visualized using IGV viewer. Parental alleles could not be separated due to a lack of single-nucleotide polymorphisms in TG-derived sperm. + KDM4D, target-specific histone editing data. e Scatter plot showing H3K9me3 enrichment peaks (n = 15,746); the peak at the targeted H19-DMR locus is highlighted. P-values were calculated using DiffBind with default parameters. Peaks with p < 0.05 and |log2 fold change | > 2 are indicated by red (gain, n = 1) or blue (loss, n = 18) dots.
To examine the role of H3K9me3 in DNA methylation recovery, we attempted to erase H3K9me3 at the H19-DMR in early embryos. To achieve this, we conducted target-specific histone editing using an H3K9me3 demethylase, KDM4D43. Mixtures of dCas9-SunTag mRNA, scFv-GFP-Kdm4d mRNA, and two guide RNAs (gRNAs) complementary to sequence upstream of H19-DMR were injected into zygotes derived from TG fathers (Fig. 5b). Since the GFP-labeled epigenome editing factor (scFv-GFP-Kdm4d) was strongly expressed for at least 3 days until the morula stage (Supplementary Fig. 7), the H3K9me3 status of epigenome-edited embryos was examined at this stage. H3K9me3 CATCH-seq analysis of TG sperm-derived, non-injected morula embryos showed that H3K9me3 was detected around H19-DMR and upstream regions (Fig. 5c). We noted that levels of H3K9me3 at this locus in TG sperm-derived embryos were lower than those in WT embryos (see Discussion) (Fig. 5c). Nevertheless, when KDM4D was recruited to the upstream of H19-DMR by target-specific histone editing, H3K9me3 around the DMR was almost completely lost over an extensive region (approximately 10 kb). This extensive effect beyond the target sites is consistent with a recent report showing that an edited region per gRNA is comparatively broad in histone modification-edited embryonic stem cells44. By contrast, H3K9me3 was retained in DMRs of other imprinted genes (Igf2r, Rasgrf1) (Fig. 5d). Genome-wide comparative analysis demonstrated that H3K9me3 was lost at only 18 regions, including H19-DMR, in epigenome-targeted morula embryos (Fig. 5eand Supplementary Data 1). Thus, H3K9 demethylation by dCas9-SunTag histone editing did not affect H3K9me3 globally.
Next, these manipulated embryos were developed to embryonic stage E11.5, and their H19-DMR DNA methylation levels were examined (Fig. 6a). Importantly, target-specific H3K9me3 removal using dCas9-SunTag-Kdm4d revealed that DNA methylation recovery at H19-DMR is inhibited (Fig. 6b). Conversely, DNA methylation recovery was observed when a KDM4D mutant (H189A, lacking enzymatic activity)45 or another histone demethylase (KDM6B, H3K27 demethylase)46 was recruited (Fig. 6b). The extent of recovery in these negative controls was less than that in the non-treated control group (Fig. 6b), which could be due to steric hindrance of the epigenome editor complexes against DNA methyltransferases. In partial support of this hypothesis, a greater degree of recovery was observed when KDM4D was recruited to a different target locus, Rosa2647 (Fig. 6b). Finally, dissection of E15.5 and E18.5 embryos derived from TG sperm-derived zygotes injected or not with target-specific KDM4D revealed that H3K9me3 removal results in more severe intrauterine growth retardation than in the non-injected controls (Fig. 6c). These data indicate that H3K9me3 around H19-DMR is involved in DNA methylation recovery during embryogenesis and is physiologically relevant to the offspring’s phenotype.
a H3K9 demethylation by target-specific histone editing using dCas9-SunTag-Kdm4d. b DNA methylation analysis (COBRA) of E11.5 embryos by target-specific histone editing. H19up, H19-DMR upstream guide RNA (gRNA); Rosa26, Rosa26 gRNA. Kdm4d, H19up gRNA (n = 8); Kdm4dMut, Kdm4d without enzymatic activity (n = 14); Kdm6b, H3K27me3 demethylase (n = 8); dCas9, dCas9 without SunTag and scFv-Kdm4d (n = 10); Kdm4d, Rosa26 gRNA (n = 10); Non-treated (n = 9). c Weight and appearance of TG father-derived embryos following target-specific histone editing. Kdm4d at E15.5 (n = 8); Non-treated at E15.5 (n = 11); Kdm4d at E18.5 (n = 8); Non-treated at E18.5 (n = 12). Scale, 1 cm. P-values were obtained using Welch’s t test (two-tailed) to compare Kdm4d to other conditions. *p < 0.05, **p < 0.01, ***p < 0.001.
To further confirm the role of H3K9me3 in DNA methylation recovery, we also removed H3K9me3 globally in zygotes by injecting Kdm4d mRNA45. Similar to target-specific removal, global loss of H3K9me3 during early preimplantation development inhibited DNA methylation recovery, whereas control embryos injected with catalytic point-mutant Kdm4d mRNA, Egfp mRNA, or buffer alone led to DNA methylation recovery (Supplementary Fig. 8). Regardless of global or target-specific editing, the inhibitory effect of H3K9me3 removal on DNA methylation recovery was more prominent at the m1 and m2 sites than that at m3/m4, possibly because DNA methylation recovery occurs more robustly at the 5’ end of the DMR. Thus, both target-specific and global removal experiments confirmed that H3K9me3 is required for DNA methylation recovery at H19-DMR.
Discussion
The germline-specific epigenome editing system developed in this study enabled us to validate the inter/transgenerational inheritance theory directly, because it involves editing of the germline epigenome alone and not the genome. Our system can be used to introduce epimutations at various loci, facilitating the study of the effects of an inter/transgenerational epimutations on offspring. Using this system, we demonstrate that artificial DNA demethylation of H19-DMR in F0 sperm can be partially transmitted to the F1 generation and mediate offspring phenotypes. Further, we used our system to demonstrate that DNA demethylation of H19-DMR is reprogrammed and normalized in F1 germline cells, and not transgenerationally inherited by F2 offspring. There have been several previous reports that epigenetic reprogramming of sperm induced by abnormal nutritional conditions or stress can be transmitted to offspring3,4,5,6,7,8,9; however, these studies were based on circumstantial evidence, and the germline-specific epigenome editing system we have developed could contribute to assessing whether there are causal relationships in these contexts.
Notably, despite successful removal of DNA methylation in sperm, we found that it was partially recovered from the 5’ end of H19-DMR (sites m1 and m2) after fertilization. We propose that H3K9me3 mediates the DNA methylation recovery that occurs after fertilization (Fig. 7). (1) In WT embryos, the paternal allele of H19-DMR is marked with H3K9me3 immediately after fertilization, which accumulates further by the morula stage. The coexistence of DNA methylation and H3K9me3 marks likely ensure robust genomic imprinting throughout development. (2) In TG father-derived embryos, the DNA hypomethyled state in sperm is transmitted to zygotes. DNA methylation is then partially restored by the morula stage, until which H3K9me3 is deposited. The level of H3K9me3 accumulation in these embryos was slightly lower than that detected in WT embryos, suggesting that there is also a mechanism by which DNA methylation recruits enzymes that mediate H3K9me3. Interestingly, the extent of DNA methylation recovery varied among F1 offspring, and there was a strong correlation between birth weight and DNA methylation level at the 3’ end of H19-DMR (sites m3 and m4) and in promoter regions. This variation could be dependent on the amount of H3K9me3, which may cause DNA methylation variation in post-implantation embryos. (3) In addition to the absence of DNA methylation, H3K9me3 erasure blocks DNA methylation recovery, resulting in persistence of the hypomethylated DNA state in postimplantation embryos.
(1) In WT embryos, the paternal allele of H19-DMR is marked with H3K9me3 immediately after fertilization, and H3K9me3 gradually accumulates until the morula stage. (2) In embryos from TG fathers, DNA hypomethylation of the DMR is inherited from sperm. H3K9me3 is marked on the paternal DMR until the morula stage, at which point DNA methylation is partially recovered. The degree of DNA methylation recovery varies among individuals. (3) Embryos from TG fathers edited using Kdm4d show loss of H3K9me3 at the paternal DMR, leading to defects in DNA methylation recovery.
Target-specific and global H3K9me3 removal experiments in zygotes revealed that H3K9me3 mediates DNA methylation recovery at the H19-DMR. By contrast, H3K9me3 is nearly absent at the H19-DMR in sperm, suggesting that H3K9me3 is not the primary intergenerational memory directly transmitted across generations, but rather that some other factor(s) upstream of H3K9me3 is involved. Identification of the upstream factors, which could be genetic or epigenetic, awaits further investigations. Future studies will be directed toward uncovering the mechanism of these upstream signals to further advance our understanding of epigenetic inheritance. Candidates include those factors known to participate in de novo H3K9me3 and subsequent DNA methylation during transposable element silencing48: Krüppel-associated box (KRAB) domain-containing zinc-finger proteins recognize transposable elements and recruit the cofactors KAP1/TRIM28, SETDB1 (H3K9 methyltransferase), and HP1, leading to formation of repressive heterochromatin and de novo DNA methylation by DNMT1, DNMT3A/B, and DNMT3L49,50,51,52,53,54,55. These factors may contribute to de novo H3K9me3 formation and DNA methylation in preimplantation embryos. Notably, Tanimoto et al. reported that a transgene encoding H19-DMR is de novo methylated after fertilization when it is paternally transmitted56. Although the underlying mechanism remains unknown, these authors showed that an 118 bp sequence upstream of H19-DMR is required for de novo DNA methylation57. Importantly, the 118 bp sequence is located in the region where H3K9me3 is deposited in zygotes. It will be interesting to examine what factors bind to this sequence, which will assist in the identification of key molecules responsible for H3K9me3 deposition and DNA methylation recovery in early embryos.
It remains unclear how H3K9me3 guides de novo DNA methylation at H19-DMR during embryogenesis. Burton et al. reported that de novo H3K9me3 catalyzed by SUV39H2 (H3K9 methyltransferase) occurs in the paternal genome immediately after fertilization58, which is consistent with our observation that H3K9me3 emerges on the paternal allele of H19-DMR in early zygotes. While sperm-derived genomes undergo widespread DNA demethylation after fertilization, Richard et al. reported that some regions, including several dozen CpG islands, are paradoxically de novo methylated by the 2-cell stage in a maternal DNMT3A-dependent manner59. Our study suggests that de novo H3K9me3 in zygotes is a prerequisite for the subsequent de novo DNA methylation, although the degree to which this mechanism is shared at loci other than the H19-DMR is currently unknown. Although H3K9me3 is generally recognized for its role in supporting DNA methylation, further research is necessary to determine whether this H3K9me3-mediated DNA methylation recovery is a general phenomenon or specific to imprinted loci.
DNA methylation recovery occurred specifically at the paternal, but not the maternal, DMR after fertilization, which raises the question of how the maternal allele resists de novo DNA methylation. The most plausible responsible factor is CTCF25,26, which binds to the maternal DMR before fertilization. Indeed, DNA methylation levels at the maternal DMR are increased by CTCF inhibition or knockdown in oocytes60,61, suggesting that the CTCF-bound maternal DMR is protected from DNA methylation. In the offspring derived from TG fathers, DNA methylation at the DMR was gradually restored pre- and post-implantation. During this time, CTCF could bind to the hypomethylated paternal DMR and inhibit DNA methylation recovery. Indeed, our previous study demonstrated that stable DNA hypomethylation of the paternal DMR by continuous expression of epigenome editing factors allows CTCF binding to the paternal DMR in newborn mice32.
Interestingly, hypomethylation of the H19-DMR and other imprinted loci has been observed in sperm from infertile patients62,63. The causal relationship between DNA methylation and sperm phenotypes could be directly elucidated in future studies using the sperm-specific epigenome editing system presented here. Further, various DNA methylation mutations occur during spermatogenesis under specific circumstances, such as aging64, which could be repaired after fertilization through the H3K9me3-mediated mechanism identified in this investigation. Advances in DNA sequencing technologies have led to the generation of an enormous number of candidate disease-causing epigenetic changes associated with various diseases, including genomic imprinting disorders, cancers, obesity, diabetes, and autism. Our epigenome editing system is a powerful tool that can be applied to determine how epimutations in sperm are transmitted or recovered across generations, as well as how they affect phenotypes.
Methods
Animals
B6D2F1 mice were purchased from CLEA Japan (Kawasaki, Japan). ICR, C57BL/6NCrl (B6N) mice were purchased from Charles River Japan (Yokohama, Japan). PWK/PhJ (PWK) mice (RBRC00213, RIKEN BioResource Research Center) originated from 003715 (The Jackson Laboratory). The mouse facility was kept at 21–25 °C and 40–60% humidity with a 12 h light–dark cycle. Animals had ad libitum access to food and water. All animal procedures were approved by the Animal Care and Experimentation Committee at Gunma University (No. 15-045) and carried out in accordance with approved guidelines.
Vector construction for germline-specific demethylation
The all-in-one epigenome editing vector, including dCas9 fused with five copies of GCN4 and an anti-GCN4 peptide antibody (scFv)-sfGFP-TET1CD fusion protein (pPlatTET-gRNA2, Addgene plasmid 82559), was previously reported by this laboratory31. An all-in-one epigenome editing vector, including nine gRNAs (Supplementary Data 2) targeting H19-DMR (pPlatTET-gRNA2-H19DMRx9), was also constructed as previously reported32. In this study, the CAG promoter of the pPlatTET-gRNA2-H19DMRx9 vector was replaced with the promoter of the Stra8 gene, which is specifically expressed during gametogenesis (pStrPlatTET-gRNA2-H19DMRx9). The vector was linearized by digestion with ApaLI before microinjection.
Generation of StrH19EpiTG mice
B6D2F1 female mice aged 8–10 weeks were induced to superovulate by injecting 7.5 units of pregnant mare’s serum gonadotropin (SEROTROPIN; ASKA Pharmaceutical, Tokyo, Japan), followed 48 h later by 7.5 units of human chorionic gonadotropin (hCG; GONATROPIN, ASKA Pharmaceutical). After administration of hCG, females were mated with B6D2F1 males and zygotes isolated from the oviduct 21 h later. After treatment with M2 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 0.1% hyaluronidase (Sigma-Aldrich) for 3 minutes, zygotes were washed with M2 medium and then transferred to drops of M16 medium (Sigma-Aldrich) supplemented with penicillin and streptomycin at 37 °C. Pronuclear injection was performed at 24–27 h post-hCG injection32. Linearized pStrPlatTET-gRNA2-H19DMRx9 vector (35 ng/μl) was injected into the pronuclei of zygotes. Injected embryos were cultured in M16 medium at 37 °C under 5% CO2 in air. The next day, embryos that had developed to the 2-cell stage were transferred into the ampulla of the oviduct (20–25 embryos per oviduct) of pseudopregnant ICR females. For vector integration analysis, genomic DNA was extracted from tail tips of obtained mice using a DNA extraction kit (DirectPCR Lysis Reagent, Mouse Tail; Viagenbiotech, CA, USA). PCR analysis using the primer set for dCas9 (Supplementary Data 2) demonstrated that 9 of 41 founder mice possessed the transgene. Three reproducible TG lines (441-2, 445-7, and 445-14) and their sub-lines were finally established; the 445-7 line was used in the present study and maintained by backcrossing WT B6D2F1 females with TG males. A TG male mouse that had been backcrossed twice with a PWK strain was used for allele-specific analysis.
Collection of sperm, oocytes, embryos, and newborn mice
Sperm (Fig. 2) were isolated from the cauda epididymis of TG or WT mice aged 10–21 weeks by dissecting tissue in 500 μl modified Human Tubal Fluid (mHTF) medium (Kyudo, Saga, Japan). Sperm were allowed to swim up for 1 h at 37 °C under an atmosphere of 5% CO2 in air. The upper 200 μl fraction was collected, and the collected sperm were then resuspended and incubated with hypotonic buffer to eliminate red blood cells for 3 min at room temperature (RT), followed by washing with phosphate-buffered saline (PBS). Newborn (P0) mice (Fig. 3) were generated by natural mating of TG or WT (B6D2F1) parents. Blastocyst, E7.5, and E11.5 embryos (Fig. 4) were generated by in vitro fertilization followed by embryo transfer. Briefly, sperm were obtained from the caudal epididymis of adult TG or WT male mice, and capacitated by 1 h incubation in mHTF medium. Cumulus–oocyte complexes were harvested from B6D2F1 female mice aged 8–10 weeks 15–17 h after hCG administration, and then inseminated with activated sperm in mHTF medium. At 4 h post-insemination, zygotes with two pronuclei were subjected to in vitro culture in KSOM medium (ARC Resource, Kumamoto, Japan) at 37 °C under 5% CO2 in air. The next day, a proportion of embryos that had developed to the 2-cell stage were transferred into the ampulla of the oviduct of pseudopregnant ICR females and harvested at E7.5 and E11.5. Wild-type preimplantation embryos (Fig. 5a) were generated by in vitro fertilization using PWK sperm and B6N oocytes65,66. Preparation of histone-edited embryos (Figs. 5b–e,6) is described below.
DNA methylation analysis
Sperm pellets were re-suspended in RSB (10 mM NaCl, 10 mM Tris pH 7.5, and 25 mM EDTA) containing 2% sodium dodecyl sulfate (SDS), 2% 2-mercaptoethanol, and 1 mg/ml Proteinase K. Embryos were re-suspended in TNES (10 mM Tris-HCl pH 7.5, 10 mM NaCl, 25 mM EDTA, and 1% SDS) containing 0.2 mg/ml Proteinase K. After incubation overnight at 55 °C, DNA was isolated by phenol/chloroform extraction followed by ethanol precipitation. Genomic DNA from whole bodies of newborn mice was extracted using an AllPrep DNA/RNA Mini Kit (QIAGEN, Venlo, The Netherlands). Purified DNA samples (500 ng) were processed with an Epitect Plus DNA Bisulfite Kit (QIAGEN) according to the manufacturer’s instructions. Modified DNA was amplified using TaKaRa Taq (TaKaRa, Kusatsu, Japan) and the PCR primers described in Supplementary Data 2. Percentages of demethylated CpG sites were determined by COBRA34. Amplified fragments were cleaved with restriction enzymes (Supplementary Data 2) with recognition sequences located in CpG sites. PCR products were separated and quantified using capillary and microchip electrophoresis (MCE-202 MultiNA, Shimadzu, Kyoto, Japan). Methylation level was calculated as the percentage of cleaved PCR products (mV・μm) among total PCR products (mV・μm). Alternatively, PCR products were cloned using the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA, USA). Around 24 positive clones were sequenced for each sample using the BigDye terminator method (ABI PRISM 3500 x L, Applied Biosystems, Foster City, CA, USA). Alignment, visualization, and quantification of bisulfite sequence data were performed using QUantification tool for Methylation Analysis (QUMA; http://quma.cdb.riken.jp/top/index.html). Single-nucleotide polymorphisms (SNPs) of PWK and B6N strains were utilized for allele-specific DNA methylation analysis.
Bisulfite amplicon sequencing analysis
For comprehensive CpG methylation analysis of H19-DMR, genomic DNA samples treated with the Epitect Plus DNA Bisulfite Kit (QIAGEN) were amplified using specific primer pairs (Supplementary Data 2). A fragment library was prepared from pooled PCR products using the NEBNext Ultra Ⅱ DNA Library Prep Kit for Illumina (NEB). Briefly, 100 ng of PCR product was sheared using the Covaris® S220 System (Covaris Inc, MA) at 5 °C for 2 cycles, treatment time 150 sec, with duty factor 10%, and 200 cycles/burst. To generate a fragment library, NEBNext Adapters (NEB) were ligated to both ends of the DNA. Barcoded libraries were amplified by PCR for 5 cycles using NEBNext Multiplex Oligos for Illumina (NEB). Prepared libraries were then sequenced on the Illumina NovaSeq 6000 platform (Illumina, San Diego, CA). Raw sequence data generated in FASTQ format were imported into CLC Genomics Workbench 24.0.1 (QIAGEN), trimmed using the Trim reads 3.0 tool, and mapped to 15 kb reference sequence regions (NC_000073, Mus musculus strain C57BL/6 J chromosome 7, GRCm38.p6 C57BL/6 J) using the Map Bisulfite Reads to Reference tool. For CpG methylation analysis, trimmed libraries were mapped to the reference sequence described above using Map Bisulfite Reads to Reference, and 5-mC percentages calculated using Call Methylation levels 1.4.
RT-qPCR
Total RNA was isolated from the whole bodies of newborn mice using an AllPrep DNA/RNA Mini Kit (QIAGEN). Isolated RNA (8.5 μl, 2 μg) was treated with DNase I (50 μ ml−1) in a total volume of 10 μl at 37 °C for 20 min. DNase I was inactivated by adding 0.8 μl 25 mM EDTA and incubating samples at 75 °C for 10 min. cDNA was produced from each RNA sample using random primers (Invitrogen, Carlsbad, CA) and SuperScript II (Invitrogen), according to the manufacturer’s instructions. Total RNA extraction and cDNA synthesis from zygotes and preimplantation embryos were performed using the superPrep Cell Lysis & RT Kit for qPCR (TOYOBO, Osaka, Japan). Reverse transcription reactions were diluted 10-fold with water before qPCR. Gene expression levels of Igf2, H19, and dCas9 were measured using a LightCycler 96 (Roche) with TB Green Premix Ex Taq II (TakaRa), according to the manufacturer’s instructions. Expression levels were normalized against those of Gapdh. Primer sequences are listed in Supplementary Data 2.
Immunohistochemistry
Testes specimens were fixed in 4% paraformaldehyde diluted in PBS, embedded in paraffin, and 4 μm sections prepared. Sections were deparaffinized, autoclaved (121 °C, 5 min) in 10 mM sodium citrate buffer pH 6.0 for antigen retrieval, and treated with methanol containing 0.3% H2O2 for 10 min. After blocking with 3% bovine serum albumin diluted in PBS containing Tween 20, an anti-GFP antibody (1:500 dilution, ab290, Lot No. GR3184825-1; Abcam, Cambridge, UK) was applied and incubated overnight at 4 °C. Sections were reacted with Histofine Simple Stain MAX-PO (R) (Nichirei, Tokyo, Japan) for 30 min at room temperature, signals visualized using 3-3’-diaminobenzidine, and samples counterstained with hematoxylin.
Preparation of RNA
Kdm4d and its enzymatic mutant, Kdm4dMut (H189A)45, Kdm5b, EGFP, dCas9, dCas9-SunTag (L15aa), scFv-Kdm4d, scFv-Kdm4dMut, and scFv-Kdm6b were amplified from template plasmid using Q5 Hot Start High-Fidelity DNA Polymerase (New England BioLabs (NEB), MA, USA) and primer sets for in vitro transcription (Supplementary Data 2). Amplified PCR products were gel-purified and subjected to in vitro transcription with an mMESSAGE mMACHINE T7 ULTRA kit (Life Technologies). In vitro transcribed RNA was eluted into RNase-free water, and the quality was checked by gel electrophoresis. Single guide RNAs (sgRNAs) for H19-DMR and Rosa26 (Supplementary Data 2) were purchased from Integrated DNA Technologies (IDT).
Histone editing by RNA injection
Zygotes derived from TG fathers were generated by in vitro fertilization. Sperm were obtained from adult TG male mouse caudal epididymis and capacitated by 1 h incubation in mHTF medium (Kyudo, Saga, Japan). Cumulus–oocyte complexes were harvested from B6D2F1 female mice 15–17 h after hCG administration, and then inseminated with activated sperm in mHTF medium. At 4 h postinsemination, zygotes with two pronuclei were subjected to cytoplasmic injection of RNA67 for histone editing. For global histone editing, in vitro transcribed Kdm4d/Kdm4dMut (1300 ng/μl) and EGFP (1300 ng/μl) mRNA samples were injected into the cytoplasm of zygotes in M2 medium. For target-specific histone editing, in vitro transcribed dCas9-SunTag mRNA (239 ng/μl), scFv-GFP-Kdm4d/Kdm4dMut mRNA (161 ng/μl), or scFv-GFP-Kdm6b mRNA (172 ng/μl), and two sgRNAs targeting sites upstream of H19-DMR (97 ng/μl each) or an sgRNA targeting Rosa26 (194 ng/μl) were injected into the cytoplasm of zygotes in M2 medium. Injected embryos were cultured in KSOM medium at 37 °C under 5% CO2 in air. The next day, a proportion of the embryos that had developed to the 2-cell stage were transferred into the ampulla of the oviduct of pseudopregnant ICR females and harvested at E11.5–E18.5 for DNA methylation analyses or weight measurement.
H3K9me3 Carrier Assisted ChIP-seq (CATCH-seq)
H3K9me3 CATCH-seq libraries were prepared as previously described41,42, with some modifications. Zygotes (n = 200–250) and morula embryos (n = 30–45) were permeabilized using Nuclei EZ lysis buffer supplemented with 0.1% Triton X-100, 0.1% deoxycholate, complete EDTA-free protease inhibitor cocktail and 1 mM phenylmethanesulfonyl fluoride on ice. Chromatin was fragmented in situ using 2 μ/μl MNase (M0247S, NEB) at 37 °C for 7.5 min; the reaction was stopped by adding 1/10 volume of 100 mM EDTA and diluted with freshly prepared immunoprecipitation buffer. Then, 30 ng of annealed I-SceI carrier DNA was added to each sample. The forward and reverse strand sequences of the carrier DNA were as follows: /5AmMC6/Gtagggataacagggtaattagggataacagggtaattagggataacagggtaattagggataacagggtaattagggataacagggtaattagggataacagggtaat*c/3AmMO/ and /5AmMC6/Gattaccctgttatccctaattaccctgttatccctaattaccctgttatccctaattaccctgttatccctaattaccctgttatccctaattaccctgttatcccta*c/3AmMO/, respectively, where asterisks represent phosphorothioate bonds. Oligos were synthesized by IDT. For each immunoprecipitation reaction, 1.0 μl of rabbit polyclonal anti-H3K9me3 antibody (Active Motif, #39162, Lot No. 30220003) conjugated to precleared Dynabeads Protein A and G mixture was used. After immunoprecipitation at 4 °C overnight, chromatin-Dynabeads were washed in low and high-salt wash buffers, and chromatin eluted in freshly prepared ChIP elution buffer at 65 °C for 1 h. DNA was recovered by phenol-chloroform extraction followed by ethanol precipitation. Adapter ligation was conducted using an NEBNext Ultra II DNA Library Prep Kit for Illumina (E7645, NEB) at half scale relative to the manufacturer’s instructions, and libraries purified using 1.8× SPRIselect beads (B23318, Beckman Colter). DNA was amplified using KAPA Hifi 2 × master PCR mix (KK2605) with indexing primers for 13–14 PCR cycles. After purification with 0.9 × SPRIselect beads, samples were digested with I-SceI (5 μ/μl, NEB, R0694) at 37 °C for 2 h, followed by heat inactivation at 65 °C for 20 min, then purified using 0.9 × SPRIselect beads. A second amplification was not performed. Libraries were sequenced on the NextSeq500 (Illumina, CA, USA) or NovaSeq (Illumina) platforms.
CATCH-seq data analysis
Allele-specific analysis of hybrid embryos (B6N × PWK) was performed as described previously66, with some modifications. Reads were trimmed using fastp68 and mapped using bowtie269 with the options “-N 1”. The modified mm10 reference genome, in which PWK strain single-nucleotide polymorphisms were masked as N, was used for mapping. Multiply mapped reads were retained for downstream analysis in this study. PCR duplicates were removed using Picard MarkDuplicates (https://broadinstitute.github.io/picard/). SNPsplit70 was used to assign reads to their parental origin. For analysis of previous data39,40, reads were aligned to the original mm10 reference genome, and subsequent processes performed as described above. For analysis of TG embryo CATCH-seq data, reads were extended to 250 bp and processed as single-end reads to minimize the difference in sequencing conditions among samples, and subsequent processes were performed as described above. To visualize H3K9me3 enrichment, reads from biological replicates were pooled, and bigwig files generated using bamCoverage from deepTools71, with the options “--normalizeUsing RPKM --binSize 50”. Scatter plots showed a strong correlation between biological replicates of H3K9me3 CATCH-seq (Supplementary Fig. 9).
To confirm the specificity of epigenome editing by KDM4D, biological replicates of WT_control were pooled for peak calling using bdgpeakcall from macs272 with options “-l 3000 -c 4”. H3K9me3 enrichment peaks were compared using DiffBind73; differences of p < 0.05 and |log2 fold change | > 2 were considered significant changes induced by KDM4D (Fig. 5eand Supplementary Data 1).
Micro-CT and 3D reconstruction
Eight-week-old mice were scanned using a computed tomography instrument (DELPetμCT100, DELBio, Taoyuan County, Taiwan (R.O.C.)). Three-dimensional cross sections were generated with a resolution of one cross-section per 22.5 μm. Images were transformed into DICOM format and landmarks placed within the 3D representation at the endpoints of limb bones in the forelimb (humerus and ulna) and hindlimb (femur and tibia) using VivoQuant2020 software (Invicro, MA, USA). Linear measurements were obtained for each pair of landmarks.
Statistical analysis
Box plots show the median (center line), the 25th and 75th percentiles (box edges), and the minimum and maximum values (whiskers). Data shown in bar and line graphs are presented as mean and standard deviation (s.d.) values. DNA methylation, mRNA expression, body weight, and food intake were analyzed by Welch’s t test (two-tailed) for pairwise comparisons or one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc HSD test for multiple comparisons. To analyze correlations between these variables, Pearson’s correlation coefficient (r) values were calculated. Fisher’s exact probability test was used to compare the frequency of body asymmetry. A p-value < 0.05 was considered significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The accession number for the raw sequencing data reported in this paper is DDBJ: PRJDB19200. Detailed sample information is shown in Supplementary Data 3. Sperm and oocyte ChIP-seq datasets were obtained from previous publications (DDBJ: DRA006537 and GSE97778)39,40. The source data and exact p-values underlying Figs. 2c, 3c–e, 4a, b, 6b, c, and Supplementary Figs. 1b, 2c, 4a–c, 5b, d, 6, and 8 are provided as a Source Data file. Source data are provided in this paper.
References
Nilsson, E. E., Sadler-Riggleman, I. & Skinner, M. K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 4, dvy016 (2018).
Cavalli, G. & Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 571, 489–499 (2019).
Wei, Y. et al. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc. Natl. Acad. Sci. USA 111, 1873–1878 (2014).
Sun, W. et al. Cold-induced epigenetic programming of the sperm enhances brown adipose tissue activity in the offspring. Nat. Med. 24, 1372–1383 (2018).
Anway, M. D., Cupp, A. S., Uzumcu, M. & Skinner, M. K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 (2005).
Dias, B. G. & Ressler, K. J. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 17, 89–96 (2014).
Carone, B. R. et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143, 1084–1096 (2010).
Martinez, D. et al. In utero undernutrition in male mice programs liver lipid metabolism in the second-generation offspring involving altered Lxra DNA methylation. Cell Metab. 19, 941–951 (2014).
Radford, E. J. et al. In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 345, 1255903 (2014).
Tomar, A. et al. Epigenetic inheritance of diet-induced and sperm-borne mitochondrial RNAs. Nature 630, 720–727 (2024).
Chen, Q., Yan, W. & Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 17, 733–743 (2016).
Lismer, A. & Kimmins, S. Emerging evidence that the mammalian sperm epigenome serves as a template for embryo development. Nat. Commun. 14, 2142 (2023).
Rassoulzadegan, M. et al. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 441, 469–474 (2006).
Chen, Q. et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016).
Takahashi, Y. et al. Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice. Cell 186, 715–731 (2023).
Sapozhnikov, D. M. & Szyf, M. Genetic confounds of transgenerational epigenetic inheritance in mice. Epigenetics 19, 2318519 (2024).
Policarpi, C., Dabin, J. & Hackett, J. A. Epigenetic editing: Dissecting chromatin function in context. Bioessays 43, e2000316 (2021).
Nakamura, M., Gao, Y., Dominguez, A. A. & Qi, L. S. CRISPR technologies for precise epigenome editing. Nat. Cell Biol. 23, 11–22 (2021).
Ferguson-Smith, A. C. & Bartolomei, M. S. The phenomenon of genomic imprinting was discovered 40 years ago. Nature 629, 763–765 (2024).
Arnaud, P. Genomic imprinting in germ cells: imprints are under control. Reproduction 140, 411–423 (2010).
Gicquel, C. et al. Epimutation of the telomeric imprinting center region on chromosome 11p15 in Silver-Russell syndrome. Nat. Genet. 37, 1003–1007 (2005).
Tremblay, K. D., Duran, K. L. & Bartolomei, M. S. A 5’ 2-kilobase-pair region of the imprinted mouse H19 gene exhibits exclusive paternal methylation throughout development. Mol. Cell Biol. 17, 4322–4329 (1997).
DeChiara, T. M., Robertson, E. J. & Efstratiadis, A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64, 849–859 (1991).
Bartolomei, M. S., Zemel, S. & Tilghman, S. M. Parental imprinting of the mouse H19 gene. Nature 351, 153–155 (1991).
Bell, A. C. & Felsenfeld, G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405, 482–485 (2000).
Hark, A. T. et al. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405, 486–489 (2000).
Davis, T. L., Yang, G. J., McCarrey, J. R. & Bartolomei, M. S. The H19 methylation imprint is erased and re-established differentially on the parental alleles during male germ cell development. Hum. Mol. Genet. 9, 2885–2894 (2000).
Plasschaert, R. N. & Bartolomei, M. S. Genomic imprinting in development, growth, behavior and stem cells. Development 141, 1805–1813 (2014).
Monk, D., Mackay, D. J. G., Eggermann, T., Maher, E. R. & Riccio, A. Genomic imprinting disorders: lessons on how genome, epigenome and environment interact. Nat. Rev. Genet. 20, 235–248 (2019).
Oulad-Abdelghani, M. et al. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J. Cell Biol. 135, 469–477 (1996).
Morita, S. et al. Targeted DNA demethylation in vivo using dCas9-peptide repeat and scFv-TET1 catalytic domain fusions. Nat. Biotechnol. 34, 1060–1065 (2016).
Horii, T. et al. Successful generation of epigenetic disease model mice by targeted demethylation of the epigenome. Genome Biol. 21, 77 (2020).
Sadate-Ngatchou, P. I., Payne, C. J., Dearth, A. T. & Braun, R. E. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis 46, 738–742 (2008).
Xiong, Z. & Laird, P. W. COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res. 25, 2532–2534 (1997).
Price, S. M., Stanhope, R., Garrett, C., Preece, M. A. & Trembath, R. C. The spectrum of Silver-Russell syndrome: a clinical and molecular genetic study and new diagnostic criteria. J. Med. Genet. 36, 837–842 (1999).
Becker, J. S., Nicetto, D. & Zaret, K. S. H3K9me3-Dependent heterochromatin: barrier to cell fate changes. Trends Genet. 32, 29–41 (2016).
Singh, P. et al. Chromosome-wide analysis of parental allele-specific chromatin and DNA methylation. Mol. Cell Biol. 31, 1757–1770 (2011).
Yang, H. et al. Allele-specific H3K9me3 and DNA methylation co-marked CpG-rich regions serve as potential imprinting control regions in pre-implantation embryo. Nat. Cell Biol. 24, 783–792 (2022).
Yamaguchi, K. et al. Re-evaluating the localization of sperm-retained histones revealed the modification-dependent accumulation in specific genome regions. Cell Rep. 23, 3920–3932 (2018).
Wang, C. et al. Reprogramming of H3K9me3-dependent heterochromatin during mammalian embryo development. Nat. Cell Biol. 20, 620–631 (2018).
Zhu, Y. et al. Genomewide decoupling of H2AK119ub1 and H3K27me3 in early mouse development. Sci. Bull. 66, 2489–2497 (2021).
Matsuwaka, M., Kumon, M. & Inoue, A. H3K27 dimethylation dynamics reveal stepwise establishment of facultative heterochromatin in early mouse embryos. Nat. Cell Biol. 27, 28–38 (2025).
Krishnan, S. & Trievel, R. C. Structural and functional analysis of JMJD2D reveals molecular basis for site-specific demethylation among JMJD2 demethylases. Structure 21, 98–108 (2013).
Policarpi, C., Munafo, M., Tsagkris, S., Carlini, V. & Hackett, J. A. Systematic epigenome editing captures the context-dependent instructive function of chromatin modifications. Nat. Genet. 56, 1168–1180 (2024).
Matoba, S. et al. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell 159, 884–895 (2014).
Agger, K. et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449, 731–734 (2007).
Friedrich, G. & Soriano, P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 1513–1523 (1991).
Ohtani, H. & Iwasaki, Y. W. Rewiring of chromatin state and gene expression by transposable elements. Dev. Growth Differ. 63, 262–273 (2021).
Friedman, J. R. et al. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 10, 2067–2078 (1996).
Peng, H. et al. Reconstitution of the KRAB-KAP-1 repressor complex: a model system for defining the molecular anatomy of RING-B box-coiled-coil domain-mediated protein-protein interactions. J. Mol. Biol. 295, 1139–1162 (2000).
Ivanov, A. V. et al. PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol. Cell 28, 823–837 (2007).
Schultz, D. C. et al. SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 16, 919–932 (2002).
Nielsen, A. L. et al. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 18, 6385–6395 (1999).
Ryan, R. F. et al. KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Kruppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol. Cell Biol. 19, 4366–4378 (1999).
Haggerty, C. et al. Dnmt1 has de novo activity targeted to transposable elements. Nat. Struct. Mol. Biol. 28, 594–603 (2021).
Tanimoto, K. et al. Genomic imprinting recapitulated in the human beta-globin locus. Proc. Natl. Acad. Sci. USA 102, 10250–10255 (2005).
Matsuzaki, H. et al. Recapitulation of gametic DNA methylation and its post-fertilization maintenance with reassembled DNA elements at the mouse Igf2/H19 locus. Epigenetics Chromatin 13, 2 (2020).
Burton, A. et al. Heterochromatin establishment during early mammalian development is regulated by pericentromeric RNA and characterized by non-repressive H3K9me3. Nat. Cell Biol. 22, 767–778 (2020).
Richard Albert, J. et al. Maternal DNMT3A-dependent de novo methylation of the paternal genome inhibits gene expression in the early embryo. Nat. Commun. 11, 5417 (2020).
Schoenherr, C. J., Levorse, J. M. & Tilghman, S. M. CTCF maintains differential methylation at the Igf2/H19 locus. Nat. Genet. 33, 66–69 (2003).
Fedoriw, A. M., Stein, P., Svoboda, P., Schultz, R. M. & Bartolomei, M. S. Transgenic RNAi reveals essential function for CTCF in H19 gene imprinting. Science 303, 238–240 (2004).
Marques, C. J. et al. Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. Mol. Hum. Reprod. 14, 67–74 (2008).
Laurentino, S. et al. Epigenetic germline mosaicism in infertile men. Hum. Mol. Genet. 24, 1295–1304 (2015).
Ashapkin, V., Suvorov, A., Pilsner, J. R., Krawetz, S. A. & Sergeyev, O. Age-associated epigenetic changes in mammalian sperm: implications for offspring health and development. Hum. Reprod. Update 29, 24–44 (2023).
Inoue, A., Jiang, L., Lu, F., Suzuki, T. & Zhang, Y. Maternal H3K27me3 controls DNA methylation-independent imprinting. Nature 547, 419–424 (2017).
Mei, H. et al. H2AK119ub1 guides maternal inheritance and zygotic deposition of H3K27me3 in mouse embryos. Nat. Genet. 53, 539–550 (2021).
Horii, T. & Hatada, I. Generation of genome-edited mice by cytoplasmic injection of CRISPR-Cas9 RNA. Methods Mol. Biol. 2637, 75–86 (2023).
Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. Imeta 2, e107 (2023).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Krueger, F. & Andrews, S. R. SNPsplit: Allele-specific splitting of alignments between genomes with known SNP genotypes. F1000Res. 5, 1479 (2016).
Ramirez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Ross-Innes, C. S. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2012).
Acknowledgements
We thank Ms. Junko Shima, Ms. Yuko Okazaki, Ms. Emi Hosoya, Ms. Nanase Yamazaki, Ms. Rie Fukuda, Ms. Marie Ishikawa and Ms. Eriko Suetomo (Gunma University) for technical support with PCR and bisulfite sequencing analyses, and Dr. Yuhkoh Satouh (Gunma University) for evaluation of testis sections. We also thank Ms. Makiko Nakagawa, Ms. Yuko Nakatani, and Dr. Kenji Watanabe (Yamaguchi University) and Ms. Mami Kumon (RIKEN) for technical support with next-generation sequencing analyses. We appreciate the technical support provided by the Yamaguchi University Science Research Center. This work was supported by funding from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 24H00679, Scientific Research (A) and 23K23802, Scientific Research (B) to T.H., 23K05727, Scientific Research (C) to S.M., and 24K02582, Scientific Research (B) to I.H.; the Japan Agency for Medical Research and Development (AMED) under Grant Number 22gm6310009h0004 to S.M.; the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Numbers JP21am0101120 and JP24ama121049 to I.H.; the Tokyo Biochemical Research Foundation to I.H.; the Takeda Science Foundation to I.H.; the Practical Research Project for Rare/Intractable Diseases from AMED under Grant Number 21ek0109489h0002 to I.H.; the Uehara Memorial Foundation to I.H.; the Joint Usage/Research Center for Developmental Medicine, IMEG, Kumamoto University to T.H.; the Life Science Institute, Inc. (LSII) to I.H.; Grant-in-Aid for Transformative Research Areas (A) under Grant Number 25H01355 to A.I.; JST FOREST Program, Grant Number JPMJFR2335 to A.I.; and the JSPS postdoctoral fellowship PD, Grant Number 24KJ0236 to H.F.
Author information
Authors and Affiliations
Contributions
T.H. and I.H. designed the experiments. T.H., S.M., S.H., Y.H., H.F., R.K., M.K., M.N., Y.M., and A.I. performed the experiments and analyzed the data. T.H., S.M., H.F., R.K., A.I., and I.H. wrote the manuscript. I.H. supervised the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that a patent application related to this work has been filed by Gunma University (Japanese Patent Application No. 2023-522326; currently under examination). The inventors are I.H., T.H., and S.M. The patent pertains to methods for germline epigenome editing. This research was funded by the LSII, Inc. The funder provided financial support to I.H. and was involved in the data analysis presented in Supplementary Fig. 4; however, the decision to publish and the preparation of the manuscript were made independently by the authors. S.H., Y.H., H.F., R.K., M.K., M.N., Y.M., and A.I. declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Albert Jeltsch, Maxim Greenberg and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Horii, T., Morita, S., Hino, S. et al. Germline epigenome editing identifies H3K9me3 as a mediator of intergenerational DNA methylation recovery in mice. Nat Commun 16, 11200 (2025). https://doi.org/10.1038/s41467-025-67488-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-67488-9