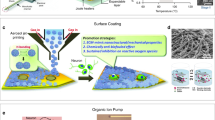
Abstract
The implantable neural probe for simultaneous recording of various brain signals is one of the key technologies for neurological science and clinics that is yet to be broken through. Here, we introduce an implantable neural probe with integrated carbon nanotube field-effect transistors which is able to perform multimodal recording of electrical and chemical signals of the brain under magnetic resonance imaging (MRI). We demonstrate here a simultaneous measurement of an electrophysiological signal with high signal-to-noise ratio up to 40.34 dB and calcium concentration with a detection limit down to 0.47 nM. We use our neural probes to detect neural activity in rats and results reveal that changes in Ca²⁺ concentration occur concurrently with the epileptiform local field potential events, providing an alternative method for accurate detection of epilepsy. Our work may provide a powerful means for the future studies of brain and holds great potential for practical diagnostic applications.
Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its supplementary files. Any additional requests for information can be directed to, and will be fulfilled by, the corresponding authors. Source data are provided with this paper.
Code availability
The custom code used in this study is available on Zenodo under the https://doi.org/10.5281/zenodo.17547948 (2025)55.
References
Babiloni, C. et al. Free copper and resting temporal EEG rhythms correlate across healthy, mild cognitive impairment, and Alzheimer’s disease subjects. Clin. Neurophysiol. 118, 1244–1260 (2007).
Veliev, F. Interfacing neurons with nanoelectronics: from silicon nanowires to carbon devices (v1, Materials. University Grenoble Alpes, 2016).
Williams, K. R. et al. Progress of graphene devices for electrochemical biosensing in electrically excitable cells. Prog. Biomed. Eng. 3, 022003 (2021).
Hébert, C. et al. Flexible graphene solution-gated field-effect transistors: efficient transducers for micro-electrocorticography. Adv. Funct. Mater. 28, 1703976 (2018).
Coles, L. et al. Origami-inspired soft fluidic actuation for minimally invasive large-area electrocorticography. Nat. Commun. 15, 6290 (2024).
Guan, S. et al. Self-assembled ultraflexible probes for long-term neural recordings and neuromodulation. Nat. Protoc. 18, 1712–1744 (2023).
Lee, K. et al. Flexible, scalable, high channel count stereo-electrode for recording in the human brain. Nat. Commun. 15, 218 (2024).
Bonaccini, C. A. et al. Full-bandwidth electrophysiology of seizures and epileptiform activity enabled by flexible graphene microtransistor depth neural probes. Nat. Nanotechnol. 17, 301–309 (2022).
Masvidal-Codina, E. et al. High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors. Nat. Mater. 18, 280–288 (2019).
Zhao, S. et al. Graphene encapsulated copper microwires as highly MRI compatible neural electrodes. Nano Lett. 16, 7731–7738 (2016).
Lu, L. et al. Soft and MRI compatible neural electrodes from carbon nanotube fibers. Nano Lett. 19, 1577–1586 (2019).
Nawaz, A. et al. Organic electrochemical transistors for in vivo bioelectronics. Adv. Mater. 33, 2101874 (2021).
Liang, Y. et al. PEDOT: PSS-based bioelectronic devices for recording and modulation of electrophysiological and biochemical cell signals. Adv. Healthc. Mater. 10, 2100061 (2021).
Liu, L. et al. Aligned, high-density semiconducting carbon nanotube arrays for high-performance electronics. Science 368, 850–856 (2020).
Zhao, S. et al. Full activation pattern mapping by simultaneous deep brain stimulation and fMRI with graphene fiber electrodes. Nat. Commun. 11, 1788 (2020).
Gao, Z. H. Advances in surface-coated single-walled carbon nanotubes as near-infrared photoluminescence emitters for single-particle tracking applications in biological environments. Polym. J. 50, 589–601 (2018).
Liu, L. et al. Carbon nanotube complementary gigahertz integrated circuits and their applications on wireless sensor interface systems. ACS Nano 13, 2526–2535 (2019).
Liang, Y. et al. Wafer-scale uniform carbon nanotube transistors for ultrasensitive and label-free detection of disease biomarkers. ACS Nano 14, 8866–8874 (2020).
Guo, Y. et al. Biocompatibility and magnetic resonance imaging characteristics of carbon nanotube yarn neural electrodes in a rat model. Biomed. Eng. Online 14, 118 (2015).
Abidian, M. R. & Martin, D. C. Multifunctional nanobiomaterials for neural interfaces. Adv. Funct. Mater. 19, 573–585 (2009).
Keefer, E. W. et al. Carbon nanotube coating improves neuronal recordings. Nat. Nanotechnol. 3, 434–439 (2008).
Gupta, A. et al. Biocompatibility of single-walled carbon nanotube composites for bone regeneration. Bone Jt. Res. 4, 70–77 (2015).
Aoki, K. & Saito, N. Biocompatibility and carcinogenicity of carbon nanotubes as biomaterials. Nanomaterials 10, 264 (2020).
Girven, K. S. & Sparta, D. R. Probing deep brain circuitry: new advances in in vivo calcium measurement strategies. ACS Chem. Neurosci. 8, 243–251 (2017).
Xiao, J. et al. Implantable probe with integrated reference electrode for in situ neural signal and calcium ion monitoring. Biodes. Manuf. 4, 591–595 (2024).
Meyer, J. C. et al. Raman modes of index-identified freestanding single-walled carbon nanotubes. Phys. Rev. Lett. 95, 217401 (2005).
Paillet, M. et al. Raman active phonons of identified semiconducting single-walled carbon nanotubes. Phys. Rev. Lett. 96, 257401 (2006).
Li, T. et al. Functionalized carbon nanotube field-effect transistor biosensor for highly sensitive detection of exosomal protein. Anal. Chim. Acta 1273, 341511 (2023).
Saito, R., Dresselhaus, G. & Dresselhaus, M. S. Phonon trigonal warping effect in graphite and carbon nanotubes. Phys. Rev. Lett. 61, 2981 (2000).
Cruttenden, C. E. et al. Carbon nano-structured neural probes show promise for magnetic resonance imaging applications. Biomed. Phys. Eng. Express 4, 015001 (2017).
Joshi, A. & Datar, S. Carbon nanostructure composite for electromagnetic interference shielding. Pramana 84, 1099–1116 (2015).
Xiao, M. & Zhang, Z. Wafer-scale biologically sensitive carbon nanotube transistors: from fabrication to clinical applications. Tech. Dig. Int. Electron Devices Meet. 17, 31–34 (2022).
Brosel-Oliu, S. et al. Single-step functionalization strategy of graphene microtransistor array with chemically modified aptamers for biosensing applications. Small 20, 2308857 (2024).
Garcia-Cortadella, R. et al. Graphene active sensor arrays for long-term and wireless mapping of wide frequency band epicortical brain activity. Nat. commun. 12, 211 (2021).
Heinemann, U. et al. Extracellular calcium and potassium concentration changes in chronic epileptic brain tissue. Adv. Neuro. 44, 641–661 (1986).
Nicholson, C. & Eva, S. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21, 207–215 (1998).
Raza, M. et al. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc. Natl. Acad. Sci. Usa. 101, 17522–17527 (2004).
Zhao, C. et al. Implantable aptamer–field-effect transistor neuroprobes for in vivo neurotransmitter monitoring. Sci. Adv. 7, eabj7422 (2021).
Yang, H. et al. Carbon nanotube array-based flexible multifunctional electrodes to record electrophysiology and ions on the cerebral cortex in real time. Adv. Funct. Mater. 32, 2204794 (2022).
Hasan, N. et al. Ion-selective membrane-coated graphene–hexagonal boron nitride heterostructures for field-effect ion sensing. ACS Omega 6, 30281–30291 (2021).
Khodagholy, D. et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 4, 1575 (2013).
Rivnay, J. et al. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 3, e1601649 (2017).
Ziemann, A. et al. Seizure termination by acidosis depends on ASIC1a. Nat. Neurosci. 11, 816–822 (2008).
Zhao, F. et al. An electrochemophysiological microarray for real-time monitoring and quantification of multiple ions in the brain of a freely moving rat. Angew. Chem. 132, 10512–10516 (2020).
Raimondo, J. V. et al. Ion dynamics during seizures. Front. Cell. Neurosci. 9, 419 (2015).
Allen, P. J. & Lemieux, L. Identification of EEG events in the MR scanner: the problem of pulse artifact and a method for its subtraction. NeuroImage 8, 229–239 (1998).
Allen, P. J. et al. A method for removing imaging artifact from continuous EEG recorded during functional MRI. NeuroImage 12, 230–239 (2000).
Du, W. et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 37, 733–738 (2005).
Staley, K. Molecular mechanisms of epilepsy. Nat. Neurosci. 18, 367–372 (2015).
Zhou, X. et al. Intracellular calcium homeostasis and its dysregulation underlying epileptic seizures. Seizure 103, 126–136 (2022).
Zhang, Y. et al. MRI magnetic compatible electrical neural interface: from materials to application. Biosens. Bioelectron. 194, 113592 (2021).
Angelone, L. M. et al. On the effect of resistive EEG electrodes and leads during 7T MRI: simulation and temperature measurement studies. Magn. Reson. Imaging 24, 801–812 (2006).
Chen, H. et al. Aptamer-functionalized carbon nanotube field-effect transistor biosensors for Alzheimer’s disease serum biomarker detection. ACS Sens. 7, 2075–2083 (2022).
Pan, J. et al. 7T Magnetic compatible multimodality electrophysiological signal recording system. Electronics 12, 3648 (2023).
Xia, J. et al. MRI electrode artifact area estimation using contour detection. https://doi.org/10.5281/zenodo.17547948 (2025).
Acknowledgements
Shurong Dong, Gang Pan, Jikui Luo, Shaomin Zhang et al. would like to thank STI2030-Major Projects (No. 2021ZD0200401). Shurong Dong would like to thank Zhejiang Province high level talent special support plan (No. 2022R52042), Zhejiang Province Key R & D programs (No. 2024C03001, No. 2024C03007). Zhen Cao would like to thank Zhejiang Province Leading Geese Plan (No. 2024C03217). Yanlan Yu would like to thank Medical Interdisciplinary Innovation Program 2024, Zhejiang University School of Medicine. The authors would like to express their gratitude to Prof. Tawfique Hasan (Department of Engineering, University of Cambridge, UK) for the collaboration and technical consultations. We also thank Jingyao Chen, Qiong Huang, Chengcheng Zhang, and Yajun Yu from the core facility platform of Zhejiang University School of Medicine for their technical support, and Xu Bin from Zhejiang University 7 T Brain Imaging Research Center for assistance. We further acknowledge Hangzhou Rong brain Technology Co., Ltd. for assisting with the LFP collection, and process engineers from Haijiexing Technology Co., Ltd. (Suzhou, China) for sharing their expertise inlaser internal modification and providing relevant equipment.
Author information
Authors and Affiliations
Contributions
J.X. and S.D. developed the methodology, acquired the data, and wrote the manuscript. L.Z., Y.Y. and F.Z. conducted the animal experiments. S.W. and L.D. performed data analysis. S.Z., L.D. and G.D. contributed to methodology development and manuscript revision. S.D. supervised the project. G.P. and S.D. provided funding and supervised the study. Z.C. contributed to manuscript revision and supervision. J.L., Y.Y.S.H. and L.O. contributed to manuscript revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Mariana Branco and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xia, J., Zhang, L., Wang, S. et al. Implantable neural probes with monolithically integrated CNTFET arrays for multimodal monitoring. Nat Commun (2025). https://doi.org/10.1038/s41467-025-67535-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-67535-5