Abstract
The hunting of large whales has shaped the lifeways of many coastal communities for millennia, yet its origins remain debated, often associated with postglacial cultures in Arctic and subarctic regions dating to approximately 3500-2500 years ago. Here, we present evidence that large baleen whales were likely hunted 5000 years ago by Indigenous groups in southern Brazil. We analysed museum collections of cetacean bones and artefacts from archaeological shellmounds, known as sambaquis, in the region of Babitonga Bay. Zooarchaeological, typological, and molecular analyses of bone remains and artefacts indicate that Sambaqui people exploited southern right whales (Eubalaena australis), humpback whales (Megaptera novaeangliae) and dolphins in coastal waters. The abundance of whale bone remains, the presence of specialised marine hunting artefacts, and the importance of whales in funerary contexts are consistent with archaeological and ethnographic evidence of whaling societies. Our results also illuminate species distributions prior to commercial exploitation, providing insights for conservation strategies. Whale exploitation was an element of Indigenous maritime knowledge in southern Brazil long before European contact; an unwritten history preserved in museum collections and in the sambaquis that have survived the impacts of modern human activities.
Similar content being viewed by others
Introduction
For millennia, coastal communities worldwide have depended on large whales through opportunistic harvesting and active hunting, a practice that, while controversial today, remains integral to the food security, cultural identity, and traditions of many coastal peoples1. Despite its historical significance, the antiquity of whale hunting remains elusive. While the use of whale products dates back to the Late Pleistocene2, there is consensus that the systematic pursuit of large whales is a more recent cultural phenomenon, emerging within the broad spectrum of postglacial coastal and maritime adaptations. Rock art depicting whale hunting scenes, such as the Bangudae Petroglyphs in Korea and various sites in the White Sea and Scandinavia, suggest that humans may have engaged in whale hunting as far back as 6000 years ago3,4,5,6,7. Nevertheless, the exact dates and cultural contexts of these artworks remain uncertain. Compelling evidence of active whaling, such as harpoon heads and whale bones bearing cut marks, has been uncovered at sites along the Northern Pacific Rim, North Atlantic, and the Arctic, dating back around 3500–2500 years8,9,10,11,12,13,14, shaping the prevailing view that the hunting of large whales first emerged among maritime foragers in the polar and cold temperate regions of the Northern Hemisphere14.
In the Southern Hemisphere, cetaceans have been used by pre-colonial Indigenous groups in Brazil since at least 8000 years ago, as attested by bone remains and objects found in numerous shellmounds, known as sambaquis15,16,17,18,19. Sambaquis are found along estuaries, bays and coastal lagoons, where abundant and predictable aquatic resources offered conditions for the establishment of dense and seemingly stable coastal-adapted populations for nearly 7000 years20,21, during a period of significant change in coastal environments due to sea-level fluctuations22. In southern Brazil, zooarchaeological studies have shown that a diversity of cetaceans were used by Sambaqui peoples, particularly taxa that occur in shallow coastal waters, such as the southern right whale (Eubalaena australis), the franciscana dolphin (Pontoporia blainvillei, the only extant species of the Pontoporiidae family) and several Delphinidae species such as the common bottlenose dolphin (Tursiops truncatus)16,17,23. Bones of large baleen whales are relatively abundant at several sites, with many bearing cut marks resulting from butchering16,17, while others were used as grave goods24,25,26, funerary structures15,27,28 or transformed into elaborate artefacts24,28,29,30. Despite this, the nature of whale product use has remained debatable. The absence of specialised harpoon technology, as well as the lack of hunting marks, has led to a general perception that whales were not intensively exploited, but rather used opportunistically when stranded16.
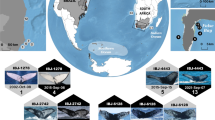
The coast of Santa Catarina state in southern Brazil has a high concentration of sambaquis, with over 200 documented sites in Babitonga Bay and nearby coastal areas31,32. Worked (artefacts) and unworked cetacean bones have been recovered from many sambaquis in the region (Fig. 1), largely by the amateur archaeologist and collector Guilherme Tiburtius between 1940 and 196025,28, during the dismantling of sites for commercial lime production and embankments, which continued in Brazil until the 1960s. Many of these sites no longer exist, with the only remaining evidence being the artefacts and human and faunal remains that have survived in Tiburtius’ collection, the majority of which is currently housed at the Museu Arqueológico do Sambaqui de Joinville (MASJ, Santa Catarina, Brazil). It is arguably the richest assemblage of pre-colonial cetacean artefacts in the country, and includes elongated, flat circular, ellipsoidal, spherical and rectangular objects, pendants, and zoomorphic figurines crafted from cetacean bones. Several of the artefact typologies appear to be unique to sambaquis in southern and southeastern Brazil, specifically those crafted from cetacean tympanic bullae (a dense bone forming part of the middle ear), and numerous worked and unworked cetacean remains were found in funerary contexts24,28,29. For all of the artefacts, their physical modifications have eliminated distinguishing anatomical features, while the fragmented nature of much of the unworked bone renders taxonomic identification difficult.
The numbers indicate those with cetacean remains and harpoons studied herein. The location of one site, Sambaqui Edgard Tiburtius-Praia Grande, is uncertain and thus has not been included in the map. The submerged coastline represents the maximum extent of sea-level rise during its highstand around 5000 years ago, gradually declining to present-day levels thereafter. Map generated using ArcGIS 10.8 and Inkscape 1.2.1, with public data from Instituto Brasileiro de Geografia e Estatística (https://www.ibge.gov.br/geociencias/downloads-geociencias.html) and Heraldry via Wikimedia Commons (https://commons.wikimedia.org/wiki/File:Cartography_of_South_America.svg). The submerged area was created using contour lines (IBGE), generating TIN and raster files, using sea level data from Toniolo et al.22.
In this study, we reassessed Tiburtius’ collection, along with artefacts and remains from other collections (Collections Lepper, Kuhlhof, Bandeira, Afonso and DeBlasis, Goulard, and Imhof) also housed at MASJ. We combined conventional zooarchaeology and collagen peptide mass fingerprinting (Zooarchaeology by Mass Spectrometry, or ZooMS) to identify the taxa of both worked and unworked bone objects from sambaqui sites in and around Babitonga Bay, and reconstructed the chronology of cetacean exploitation in the region by expanding radiocarbon dating of human and cetacean bone specimens. Significantly, comparison with archaeological and ethnographic literature shows that some enigmatic artefacts recovered from the sambaquis in the second half of the last century are in fact harpoon foreshafts made of whale bone, among the largest documented from archaeological sites in South America. Their association with whale bone remains and artefacts reveals that Sambaqui populations possessed the technology for hunting large baleen whales and dolphins as early as 5000 years ago. This study positions southern Brazil as one of the earliest centres of large baleen whale exploitation, potentially extending the antiquity of whaling by more than a thousand years.
Results
Marine mammal diversity
Marine mammal bones have been extensively found in sambaquis in southern Brazil, but the vast majority remain unidentified beyond the order or suborder level (Cetacea, Mysticeti for baleen whales), or the family level in the case of Otariidae and Delphinidae (Fig. 2A, B). Combining conventional zooarchaeology and ZooMS, our results expand the taxonomic list of exploited species and offer glimpses of cetacean diversity in Babitonga Bay between approximately 5700 and 500 cal BP (Supplementary Data 1). Zooarchaeological analysis of worked and unworked bones (n = 162) from 18 sambaquis taxonomically resolved several Delphinidae species, such as the bottlenose dolphin (T. truncatus), the Guiana dolphin (Sotalia guianensis), and the genus Stenella, along with generic Delphinidae (n = 10). Other remains could be identified only to the suborder level, as Mysticeti (n = 62) and Odontoceti (n = 12, including a probable franciscana dolphin (P. blainvillei)), or more generically as cetacean (n = 75) (Supplementary Data 2). Teeth from Delphinidae species were also identified at several sites, and a sperm whale (Physeter macrocephalus) tooth was identified at the site of Morro do Ouro, which has been dated between 4900-4710 and 3760-3520 cal BP (68.3% confidence interval, Supplementary Data 1). A large number of bones were either fragmented or modified during the manufacturing of artefacts, preventing further taxonomic identification using morphological features.
A, B Number and relative abundance of identified marine mammal remains from pre-colonial archaeological sites in southern Brazil using data in ref. 23. C Taxonomic identification of unworked and worked cetacean bones from sambaquis in Babitonga Bay using ZooMS. Failed samples and those with non-cetacean ZooMS identifications (n = 4) have not been included.
A total of 155 samples (85 unworked, 70 worked) were analysed via ZooMS, of which 79% (n = 122) provided taxonomic identifications of varying degrees (Fig. 2C). Of the unworked remains, 98% (n = 83) were able to be identified, while the worked objects had a lower success rate, with 56% (n = 39) providing usable peptide markers (Supplementary Data 2 and 3). The lower success rate of the worked objects was expected, as the majority of these were sampled using minimally-invasive methods, which are known to be less effective than destructive techniques33,34,35. Considering that nearly half of the worked objects were made from highly mineralised bone elements, such as tympanic bullae (n = 34, plus 3 additional unworked)36, and the majority were highly polished, the available surface collagen that minimally-invasive techniques rely upon would have been significantly reduced through manufacturing and subsequent taphonomic processes in the burial environments, and even more so by post-excavation cleaning and storage methods. As such, a minimally invasive success rate of 56% was better than anticipated.
Of the 122 identifiable samples, 118 were cetaceans, with ZooMS confirming the presence of at least six different taxa, including southern right whale (E. australis), the Delphinidae/Phocoenidae families, sperm whale (P. macrocephalus), and taxa previously undocumented through conventional zooarchaeology in coastal sites in Santa Catarina state23, such as humpback whale (Megaptera novaeangliae), sei whale (Balaenoptera borealis), and blue whale (Balaenoptera musculus) (Fig. 2C and Supplementary Data 3). While ZooMS is effective at distinguishing between many whale species, this is not the case for species within the Delphinidae and Phocoenidae families. Many species from these two families are currently missing from available ZooMS databases, and those that are available typically have highly similar collagen sequences, meaning ZooMS can only identify them into larger groupings. The Balaenidae species, including the three species of the genus Eubalaena (E. australis, E. japonica, and E. glacialis) and the bowhead whale (Balaena mysticetus), cannot be distinguished using ZooMS; however, since the southern right whale is the only species found in the South Atlantic ocean37, the others can be ruled out. Additionally, nine samples (three unworked and six worked) could only be identified as either right whale or fin whale (Balaenoptera physalus), as only a single peptide marker, COL1ɑ2 292 or the P2 marker, is used to distinguish them (Supplementary Fig. 3). As no sample was definitively identified as being fin whale, it is very likely that those identified as right/fin whales are in fact southern right whales, and we have therefore assumed this to be the case in the following results.
The southern right whale was by far the most identified species, with 51% (n = 60) of all cetacean remains. Of the 83 identifiable unworked remains, 81 were found to be of cetacean origin, with 49% identified as southern right whale (n = 40, of which 3 were identified as right/fin whale), 35% as Delphinidae (n = 28, of which 3 were identified as Delphinidae/Phocoenidae), 11% as humpback whale (n = 9), and a single sample was identified each for sei whale and sperm whale, with two additional samples only identifiable as Mysticeti or Cetacea. For the 39 identifiable worked objects, 37 were identified as cetaceans, with 54% as southern right whale (n = 20, of which 6 were identified as right/fin whale), 22% as humpback whale (n = 8), 19% as Cetacea (n = 4) or Mysticeti (n = 3), and one object for each of Delphinidae and blue whale (Fig. 2C). No other marine mammal taxa were found with either zooarchaeological or ZooMS analyses, even though other marine mammal species have been recovered from different sites in the region23,32. Several bones had visible cut marks likely resulting from butchering (46 out of 162, 28.4%, Supplementary Data 2). For example, a bone of a southern right whale (Col. Tiburtius 3786) from Areias Pequenas had deep transverse incisions and chop marks in the mid-shaft, and was radiocarbon dated to 1450–1300 cal BP (68.3% confidence interval, Supplementary Data 1). These types of cut marks are typically associated with the removal of blubber and meat38.
Harpoon technology
Tiburtius’ notebooks about his collection list more than 200 objects reported as whale, whalebone, or ‘ear bone’ (referring to whale tympanic bullae) from sambaqui sites in the region of Babitonga Bay. Of these, a distinct group of worked bones (12 from Tiburtius’ collection, plus two from the Kuhlhof collection and one from the Lepper collection), generically reported as bastões (sticks or rods), have been found in three sambaquis in the region21,25,28. These objects show a high level of standardisation in size, shape, and material used, and could be differentiated into two distinct typological classes. The first class, manufactured from cetacean rib bone, comprises four objects (three of which were broken at one extremity) showing similarities with harpoon socket pieces documented among ethnographic coastal Indigenous populations in South and North America39,40,41. One extremity of the long bone has been hollowed out for the insertion of a detachable head that was likely made of stone, bone or wood (Fig. 3A–D). The other extremity may have been inserted and hafted into a main shaft piece (fixed), as described among some ethnographic groups40,42. The four objects, two of which were identified as southern right whales, were found at Conquista, where seven human individuals presented radiocarbon dates ranging from 5450–5280 to 2040–1880 cal BP (68.3% confidence interval, Supplementary Data 1).
The second type, also manufactured from cetacean rib bone, was longer (26.4–52 cm) and exhibited a carved and polished bevelled distal extremity. Perpendicular fine grooves that run along the bevelled extremity would have facilitated the hafting of a side barb-point with animal hide, sinew or plant fibres (Fig. 4A–G). In all objects, the other proximal extremity is slightly conical, possibly to fit into the end of a main shaft. Their shape and size show similarities with simple-barbed harpoon heads made of wood reported by Mason (Peruvian and Chilean harpoon)39 and Ballester43,44 (Type B and D) for coastal sites in Chile, dated from ca. 2500 cal BP. Eight complete or nearly complete foreshafts were found at the sambaquis of Morro do Ouro (n = 5), Cubatãozinho (n = 2), and Conquista (n = 1), along with at least an additional six objects (Col. Tiburtius 4595, 4597, 4920, 5073, 8306, 8472) from the aforementioned sites, which are probable fragments of such foreshafts. ZooMS analysis revealed that the foreshafts were manufactured using bones of southern right whales and humpback whales. Two of these objects, Col. Tiburtius 4215 from Morro do Ouro (Fig. 4F) and Col. Tiburtius 8273 from Conquista (Fig. 4G, lower portion), both identified as southern right whales, were directly radiocarbon dated to 4900-4710 and 4970-4780 cal BP (68.3% confidence interval), respectively. Their radiocarbon ages match the chronology of human, charcoal and faunal remains from these sites (Supplementary Data 1). In the case of Conquista, the foreshafts were recovered from deposits containing marine mammal remains, along with other artefacts manufactured from marine mammal bones24.
Harpoon foreshafts from Cubatãozinho (A—Col. Tiburtius 4329; B—Col. Lepper 75.12.01), Morro do Ouro (C—Col. Khulhof 72.06.16; D—Col. Tiburtius 4597 A, Cetacean; E—Col. Khulhof 72.06.17, not available for sampling; F—Col. Tiburtius 4215), and Conquista (G—Col. Tiburtius 8273-8062); H—bevelled projectile point with hafting notches from Conquista (Col. Tiburtius 8400); I— depiction of how point H may have been attached to shaft G found at the same site. Photos and illustrations (created using Inkscape 1.2.1) by the authors.
One large bevelled bone point from Conquista (Col. Tiburtius 8400, Fig. 4H), identified as a southern right whale, contained three perpendicular notches that were possibly used for hafting the point at the bevelled extremity of the foreshafts (Fig. 4I). Bevelled points are widely documented in sambaqui sites, where they formed part of the hunting and fishing arsenal21,45. Their use as composite side barbs on projectile points is also well attested among Indigenous groups in tropical South America46,47,48. As observed with Chilean harpoons, both types were probably mounted at the distal end of a main shaft and may have included lines for the retrieval of the prey by the hunter. The main shaft was likely made of wood, while the lines would have been plant fibre or sinew, all of which rarely survive in subtropical burial environments except under exceptional circumstances (Fig. 5A–F). Significantly, three foreshafts (Col. Tiburtius 4595 and 4596, Col. Kuhlhof 72.06.16) were reported by Tiburtius as grave goods in human burials at Morro do Ouro25, with the Col. Kuhlhof foreshaft identified as a southern right whale (the other two were not available for sampling). Additionally, Tiburtius reported that one foreshaft from Cubatãozinho was also found associated with a human burial24. Although there is no information about the human individuals associated with these foreshafts, their funerary contexts suggest they were prised possessions with significant ritualistic, symbolic and/or spiritual meanings.
A Chilean harpoon of type B and B type D, both with foreshafts made of wood, fitted with a bone barb and, in some cases, a stone point (type D); illustration created by the authors of an artefact published in Ballester43. C Hypothetical reconstruction of a Sambaqui harpoon foreshaft with a hafted bevelled bone projectile point (Col. Tiburtius 8400). The barb of this bevelled point is broken and would have originally extended further to allow for effective retention. D Hypothetical reconstruction of a Sambaqui socket-piece assemblage with a bone point and a lateral barb (bevelled bone point). E, F illustrate reconstructions of how both the harpoon foreshafts and socket-pieces likely would have been assembled into a complete harpoon, with the main shaft made of wood and the line made of sinew or plant fibre (based on examples of preserved Chilean harpoons43). Photos and illustrations of harpoons (created using Inkscape 1.2.1) by the authors.
Other worked objects previously reported by Tiburtius25,28 and analysed herein included decorated long bones with zoomorphs (n = 2), possible pendants (n = 6), centrally perforated and non-perforated flat circular (n = 6), ellipsoidal (n = 2) and rectangular (n = 1) disk-like artefacts (some with notched outer edges), solid spheres (n = 12), double-ended points (n = 3), and zoomorph figurines (n = 3), among others (Fig. 6A–J). The two decorated long bones were manufactured from cetacean ribs, with one identified as a humpback whale (Fig. 6B, Col. Lepper 75.12.03). The other was found next to the femur of a human individual in Conquista (Fig. 6A, Col. Tiburtius 8097)24, and was earlier interpreted as a possible atlatl21. Several rectangular pendants and other artefacts were also made from long bones, with the majority identified as originating from either southern right or humpback whales; however, one rectangular object from Conquista was also identified as a blue whale (Col. Tiburtius 7383). The large majority of the other artefacts were made from tympanic bullae (Fig. 6C–J), generically attributed to southern right whale or Mysticeti, and found at the sambaqui sites of Areias Grandes, Areias Pequenas, Barra do Sul, Conquista, Harmonia Lyra, Itacoara, Linguado, Morro do Ouro, Rio Pinheiros I, and Edgard Tiburtius-Praia Grande, with calibrated radiocarbon dates between 5880-5560 and 630-510 BP (68.3% confidence interval, Supplementary Data 1). Assuming that only one artefact could be generated from a single tympanic bulla, it is possible to estimate that more than 15 individuals are represented by the bullae objects analysed herein (many more exist from the aforementioned sites but were not subjected to ZooMS). Two near-complete unworked bullae, one from a southern right whale (Col. Tiburtius 4093) and the other a sei whale (Col. Tiburtius 4903), were identified from Barra do Sul, while an additional broken one, identified as Mysticeti (Col. Tiburtius 8042), was found at Conquista. Nevertheless, according to Tiburtius24, Conquista alone yielded 60 artefacts manufactured from tympanic bullae, of which 22 were fully finished, 18 were broken, 15 were in preparation, and 5 were nearly finished. This suggests that a minimum of 30 individual whales, obtained through hunting or scavenging, were processed at this site alone.
Decorated ribs with zoomorphic designs from Conquista (A—Col. Tiburtius 8097) and Cubatãozinho (B—Col. Lepper 75.12.03), and objects made from tympanic bullae such as drop-shaped artefacts (C—Col. Tiburtius 5281, Barra do Sul), perforated flat ellipsoidal (D—Col. Tiburtius 4392, Morro do Ouro), circular (E—Col. Tiburtius 7825, Conquista) and rectangular (F—Col. Tiburtius 2194, Areias Grandes) disks, solid spheres (G—Col. Tiburtius 5536-A, Morro do Ouro), zoomorph figurines representing a whale (H—Col. Tiburtius 8381, Conquista) and a bird (I—Col. Tiburtius 4311, Barra do Sul), and rectangular pendants (J—Col. Tiburtius 7810, Conquista). Bone of southern right whale with cut marks from Areias Pequenas (K—Col. Tiburtius 3786). Photos and illustrations (created using Inkscape 1.2.1) by the authors.
While the use of some object types can be hypothesised, such as some drop-shaped artefacts resembling net weights (Col. Tiburtius 5281, identified as humpback whale, Fig. 6C) and perforated disks resembling spindle whorls49 (Fig. 6D, E), the function of the majority of these objects remains unknown and several types, specifically those originating from tympanic bullae such as spheres, unperforated disks, and doubled-ended points (‘gotas’), are seemingly unique to the Sambaqui populations. Artefacts crafted on tympanic bullae were also found in sites to the north, from the coasts of São Paulo (e.g. Piaçaguera50) and Paraná (e.g. Gomes, Matinhos, Guaraguaçu, Ramal, Araujo II19,28,29,51,52,53,54) and south to Santa Catarina Island (e.g. Pantano do Sul28,55), spanning approximately 700 kilometres of coastline. Even the possible spindle whorls are unique in that they derive from tympanic bullae, while spindle whorls from coastal groups in the Northwest coast of North America are largely manufactured out of other marine mammal bone elements (e.g. epiphysis)49. Several other worked and unworked cetacean bone objects were also found associated with human burials at the sambaquis of Morro do Ouro (Col. Tiburtius 4691) and Rio Pinheiros I (Col. Tiburtius 4689, 4699, 4740, 5016), again pointing to the important ritualistic and spiritual role cetaceans held for early Sambaqui societies, as does the presence of the ‘cetacean’ zoomorph (Fig. 6G, Col. Tiburtius 8381) found at Conquista and made from a cetacean tympanic bulla.
Discussion
The antiquity of subsistence whaling has been the subject of contentious debate for decades, particularly due to the difficulty of using often fragmentary and ephemeral archaeological evidence to determine whether whales were exploited opportunistically as stranded animals or drifted carcasses, or actively hunted14,56. Today, multiple lines of evidence are generally invoked as likely indicators of active whaling among prehistoric groups. These include, for example, the abundance of whale bones, the presence of specialised hunting technology, bones with butchering and harpoon strike marks, bones with embedded points, the repeated occurrence of inshore and slow moving species across multiple sites, a high quantity of artefacts crafted from whale bone such as figurines representing whales, whale-derived objects used as grave goods, and the depiction of hunting scenes, among others8,44,57,58,59. With the exception of bones bearing harpoon strikes or embedded points and depictions of whaling, all other key indicators are present in sambaqui sites in southern Brazil. Significantly, our study shows that Sambaqui populations in Babitonga Bay also produced whale bone harpoons for the pursuit of large marine prey as early as 5000 years ago. It is worth noting that the collection studied was specifically searched for cetacean remains and while pinniped remains were absent in Tiburtius’ collection, their occurrence in sites in southern Brazil17,23,24,60 suggests these animals may also have been targeted by Sambaqui groups in Babitonga Bay through harpooning. The use of harpoons likely extended to other marine animals as well, such as sharks, rays and other large fish, which are well represented in the archaeological record32, and beached cetacean carcasses or stranded individuals would have certainly been viewed as valuable resources.
To date, large harpoon foreshafts have been documented at only three sites in Babitonga Bay (Morro do Ouro, Conquista, Cubatãozinho) and the distinctive features of a few localised sites cannot be taken to represent all Sambaqui populations. Nevertheless, the broader archaeological record reveals socio-ecological traits consistent with those expected in prehistoric whaling societies8. These include reliance on predictable fish resources, community-based exploitation strategies that enable cooperative hunting31, reduced residential mobility and locally high population densities61. In particular, faunal32,62 and stable isotope analyses31,63,64 of hundreds of human individuals recovered from dozens of sites indicate dietary dependence on marine and brackish organisms, primarily fish, but also marine mammals16,17 obtained either through active hunting or opportunistic acquisition. Terrestrial mammals, by contrast, are relatively rare in sambaqui sites23 and their dietary contribution is estimated to have been minor31. The large harpoons analysed in this study were therefore designed to target large marine prey, with baleen whales representing the largest of these targets. While more research is needed to resolve the earliest origins of whaling, with previous consensus pointing to whaling cultures in cold temperate and Arctic regions between approximately 3500 and 2500 years ago14, the lines of evidence presented herein would push back the earliest known record of active whaling by at least 1000 years. Early harpoon heads have been recovered from coastal sites in Tierra del Fuego and the Atacama Desert, dated to around 7000 and 4000 years ago43,44,65, but their association with the hunting of large whales remains uncertain.
The prevalence of southern right whales across all sites, as both unworked bones and artefacts, indicates that they were procured during their migratory seasons. Southern right whales are the most common whale species in southern Brazil. Today, and historically, from June to November, the coast of Santa Catarina becomes a breeding hotspot for southern right whales, where cow-calf pairs and lone adults spend days or weeks moving slowly between protected shallow bays close to the shoreline66. This particular coastal habit, along with their slow swimming and high buoyancy and floatability after death67,68, would have facilitated their capture with relatively simple technologies, in the same way this species was pursued by local coastal whalers in colonial times using row boats and hand-thrown harpoons69. Young animals and females would have offered relatively accessible prey for hunters operating in inshore waters such as Babitonga Bay and the adjacent coastal areas. The hunting of breeding stocks is supported by the presence of young individuals in sambaqui sites in southern Brazil17, which suggests active selection rather than passive collection. Sambaqui groups would have undoubtedly had access to and made use of stranded individuals; however, stranding events are unpredictable and while the stranding rates over the past 5000 years are unknown, today they are a rare occurrence for southern right whales in this region70.
In addition to southern right whales, ZooMS identified three other species of mysticetes (humpback, blue and sei whales) from several sambaquis. The relative abundance of humpback whale remains suggests that this species may previously have been more common in the region and that it is likely they were also pursued by Sambaqui groups in the shallow coastal waters around Babitonga Bay, though to a lesser extent than southern right whales. There is no information about the southern distribution of humpback whales prior to their commercial exploitation during colonial times. The results presented here provide the first indisputable evidence that humpback whales once occupied the coastal waters of southern Brazil, extending at least as far as the northern coast of Santa Catarina state. Current knowledge accumulated over the past few decades highlights a reproductive concentration area for this species in Abrolhos Bank, off the coast of the northeastern Brazilian state of Bahia, with a southerly oceanic distribution considered merely part of their migratory route71. Over the past decades, however, Brazil’s southeastern and southern regions have seen an increasing occurrence of humpback whales during the breeding season, which has been attributed to recent rapid population growth72,73, but may in fact represent a reoccupation of areas previously inhabited by the species. Recognising this expansion as a reoccupation of areas previously inhabited has important implications for humpback whale conservation, which is particularly relevant considering the growing conflicts between humpback whales and marine vessels, with increasing records of entanglements in gillnets and collisions in the region74.
Blue, sei and sperm whales, by contrast, are typically oceanic species that do not occur near the coast and strandings are rare along the Santa Catarina coast75, which is also reflected in our results with these species represented by only a single specimen each. These species were probably exploited as drift carcasses or stranded individuals rather than actively hunted. Similarly, if any fin whale is present in the few samples which were only able to be resolved to the level of right/fin whale, it is likely these would also represent strandings rather than actively hunted individuals considering their offshore distribution on the Atlantic coast, even though the species was heavily exploited by the whaling industry in Brazil between the 1960s and 1980s in regions further north, between the states of Rio de Janeiro and Paraíba76.
A variety of dolphin species, found primarily as unworked bones in sambaquis, may have been hunted with harpoons and nets close to shore16. As previously mentioned, ZooMS cannot effectively separate most dolphin and porpoise species and conventional zooarchaeological analysis can often provide greater taxonomic resolution with good bone preservation. For example, a sternum from Porto do Rei (Col. Tiburtius 6847) identified as Delphinidae via ZooMS was determined zooarchaeologically to be a Guiana dolphin. This species is associated with bays and estuaries along the coast, where it forms resident populations, as is the case with Babitonga Bay and may have constituted a resource available year-round. Similarly, also from Porto do Rei, a caudal vertebra (Col. Tiburtius 6836) with a ZooMS identification of Delphinidae, was identified zooarchaeologically as a franciscana dolphin, another common coastal species with a resident population in Babitonga Bay and recorded in sambaqui sites, however, in smaller numbers77. The combined zooarchaeological and ZooMS results highlight the complementary nature of these two techniques and the increased effectiveness when used together.
Drawing parallels from ethnographic and archaeological records10,44,57, whale exploitation could have been central to Sambaqui social organisation, settlement patterns and resource management. The rarity of bone harpoons in sambaquis alludes to the importance of such artefacts that must have been maintained and curated and hence less frequently produced56; to their specific function, with harpoons potentially often lost at sea or broken during the hunt; to the prestige of whaling; and to the social prominence of the individuals involved in these practices58. The direct association of harpoons and whale bones with human burials, in fact, evokes individuals invested with various roles related to acquiring, processing and distributing whale products57. As documented among whaling cultures of North America57, the pursuit of a whale may have been restricted to a few individuals, passed through chiefly lineages that fostered social status, rights and privileges9,57,78, while the processing of the animal could have involved the whole community79. European travellers of the 16th to 18th centuries reported the killing of large whales by Indigenous groups in Tierra del Fuego, the Atacama Desert and the Gulf Coast, either for meat and oil44,79, or specifically to recover tympanic bullae used in the funerary practices of deceased chiefs80, with whales being targeted by both individuals and organised groups. In southern Brazil, the hunting, particularly in winter, may have catalysed social gatherings along specific whaling sites holding political and ideological significance20,61. Large coastal sambaquis may have served as communal gathering places for whale sighting and monitoring, hunting ceremonies, rituals of ancestral worship and other aspects related to the reproduction of whaling culture. Access to whaling grounds could have been regulated through exclusive ownership, with particular groups or individuals having rights over specific areas57.
Whaling relies on maritime technologies, including watercraft, harpoon lines and flotation devices, that rarely survive in tropical and subtropical regions due to being crafted from perishable materials such as wood, plant fibres, internal organs (bladders) and hides10,40,43,79. A single cetacean would provide large quantities of raw materials, the majority of which would leave no trace in the archaeological record, or may not even have formed part of it, such as bones left at butchering sites on the shore10,57,81. Skin from cetaceans could have been used for boats, tents and clothing; internal organs would have provided containers and float devices such as those used to create drag in whaling; and baleen may have been used for mats, baskets, nets, weirs and snares, among others8,58,82. Large quantities of oil, which may represent 25% to 50% of cetacean body weight depending on species83, may have been extracted from blubber and bones and used as fuel in domestic and ceremonial activities16,79. Bones could have been employed for architectural purposes84 and, as shown here, for the production of numerous tools and objects, including spindles for weaving and making cordage.
Whales and dolphins may have played a much larger role in the diet of Sambaqui people than estimated by studies of faunal remains16,17,32 and stable isotope analyses of human individuals, which have mostly focused on the contribution of fish in dietary models31,63,64. This has possibly been exacerbated by the highly fragmented and weathered condition of marine mammal bones, which often characterises the bone assemblages at these sites17,24. Cetacean meat, blubber and bone contain significant amounts of proteins, mineral nutrients, oils and fats83,85, which must have been particularly valuable during colder months of the year. The cut marks on a number of the cetacean bones studied herein are possible evidence of such exploitation38,86,87. It is also important to note that, along with bone remains being left at butchering sites and thus not entering the archaeological record, cetacean bones have a history of being disregarded during archaeological excavations, particularly those that are highly fragmented. In addition, many of the remains studied herein were not excavated following modern standards or were collected under varying circumstances and thus, a large portion of the cetacean remains at any given site likely went unnoticed. Together, this implies that the cetacean remains that make up the studied collections only represent a small fraction of the actual level of exploitation.
Sambaqui populations were initially perceived as mollusc gatherers88, then later as fishers89 and now also as possible whalers. This represents a paradigm shift in Sambaqui archaeology, one that opens new avenues of research and unlocks diverse interpretative models of social organisation, technology and cosmology that place interactions with large marine mammals at the centre of the equation. The evolution, scale and geographic spread of whale hunting in pre-colonial Brazil, however, remain unknown, as does its eventual decline. Interestingly, the largest accumulations of cetacean remains and the majority of cetacean bone artefacts derive from early sambaqui sites such as Conquista, Morro do Ouro, and Barra do Sul, largely dating between 5700 and 3600 cal BP. More recent sites with cetacean remains, such as Espinheiros II and Bupeva II, which date to 3000 and 500 cal BP, respectively, generally only have unworked cetacean remains and little to no artefacts. Related Indigenous knowledge seems to have vanished sometime prior to European contact, as there appears to be no record of it in European accounts from the 16th century AD90. Unfortunately, much of this unwritten history has been lost due to escalating anthropogenic impacts on sambaquis and other archaeological sites over the last centuries. The limited evidence that survives has reached us thanks to the efforts of those who strived to preserve this invaluable Indigenous cultural heritage. We suspect that there are many more such objects sitting in archive boxes in museums, unseen and untouched for decades, just waiting for their stories to be rediscovered. Our study highlights the crucial role these institutions and their collections play in addressing fundamental questions about the origins and evolving nature of past human coastal adaptations.
Methods
Permissions and permits
Prior to analysis, permits for proteomic analysis and radiocarbon dating of these specimens were obtained from the Instituto do Patrimônio Histórico e Artístico Nacional (IPHAN, Processes 01450.006223/2024-21, 01510.000059/2021-43, 01510.000039/2022-53).
Inclusion and ethics
The study included several Brazilian researchers (A.C.C., T.A.K.S.M., D.B., M.J.C., F.B., T.F. and A.P.K.R.) who contributed to various aspects of the research project. The research is locally relevant and the local institutions involved in the article (MASJ, UNIVILLE) have repeatedly expressed public support. We have a collaboration agreement with MASJ and UNIVILLE and throughout the ERC project TRADITION, we have trained local students and researchers in zooarchaeology (TF), stable isotopes (TF) and ZooMS analysis (TF, TAKSM). These researchers are now applying for grants to establish laboratories in Brazil. The type of study we conducted did not require approval from a local ethics review committee.
Archaeological setting
The estuarine environment of Babitonga Bay and the adjacent coastal region, in the northeast corner of the state of Santa Catarina, is referred to as the Babitonga Ecosystem91. It contains a high concentration of known pre-colonial coastal archaeological sites, with over 200 officially recorded. Of these, approximately 25 sambaquis preserved worked (artefacts) and/or unworked remains of marine mammals. This study analysed cetacean remains from 18 sambaqui sites (Fig. 1) with archaeological materials housed at the Museu Arqueológico de Sambaqui de Joinville (MASJ) in Joinville, Santa Catarina. The sites include Areias Grandes, Areias Pequenas, Barra do Sul, Bupeva II, Conquista, Costeira, Cubatãozinho, Edgar Tiburtius-Praia Grande, Enseada I, Espinheiros II, Harmonia Lyra, Ilha dos Espinheiros II, Itacoara, Linguado I, Morro do Ouro, Pernambuco, Porto do Rei and Rio Pinheiros I. The archaeological material was largely collected by Guilherme Tiburtius from 1940 to 1960 through a combination of controlled excavations and rescue interventions when sites were being dismantled for the lime industry, roads and residential embankments25,92. Many of the sites no longer exist and those that do have been substantially altered; thus, the specific chronological context of some material is limited. Most of the material comes from the Collection Tiburtius; however, some material from other excavations/collections (e.g. harpoons from the Collection Lepper and the Collection Kuhlhof) also housed in MASJ and visually identified as cetacean, were included in the study (Supplementary Data 2). For most of these sites, the faunal remains were handpicked during excavations that did not follow modern standards (such as sediment sieving using different mesh sizes, recovery of all remains, etc.), with a focus on visible specimens (large bones, bone and stone artefacts, human materials). As a result, some of the collections lack the comprehensiveness (including small, fragmented and non-identifiable remains) expected in modern archaeological practice. The cetacean bones analysed herein likely represent only a very small fraction of the total amount of marine mammal bone originally present within the sambaquis. For example, at Conquista, Tiburtius reported (but did not recover) a large number of whale bones, often showing signs of percussion by stone tools, as well as small hearths of piled stones surrounded by partially charred fragments of whale bones, probably the remains of meals24.
Sampling
The Collection Tiburtius at MASJ consists of more than 9000 specimens, while Tiburtius’ notebooks (which unfortunately are not complete and do not include all of his collected items) list over 200 whale bone specimens from pre-colonial sites in Babitonga Bay; however, in some cases, the sites and/or specific contexts are not clear. Initial zooarchaeological analysis was performed to first identify potential cetacean remains, generally avoiding those from unknown archaeological contexts. Marine mammal bones tend to be fairly distinct when compared to those of terrestrial mammals and large fish because of their high porosity. Nevertheless, when dealing with highly fragmented bone assemblages, they can be confused with those of certain large terrestrial mammals; however, such mammals tend to make up a small proportion of the fauna found in sambaquis23,93. Once separated, the potential cetacean remains were classified based on artefact types94 and further taxonomic identifications were assigned when possible through side-by-side comparison with modern specimens of the Iperoba Biological Collection (ABI) at the Universidade da Região de Joinville (UNIVILLE) in São Francisco do Sul, along with specialised literature (Supplementary References). From the preliminary zooarchaeological analysis, 155 samples comprising 85 unworked remains and 70 worked objects were selected for ZooMS analysis using a combination of destructive (unworked) and minimally-invasive (worked and unworked) sampling methods (Supplementary Data 2). Several studies have shown that destructive ZooMS analysis results in a greater success rate when compared to minimally-invasive methods33,35,95, however, the near-complete state of preservation of many of the objects meant physical removal of even small samples was undesirable. Some remains identified anatomically as cetacean were not selected for ZooMS due to either being deemed inappropriate for biomolecular analysis (ie, evidence of burning, obvious presence of consolidation material, etc.) or to avoid unnecessary sampling when successful collagen extraction was unlikely, as was the case for a number of artefacts made from tympanic bullae. These artefacts tended to be highly polished, often having the appearance of ivory and could not be sampled destructively; therefore, sampling was performed using minimally-invasive techniques which rely on the availability of surface collagen. The combination of highly dense and polished bone equates to less available surface collagen, which would have been even further reduced through post-depositional and post-excavation activities. As such, only a subset of these artefacts was selected for ZooMS analysis.
Destructive ZooMS
Subsamples of 15 to 40 mg of bone (taken from areas without obvious consolidant, when evident) were demineralised in 250 µL of 0.6 M hydrochloric acid (HCl, 4 °C). Once demineralised, the acid was discarded and samples were washed three times with 200 µL of 50 mM ammonium bicarbonate (AmBic, NH4HCO3, pH 8.0). A final 100 µL of AmBic was added and the samples were gelatinised at 65 °C for 1 h. 50 µL of the gelatinised supernatant was transferred to a new tube and 0.4 µg of trypsin was added and incubated overnight at 37 °C to digest the collagen. 1 µL of 5% trifluoroacetic acid (TFA) solution was added to stop the trypsin and peptides were desalted using Pierce™ C18 ZipTip® pipette tips (Thermo Scientific™) for a final elution of 50 µL. 1 µL of eluted peptides was combined with 1 µL of matrix solution (α-cyano-4-hydroxycinnamic acid) and spotted in triplicate onto a Bruker ground steel target plate, along with calibration standards, then analysed on a Bruker Ultraflex III MALDI-ToF-MS in reflectron mode. Triplicate spectra were averaged and analysed using mMass (version 5.5.0) software96 and compared to a database of known collagen peptide markers97,98,99.
Minimally-invasive ZooMS
Two different minimally-invasive methods were employed. Initially, the forced bag method33 was used and later, after the (expected) limited success of the forced bag samples, a number of samples were selected for microfilm sampling100, which has been shown to be the most effective minimally-invasive method to date35,95.
Forced Bag ZooMS
The forced bag method was followed as described in McGrath et al.33. Objects were placed into a new, previously unused zip-seal bag and gently rubbed in the bag (from here referred to as the ‘forced bag’). The objects were removed from the forced bag and returned to their original storage bag/box. 200 µL of AmBic (65 °C) was added to the forced bag and spread around the bag surface, where possible, focusing on areas of the bag that had visible residue and then transferred to a new tube. In some instances, very small bone fragments could be seen in bags that had fallen off the object while it was being rubbed in the bag. In these cases, the bone pieces were collected and included in the AmBic. The AmBic was incubated at 65 °C for 20 min and 0.4 µg of trypsin was added, then incubated at 37 °C for 6 hours. 1 µL of 5% TFA solution was added to terminate trypsin activity and peptides were desalted, eluted and analysed via MALDI-ToF-MS as described above.
Microfilm ZooMS
Previously cut pieces of polishing microfilm (30 µm grit, PFL-2RAO-30-PSA, Precision Fibre Products) were gently rubbed on the objects using a clean pair of tweezers and the microfilm was placed into a new tube. Extraction followed that as outlined in refs. 95,100. 100 µL of AmBic (65 °C) was added to the microfilm, then vortexed for several minutes and centrifuged briefly. The supernatant was transferred to a new tube and gelatinised at 65 °C for 30 min then 0.4 µg of trypsin was added and incubated at 37 °C for 6 h to digest the collagen. Trypsin activity was terminated and peptides were desalted, eluted and analysed via MALDI-ToF-MS as described above.
Radiocarbon dating
Many of the studied sites have limited chronological information and lack radiocarbon dates. Due to the uniqueness of the artefacts, sampling for radiocarbon dating was not permitted, with the exception of two whalebone harpoons (see below). Therefore, radiocarbon analyses were performed on a range of other associated material from the sambaqui sites. These included unworked cetacean remains from Areias Pequenas (n = 1), Barra do Sul (n = 2), Costeira (n = 1), Espinheiros II (n = 4), Ilha dos Espinheiros II (n = 2) and Morro do Ouro (n = 2) and human remains from the sites of Areias Pequenas (n = 2), Barra do Sul (n = 1), Conquista (n = 7), Enseada I (n = 5), Espinheiros II (n = 2), Itacoara (n = 8) and Linguado (n = 2). The cetacean bones, and some of the humans selected for radiocarbon dating, were first analysed using ZooMS to rule out possible contamination of animal-derived consolidants that were commonly used in 19th and early 20th-century curation practices101. Permission was granted to sample two broken harpoon foreshaft pieces for dating, one from Morro do Ouro (Col. Tiburtius 4215) and one from Conquista (Col. Tiburtius 8273). These new dates were then supplemented with previously published radiocarbon dates (Supplementary Data 1).
Unworked bones of 12 cetaceans were pretreated and extracted at the Higham Lab, Faculty of Life Sciences, University of Vienna (Austria). The samples were drilled using tungsten carbide drill bits, with samples weighing between 380 and 1540 mg. Collagen was extracted using a modified Longin collagen method outlined in refs. 102,103. The samples were gelatinised in weakly acidic pH3 water and ultrafiltered using 30 kD Sartorius ultrafilters, before being freeze-dried. A 0.3 mg portion of each sample was measured for δ13C and δ15N values using an Elemental Analyser-Isotope Ratio Mass Spectrometer (EA-IRMS) at the Faculty of Life Sciences Silver Laboratory to a precision of ± 0.3‰ relative to V-PDB and AIR, respectively. The remaining collagen was combusted, graphitised and measured at the Keck AMS Facility, University of California at Irvine (USA). Samples were combusted with CuO and silver, after which the CO2 was graphitised on a hydrogen reduction line with pre-baked Fe as a catalyst at 550 °C. Targets were pressed and measured on the AMS system (National Electrostatics Corporation compact 0.5MV accelerator (NEC 1.5SDH-1) with several upgrades). Samples were normalised and corrected for fractionation using the AMS δ13C value. More specific information of target preparation and AMS measurement can be found in refs. 104,105. The fraction modern values were corrected using blanks prepared in Vienna from a beyond radiocarbon background (the Hollis mammoth bone106), which yielded values of 0.0019 and 0.0017 (± 0.0000) FmC, or 50.3 and 51.2 ka BP.
Bone collagen of 17 human samples was extracted and radiocarbon dated at the Centre for Applied Physics, Dating and Diagnostics (CEDAD) at the University of Salento (Italy), following the protocol reported in Quarta et al.107, with collagen extracted following the Login method108, then dried and vacuum-sealed in pre-evacuated quartz tubes together with CuO and silver wool. Samples were converted to CO2 by combustion in sealed quartz tubes and the CO2 was converted at 550 °C into graphite by using ultrahigh purity hydrogen as a reducing medium and 2 mg Fe powder as a catalyst. Samples yielding an optimal amount of graphite were then pressed in the aluminium cathodes of the AMS system (3 MV TandetronTM Mod. HVEE 4130HC) for the measurement of the isotopic ratios. Measured 14C/12C were corrected for mass fractionation by using the δ13C term measured online with the AMS system and for machine and chemical processing background. Uncertainty in measured isotopic ratios was calculated by considering both the scattering of the 10 repeated determinations performed on each sample and the radioisotope counting statistics.
Two cetacean bone harpoon foreshafts and 10 human bones were extracted and radiocarbon dated at the CIRAM Radiocarbon Facility (France). In short, collagen was demineralised and gelatinised following the Login method108, then combusted at 920 °C and transformed into gas using an elemental analyser (Elementar Vario ISOTOPE Select). The residual CO2 from the EA outlet was absorbed in the zeolite trap of an AGE automated graphitisation system (AGE 3, Ion Plus) and released to the given reactor for graphite transformation by catalysis following the method described by Vogel et al.109. The δ13C and δ15N values were measured using an EA-IRMS (Elementar Isoprime precision) with an error below 0.1‰. The different carbon isotopes were separated using a 250 kV accelerator mass spectrometer in a joint venture with JSC Barnas (ISO 9001 and ISO 14001). 14C content was determined by comparing the simultaneously collected 14C, 13C and 12C beams with those of control products (oxalic acid, CO2 standard, charcoal). In all three labs, the dates were reported in radiocarbon years BP (Before Present, 1950 AD, using the half-life of 5568 years).
Radiocarbon calibration
Radiocarbon dates of Delphinidae bone collagen were calibrated using the 100% Marine20 curve110, applying an estimated average local marine radiocarbon reservoir correction value (ΔR) of −126 ± 29 for the coasts of the Brazilian states of São Paulo, Paraná, Santa Catarina and Rio Grande do Sul, generated from eight reference points between latitudes 32.00°S and 23.73°S111,112,113 according to the Marine Reservoir Correction database (http://calib.org/marine/). Because ΔR varies as a function of coastal environments and oceanographic dynamics through time114, we assume that the local ΔR remained relatively stable throughout the period under consideration. This assumption is supported by the fact that the ΔR value applied in this study falls within the range of values recorded for Santa Catarina between approximately 4000–3500 years ago and the present day (–263 ± 46, –244 ± 53, –205 ± 80, –114 ± 25)115,116. However, we acknowledge that the available ΔR values for the Brazilian coast were obtained through pairwise comparisons between marine shells and terrestrial plant materials, and uncertainties remain as to whether these corrections can be reliably applied to fish and marine mammals, which feed across distinct ecological niches.
The radiocarbon dates of the human bone collagen samples were modelled using a mixed curve (SHCal20117 and Marine20) to take into account the variable intake of marine resources by these populations. As observed by others31,63,118,119, the δ13C and δ15N values of human bone collagen reflect three main dietary protein sources: marine resources, terrestrial C3 animals and terrestrial C3 plants (Supplementary Fig. 1). Based on the distribution of δ13C values and assuming that the highest (–11.22‰ and –11.74‰) and lowest (–22.80‰ and –22.60‰) values correspond to 100% and 0% marine protein intake respectively (here assumed to be the equivalent of the contribution of marine carbon to collagen), a linear equation was obtained to estimate the percentage contribution of marine protein to the diet of each individual analysed in this study (Marine protein (%) = 89,034δ13C + 202.14). Although this represents a relatively simple model, it allows for individual-level estimations rather than relying on generalised dietary assumptions. To account for uncertainties in this estimation, we applied a deviation of ±9% as reported by Toso et al.31. For one individual with no stable isotope data published by Colonese et al.120, the contribution of marine carbon to collagen was assumed to be 52 ± 9% as proposed by Toso et al.31. For samples analysed by others, we used the published estimates of marine carbon contribution to collagen reported in their studies64. The modelled radiocarbon dates used the same local ΔR value of −126 ± 29, as applied to the Delphinidae bone collagen.
Southern Hemisphere humpback whales feed primarily on krill (Euphausia superba) in Antarctic waters before migrating to lower latitude breeding regions121. Bone collagen δ13C values of samples herein analysed ranged from –22.39‰ to –19.39‰ after correcting for trophic fractionation (–3.11‰)122, matching values reported for krill collected between the western Antarctic Peninsula121 and the southern Patagonian Shelf123. Radiocarbon dates of humpback whale bone collagen were thus calibrated using the 100% Marine20 curve and the average radiocarbon reservoir correction value (ΔR) of 443 ± 135 for the western Antarctic Peninsula124. Southern right whales feed on pelagic copepods and krill across the Southern Hemisphere125,126. Bone collagen δ13C values herein ranged from −18.62‰ to −17.05‰ after correcting for trophic fractionation, reflecting food sources in the Patagonian shelf123. Radiocarbon dates of southern right whale bone collagen were therefore calibrated using the 100% Marine20 curve and the average radiocarbon reservoir correction value (ΔR) of 448 ± 33, obtained from 10 open sea reference points along the Argentinean Patagonia coast127. Calibrations were performed in OxCal 4.4 online128,129 and radiocarbon dates were rounded to 10 years. Radiocarbon results are presented in Supplementary Data 1 and plots of reservoir-corrected calibrated radiocarbon ages for human and marine mammal bones are reported in Supplementary Fig. 2.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files). MALDI spectra have been deposited in Zenodo: https://doi.org/10.5281/zenodo.15024595. All analysed samples, including those used for radiocarbon dating, are curated at the Museu Arqueológico de Sambaqui de Joinville (MASJ). Fernanda Mara Borba serves as the main curator and is a co-author of this manuscript.
References
Reeves, R. R. The origins and character of ‘aboriginal subsistence’ whaling: a global review. Mamm. Rev. 32, 71–106 (2002).
McGrath, K. et al. Late Paleolithic whale bone tools reveal human and whale ecology in the Bay of Biscay. Nat. Commun. 16, 4646 (2025).
Kang, B. W. Reexamination of the chronology of the bangudae petroglyphs and whaling in prehistoric Korea: a different perspective. J. Anthropol. Res. 76, 480–506 (2020).
Janik, D. L. Visual narratives and the depiction of whaling in north European rock art: the case of the White Sea. Nouv. l archéol. 166, 51–63 (2022).
Bland, R. Another look at the Pegtymel’ petroglyphs. Arct. Anthropol. 47, 22–31 (2011).
Helskog, K. Landscapes in rock-art: rock-carving and ritual in the old European North. TheFigured Landscapes Of Rock Art. Looking At Pictures In Place. 265–288 (Cambridge University Press, 2004).
Gjerde, J. M. Stone Age rock art and beluga landscapes at River Vyg, north-western Russia. Fennosc. Archaeol. 30, 37–54 (2013).
Whitridge, P. The prehistory of Inuit and Yupik whale use. Rev. Arqueol. Am. 16, 99–154 (1999).
Monks, G. G., McMillan, A. & St. Claire, D. E. Nuu-chah-nulth whaling: archaeological insights into antiquity, species preferences, and cultural importance. Arct. Anthropol. 38, 60–81 (2001).
Huelsbeck, D. R. Whaling in the precontact economy of the central northwest coast. Arct. Anthropol. 25, 1–15 (1988).
Dikov, N. Earliest sea mammal hunters of Wrangell Island. Arct. Anthropol. 25, 80–93 (1988).
McCartney, A. History of native whaling in the Arctic and subarctic. In Proc. International Symposium Arctic Whaling February 1983 (eds. Jacob, H.K.S., Snoeijing, K., Vaughan, R.) 79–112 (University of Groningen Press, 1984).
Fitzhugh, B. The Origins and Development of Arctic Maritime Adaptations in the Subarctic and Arctic Pacific. (Oxford University Press, 2016).
Subsistence Whaling: Past History and Contemporary Issues. (Springer Nature, 2025).
Lessa, A., Silveira dos Santos, C. & Paulino da Silva, O. Sítio Arqueológico Rua do Papagaio: Uma ocupação diferenciada e muito antiga no litoral sul do Brasil. Rev. Arqueol. 37, 56–83 (2024).
de Castilho, P. V. Utilization of cetaceans in shell mounds from the southern coast of Brazil. Quat. Int. 180, 107–114 (2008).
Mendes, A. B. & Rodrigues, T. Tetrapod biodiversity in sambaquis from southern Brazil. Acad. Bras. Cienc. 96, e20230901 (2024).
Cunha, F. L. et al. Ocorrência de vertebrados holocênicos marinhos, Elasmobranchii e Cetacea, no ‘Sambaqui de Camboinhas’, Itaipu, Niterói, estado do Rio de Janeiro. Rev. Arqueol. 3, 52–56 (1986).
Chmyz, I., Sganzerla, E. M. & Chmyz, J. C. G. Novas contribuições para o estudo do Sambaqui de Matinhos, no Estado do Paraná. Arqueol. N.úmero Espec. 1, 1–55 (2003).
Gaspar, M. D. Considerations of the sambaquis of the Brazilian coast. Antiquity 72, 592–615 (1998).
Prous, A. Arqueologia Brasileira. (Universidade de Brasília, 1991).
Toniolo, T. et al. Sea-level fall and coastal water cooling during the Late Holocene in Southeastern Brazil based on vermetid bioconstructions. Mar. Geol. 428, 106281 (2020).
Fossile, T., Ferreira, J. & Colonese, A. C. Brazilian Zooarch Database: banco de dados da fauna arqueológica do Brasil. Rev. Arqueol. 36, 311–331 (2023).
Tiburtius, G. O Sambaqui Conquista (NR. 9). Bol. Parana. Geogr. 18, 71–126 (1966).
Tiburtius, G. Arquivos de Guilherme Tiburtius I. (Fundação Cultural de Joinville. Joinville: Museu Arqueológico de Sambaqui de Joinville, 1996).
Rorh, P. J. A. & Andreatta, M. D. O. Sítio arqueológico da Armação do Sul (nota prévia). Pesqui. Antropol. 20, 135–138 (1969).
Rorh, P. J. A. Pesquisas paleo-etnográficas na Ilha de Santa Catarina e sambaquis do litoral sul-catarinense—IV (1961). Pesqui. Antropol. 14, 5–27 (1962).
Tiburtius, G. & Bigarella, I. K. Objetos Zoomorfos Do Litoral De Santa Catarina E Paraná. 7 (Instituto Anchietano de Pesquisas, 1960).
Tiburtius, G., Leprevost, A. & Bigarella, J. J. Sobre a ocorrência de bula timpânica de baleia e artefatos derivados nos sambaquis dos estados do Paraná e Santa Catarina. Arq. Biol. Tecnol. IV, 88–102 (1949).
Tiburtius, G. Schmuckgegenstände aus den Muschelbergen von Paraná und Santa Catarina, Südbrasilien. 6 (Pesquisas, Antropologia, 1960).
Toso, A. et al. Fishing intensification as response to Late Holocene socio-ecological instability in southeastern South America. Sci. Rep. 11, 23506 (2021).
Fossile, T. et al. Bridging archaeology and marine conservation in the Neotropics. PLoS ONE 18, e0285951 (2023).
McGrath, K. et al. Identifying archaeological bone via non-destructive zooms and the materiality of symbolic expression: examples from Iroquoian bone points. Sci. Rep. 9, 11027 (2019).
Coutu, A. N. et al. Palaeoproteomics confirm earliest domesticated sheep in southern Africa ca. 2000 BP. Sci. Rep. 11, 6631 (2021).
Hansen, J. et al. A comparative study of commercially available, minimally invasive, sampling methods on Early Neolithic humeri analysed via palaeoproteomics. J. Archaeol. Sci. 167, 106002 (2024).
Ekdale, E. G., Berta, A. & Deméré, T. A. The comparative osteology of the petrotympanic complex (ear region) of extant baleen whales (Cetacea: Mysticeti). PLoS ONE 6, e21311 (2011).
Jefferson, T. A., Webber, M. A. & Pitman, R. L. Marine Mammals of the World: A Comprehensive Guide to Their Identification. (Academic Press, 2015).
Monks, G. G. Quit blubbering: an examination of Nuu’chah’nulth (Nootkan) whale butchery. Int. J. Osteoarchaeol. 11, 136–149 (2001).
Mason, O. T. Aboriginal American Harpoons: A Study in Ethnic Distribution and Invention. Report of U.S. National Museum, (US Government Printing Office, 1902).
Driver, H. E. & Massey, W. C. Comparative Studies of North American Indians. Tran. Am. Philos. Soc. 47, 165–456 (1957).
Bennyhoff, J. A. Californian fish spears and harpoons. Anthropol. Rec. 9, 295–337 (1950).
Arnold, C. D. Arctic harpoons. Arct. Profiles 42, 80–81 (1989).
Ballester R., B. Tecnología de arponaje en la costa del desierto de Atacama, norte de Chile. Estud. Atacameños. 57, 65–950 (2018).
Gallardo, F., Ballester, B. & Calás, E. Caza de grandes presas marinas en la costa de Antofagasta y los canales de Tierra del Fuego. Rev. Arqueol. Am. 293, 327 (2022).
Beck, A. A Variação Do Conteúdo Cultural Dos Sambaquis: Litoral De Santa Catarina. (Universidade de São Paulo, 1972).
Marsh, E. J., Llano, C., Cortegoso, V., Castro, S. & Yebra, L. The bow and arrow in South America. J. Anthropol. Archaeol. 69, 101471 (2023).
Meyer, H. Bows and arrows in Central Brazil. Annual Report of the Smithsonian Institution 1896. 549–590 (1898).
Métraux, A. Weapons. In Handbook of South American Indians (ed. Steward, J) 229–263 (Smithsonian, 1949).
Johnson, L. M. Revisiting the origins of northwest coast packstraps. Mus. Anthropol. 36, 128–146 (2013).
Uchôa, D. P. Arqueologia de Piaçaguera e Tenório: Análise de Dois Tipos de Sítios Pré-Cerâmicos do Litoral Paulista. (Habilis, 2007).
Menezes, M. J. Notas parciais sobre as pesquisas realizadas no litoral do Paraná. Pesqui. Antropol. 9, 53–64 (1968).
Rauth, J. W. O. Sambaqui do Gomes, S-11-B. - Paraná - Brasil. Arqueologia 4, 1–99 (1968).
Orssich, A. O sambaqui do Arújo II, nota prévia. Cad. Arqueol. Mus. Arqueol. Artes. Pop. 2, 1–76 (1977).
Rauth, J. W. Nota prévia sobre o sambaqui do Ramal. Mus. Para. Emílio Goeldi 4, 115–128 (1971).
Rohr, J. A. O Sítio Arqueológico do Pântano do Sul SC-F-10. (Secretaria da Educação e Cultura, 1977).
McCartney, A. The nature of Thule Eskimo whale use. Arctic 33, 517–541 (1980).
McMillan, A. Whales and Whalers in Nuu-chah-nulth Archaeology. BC Studies: B.C.Q. https://doi.org/10.14288/BCS.V0I187.186163 (2015).
Charpentier, A. et al. What’s in a whale bone? Combining new analytical methods, ecology and history to shed light on ancient human-whale interactions. Quat. Sci. Rev. 284, 107470 (2022).
Wellman, H. P. et al. Native American use of cetaceans in pre-contact Oregon: biomolecular and taphonomic analyses illuminate human–cetacean relationships. J. Isl. Coast. Archaeol. 20, 1–24 (2024).
Castilho, P. V. de & Lopes, P. C. S. Sea mammals in archaeological sites on the southern coast of Brazil. Rev. Mus. Arqueol. Etnol. 18, 101–113 (2008).
Fish, P. R., Fish, S. K., DeBlasis, P. & Gaspar, M. D. Monumental shell mounds as persistent places in southern coastal Brazil. Archaeol. Hist. Ecol. Small-Scale Econ. 120–140 (University Press of Florida, 2013).
Klokler, D. Zooarchaeology of Brazilian shell-mounds. In The Oxford Handbook of Zooarchaeology (eds. Albarella, U., Rizzetto, M., Russ, H., Vickers, K. & Viner-Daniels, S) (Oxford University Press, 2017).
Colonese, A. C. et al. Long-term resilience of late Holocene coastal subsistence system in Southeastern South America. PLoS ONE 9, e93854 (2014).
Pezo-Lanfranco, L. et al. Middle Holocene plant cultivation on the Atlantic Forest coast of Brazil? R. Soc. Open Sci. 5, 180432 (2018).
Orquera, L. A. Advances in the archaeology of the Pampa and Patagonia. J. World Prehist. 1, 333–413 (1987).
Pires Renault-Braga, E., Rejane Groch, K., de Carvalho Flores, P. A., Secchi, E. R. & Dalla-Rosa, L. Area usage estimation and spatiotemporal variability in distribution patterns of southern right whales, Eubalaena australis, of southern Brazil. Mar. Ecol. 39, e12506 (2018).
Nowacek, D. P. et al. Buoyant balaenids: the ups and downs of buoyancy in right whales. Proc. Biol. Sci. 268, 1811–1816 (2001).
Morais, I. O. B. et al. From the southern right whale hunting decline to the humpback whaling expansion: a review of whale catch records in the tropical western South Atlantic Ocean. Mamm. Rev. 47, 11–23 (2017).
Ellis, M. A Baleia No Brasil Colonial: Feitorias, Baleeiros, Técnicas, Monopólio, Comércio, Iluminação. (Edições Melhoramentos, 1969).
B. Greig et al. Stranding events of southern right whales, Eubalaena australis, in southern Brazil. J. Cetacean Res. Manag. 2, 157–160 (2020).
Zerbini, A. N. et al. Satellite-monitored movements of humpback whales Megaptera novaeangliae in the Southwest Atlantic Ocean. Mar. Ecol. Prog. Ser. 313, 295–304 (2006).
Landine, A., Zerbini, A. N., Danilewicz, D., Sucunza, F. & Andriolo, A. Humpback whales in the Southwest Atlantic Ocean: investigating their breeding movements by satellite tracking. Mar. Mamm. Sci. https://doi.org/10.1111/mms.13146 (2024).
Wedekin, L. L. et al. Running fast in the slow lane: rapid population growth of humpback whales after exploitation. Mar. Ecol. Prog. Ser. 575, 195–206 (2017).
Siciliano, S., Cardoso, J., Francisco, A. & Moreira, S. C. A STOP FOR A SNACK: apparent humpback whale (Megaptera novaeangliae) feeding behavior and association with gillnets during migration off south-eastern Brazil. Bol. Lab. Hidrobiol. 29, 41–49 (2019).
Milmann, L. et al. Overview of Balaenoptera whale strandings in Southern Brazil from 1993 to 2018. Lat. Am. J. Aquat. Mamm. 18, 186–195 (2023).
Zerbini, A. N., Secchi, E. R., Siciliano, S. & Simões-Lopes, P. C. A review of the occurrence and distribution of whales of the genus Balaenoptera along the Brazilian coast. Rep. Int. Whal. Comm. 47, 407–417 (1997).
Cremer, M. J. et al. Behavior and ecology of endangered species living together: long-term monitoring of resident sympatric dolphin populations. In Advances in Marine Vertebrate Research in Latin America 477–508 (Springer International Publishing, 2018).
Cote, C. Spirits of Our Whaling Ancestors (University of Washington Press, 2017).
Ballester, B. La caza de cetáceos en la costa del desierto de Atacama: relatos escritos, pinturas rupestres, artefactos y restos óseos. In Baleeiros do Sul II, Antropologia e História da Indústria Baleeira nas Costas Sul-Americanas (eds. Castellucci, W. & Quiroz, D.) 59–84 (Editora da Universidade do Estado da Bahia, 2018).
Worth, J. E. Discovering Florida: First-Contact Narratives from Spanish Expeditions along the Lower Gulf Coast (University Press of Florida, 2014).
Smith, A. & Kinahan, J. The invisible whale. World Archaeol. 16, 89–97 (1984).
Ballester, B. El Médano rock art style: Izcuña paintings and the marine hunter-gatherers of the Atacama Desert. Antiquity 92, 132–148 (2018).
Bloch, D., Dam, M. & Hanusardóttir, M. MARINE FOODS, marine mammals as meat sources. Encyclopedia of Food Sciences and Nutrition 3733–3739 (Elsevier, 2003).
Savelle, J. M. The role of architectural utility in the formation of zooarchaeological whale bone assemblages. J. Archaeol. Sci. 24, 869–885 (1997).
Higgs, N. D., Little, C. T. S. & Glover, A. G. Bones as biofuel: a review of whale bone composition with implications for deep-sea biology and palaeoanthropology. Proc. R. Soc. B. 278, 9–17 (2011).
Monks, G. An oil utility index for whale bones. In The Exploitation and Cultural Importance of Sea Mammals (ed. Monks, G) 138–153 (Oxbow Books, 2005).
Monks, G. G. The cultural taphonomy of Nuu-chah-nulth whale bone assemblages. In (ed. Matson, R.G., Coupland, G., Mackie, Q) Emerging from the Mist: Studies in Northwest Coast Culture History 188–212 (University of British Columbia Press, 2003).
Schmitz, P. I. Prehistoric hunters and gatherers of Brazil. J. World Prehist. 1, 53–126 (1987).
Figuti, L. O. Homem pré-histórico, o molusco e os sambaquis: considerações sobre a subsistência dos povos sambaquieiros. Rev. Mus. Arqueol. Etnol. 3, 67–80 (1993).
Vieira, N. A comparative approach to historical whaling techniques: Transfer of knowledge in the 17th century from the Biscay to Brazil. In (eds. Polónia, A., Bracht, F., Conceição, G. C. & Palma, M.) 125–143 (CITCEM – Centro de Investigação Transdisciplinar Cultura, Espaço e Memória, Porto, 2016).
Gerhardinger, L. C. et al. Diagnóstico socioambiental do Ecossistema Babitonga. Rev. CEPSUL 10, e2021002. Biodiversidade e Conservação Marinha: Rev. CEPSUL (2021).
Bigarella, J. J. Sambaquis (Posigraf, 2011).
Fossile, T. et al. Integrating zooarchaeology in the conservation of coastal-marine ecosystems in Brazil. Quat. Int. https://doi.org/10.1016/j.quaint.2019.04.022 (2019).
Fossari, T. D. Indústria óssea na arqueologia brasileira: estudo piloto do material de Enseada-SC e Tenório-SP (University of São Paulo, 1985).
Evans, Z., Paskulin, L., Rahemtulla, F. & Speller, C. F. A comparison of minimally invasive sampling techniques for ZooMS analysis of bone artifacts. J. Archaeol. Sci. Rep. 47, 103738 (2023).
Strohalm, M., Hassman, M., Kosata, B. & Kodícek, M. Mass data miner: an open source alternative for mass spectrometric data analysis. Rapid Commun. Mass Spectrom. 22, 905–908 (2008).
Kirby, D. P., Buckley, M., Promise, E., Trauger, S. A. & Holdcraft, T. R. Identification of collagen-based materials in cultural heritage. Analyst 138, 4849–4858 (2013).
Buckley, M. et al. Species identification of archaeological marine mammals using collagen fingerprinting. J. Archaeol. Sci. 41, 631–641 (2014).
Welker, F., Soressi, M., Rendu, W., Hublin, J.-J. & Collins, M. Using ZooMS to identify fragmentary bone from the Late Middle/Early Upper Palaeolithic sequence of Les Cottés, France. J. Archaeol. Sci. 54, 279–286 (2015).
Kirby, D. P., Manick, A. & Newman, R. Minimally invasive sampling of surface coatings for protein identification by peptide mass fingerprinting: a case study with photographs. J. Am. Inst. Conserv. 59, 235–245 (2020).
van der Sluis, L. G. et al. Identification and tentative removal of collagen glue in Palaeolithic worked bone objects: implications for ZooMS and radiocarbon dating. Sci. Rep. 13, 22119 (2023).
Higham, T. F. G., Jacobi, R. M. & Ramsey, C. B. AMS radiocarbon dating of ancient bone using ultrafiltration. Radiocarbon 48, 179–195 (2006).
Brock, F., Higham, T., Ditchfield, P. & Ramsey, C. B. Current pretreatment methods for AMS radiocarbon dating at the Oxford Radiocarbon Accelerator Unit (Orau). Radiocarbon 52, 103–112 (2010).
Santos, G. M. et al. AMS 14C sample preparation at the KCCAMS/UCI facility: Status report and performance of small samples. Radiocarbon 49, 255–269 (2007).
Beverly, R. K. et al. The Keck carbon cycle AMS laboratory, University of California, Irvine: status report. Radiocarbon 52, 301–309 (2010).
Martínez, D.E.L. et al. Permafrost-preserved wood and bone: radiocarbon blanks from Yukon and Alaska. Nucl. Instrum. Methods Phys. Res. B 455, 154–157 (2019).
Quarta, G. et al. 14C Intercomparison exercise on bones and ivory samples: implications for forensics. Radiocarbon 63, 533–544 (2021).
Longin, R. New method of collagen extraction for radiocarbon dating. Nature 230, 241–242 (1971).
Vogel, J. S., Southon, J. R., Nelson, D. E. & Brown, T. A. Performance of catalytically condensed carbon for use in accelerator mass spectrometry. Nucl. Instrum. Methods Phys. Res. B 5, 289–293 (1984).
Heaton, T. J. et al. Marine20—the marine radiocarbon age calibration curve (0–55,000 cal BP). Radiocarbon 62, 779–820 (2020).
Alves, E. et al. Radiocarbon reservoir corrections on the Brazilian coast from pre-bomb marine shells. Quat. Geochronol. 29, 30–35 (2015).
Angulo, R. J., de Souza, M. C., Reimer, P. J. & Sasaoka, S. K. Reservoir effect of the southern and southeastern Brazilian coast. Radiocarbon 47, 67–73 (2008).
De Masi, M. A. N. Prehistoric Hunter-Gatherer Mobility on the Southern Brazilian Coast: Santa Catarina Island. Unpublished Ph.D. dissertation (Stanford University, 1999).
Macario, K. D. et al. The variable nature of the coastal 14C marine reservoir effect: a temporal perspective for Rio de Janeiro. Quat. Sci. Adv. 11, 100086 (2023).
Alves, E. Q. et al. Assessing the 14C marine reservoir effect in archaeological contexts: data from the Cabeçuda shell mound in Southern Brazil. Radiocarbon 65, 1–27 (2023).
Alves, E. Q. et al. Nineteenth-century expeditions and the radiocarbon marine reservoir effect on the Brazilian coast. Geochim. Cosmochim. Acta 297, 276–287 (2021).
Hogg, A. G. et al. SHCal20 southern hemisphere calibration, 0–55,000 Years cal BP. Radiocarbon 62, 759–778 (2020).
Bastos, M. Q. et al. Elucidating pre-Columbian tropical coastal adaptation through bone collagen stable isotope analysis and Bayesian mixing models: insights from sambaqui do moa (Brazil). Ramer 7, 1–10 (2022).
Bastos, M. Q. R., Lessa, A., Rodrigues-Carvalho, C., Tykot, R. H. & Santos, R. V. Carbon and nitrogen isotope analysis: diet before and after the arrival of ceramics at Forte Marechal Luz Site. Rev. Mus. Arqueol. Etnol. 0, 137–151 (2014).
Colonese, A. C. et al. New insights into the formation process and chronology of the sambaqui Morro do Ouro (Joinville, Santa Catarina, Brazil). Bol. Mus. Para. Emílio Goeldi. Ciênc. Hum. 20, e20240031 (2025).
Groß, J. et al. No distinct local cuisines among humpback whales: a population diet comparison in the Southern Hemisphere. Sci. Total Environ. 931, 172939 (2024).
Borrell, A., Abad-Oliva, N., Gómez-Campos, E., Giménez, J. & Aguilar, A. Discrimination of stable isotopes in fin whale tissues and application to diet assessment in cetaceans: diet-tissue discrimination factors in fin whale. Rapid Commun. Mass Spectrom. 26, 1596–1602 (2012).
Valenzuela, L. O., Rowntree, V. J., Sironi, M. & Seger, J. Stable isotopes (δ15N, δ13C, δ34S) in skin reveal diverse food sources used by southern right whales Eubalaena Australis. Mar. Ecol. Prog. Ser. 603, 243–255 (2018).
Divola, C. et al. Constraining the radiocarbon reservoir age for the Southern Ocean using whale bones salvaged from early 20th-century whaling stations. Quat. Sci. Rev. 336, 108756 (2024).
Valenzuela, L. O., Sironi, M., Rowntree, V. J. & Seger, J. Isotopic and genetic evidence for culturally inherited site fidelity to feeding grounds in southern right whales (Eubalaena australis). Mol. Ecol. 18, 782–791 (2009).
D’Agostino, V. C. et al. Foraging dives of southern right whales (Eubalaena australis) in relation to larger zooplankton size prey availability in Golfo Nuevo, Península Valdés, Argentina. Sci. Rep. 14, 14211 (2024).
Cordero, R. R., Panarello, H., Lanzelotti, S. & Dubois, C. M. F. Radiocarbon age offsets between living organisms from the marine and continental reservoir in coastal localities of Patagonia (Argentina). Radiocarbon 45, 9–15 (2003).
Ramsey, C. B. & Lee, S. Recent and planned developments of the program OxCal. Radiocarbon 55, 720–730 (2013).
Ramsey, C. B. Radiocarbon calibration and analysis of stratigraphy: the OxCal program. Radiocarbon 37, 425–430 (1995).
Acknowledgements
We thank the York Centre for Excellence in Mass Spectrometry (University of York) and the Laboratori de Proteòmica CSIC (Universidad Autònoma de Barcelona) for allowing access to their respective MALDI-TOF-MS. This work was funded by the ERC Consolidator project TRADITION, which has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No 817911 (A.C.C.). This work contributes to the ICTA-UAB ‘María de Maeztu’ Programme for Units of Excellence of the Spanish Ministry of Science, Innovation and Universities (CEX2024-001506-M funded by MICIU/AEI/10.13039/501100011033, A.C.C.) and EarlyFoods (SGR-Cat 2021, 00527, A.C.C)., which has received funding from the Agència de Gestió d’Ajuts Universitaris i de Recerca de Catalunya. This grant is also supported by the Productivity grant (PQ314957/2023-5) of Brazil’s Conselho Nacional de Desenvolvimento Científico e Tecnológico (M.J.C.).
Author information
Authors and Affiliations
Contributions
K.M., T.A.K.S.M., A.C.C., D.B. and M.J.C. conceived and designed the work; K.M., T.A.K.S.M., A.C.C., L.G.S. and T.H. acquired and analysed the data; K.M., T.A.K.S.M., A.C.C. and M.J.C. interpreted the data; K.M., T.A.K.S.M., A.C.C., M.J.C., F.M.B., T.F., A.P.K.R. and M.S. drafted the work. All the authors approved the submitted version and agreed to be personally accountable for their own contributions and to ensure accuracy and integrity of all parts of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Bente Philippsen and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
McGrath, K., Montes, T.A.K.d.S., Fossile, T. et al. Molecular and zooarchaeological identification of 5000 year old whale-bone harpoons in coastal Brazil. Nat Commun 17, 48 (2026). https://doi.org/10.1038/s41467-025-67530-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-67530-w