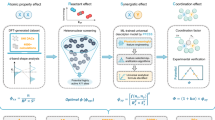
Abstract
Despite that enhancing site utilization (Usite) of single atoms (SAs) is crucial in electrocatalysis beyond increasing intrinsic activity and site density, numerous SAs are inaccessible during reaction due to the dense micropores and disordered stacking of carbon particles. Here, the enhancement for Usite of SAs is achieved through a surfactant-assis'ted freeze-casting (SAFC) strategy. The sodium dodecyl sulfate (SDS)-modified Fe-doped zeolitic imidazolate framework-8 (Fe/ZIF-8) particles assemble into single-layer two-dimensional (2D) superstructures along the ice-crystal. During pyrolysis, the dual-stress effect derived from SDS shell and tight alignment of particles induces Fe/ZIF-8 to transform into concave and mesopore-rich carbon. 2D FeNC with optimized structures at both macro- and micro-scales exhibits enhanced electron/mass transport capabilities, achieving near-100% Usite of SAs and a half-wave potential of 0.958 V for oxygen reduction in alkali. This SAFC strategy demonstrates its universality in improving the catalytic performance of various 2D MNCs across different electrocatalytic reactions.
Similar content being viewed by others
Introduction
Carbon-supported single-atom catalysts, especially zeolitic imidazolate framework-8-derived metal-nitrogen-carbon (MNC), are viable candidates to replace precious metals for electrocatalysis due to their low cost and high efficiency1,2. Beyond the current primary methods to improve their catalytic performance, that is, elevating the intrinsic activity (turnover frequency (TOF) per active site) and/or mass site density (SDmass, number of active sites per gram of catalyst)3,4,5, increasing the site utilization (Usite) of single atoms (SAs) plays an equally pivotal role. Although SAs are theoretically predicted to achieve 100% atomic utilization, not all active sites are accessible due to the numerous embedded sites and dead volumes resulting from the dense micropores and disordered stacking of carbon particles, which critically hinder the mass/electron transfer (MT/ET) and the exposure of SAs. The regulation of the size, framework, or hollow structures through hard/soft templating or organic acid etching methods has been proposed to mitigate these issues6,7,8,9. Yet, their predominant emphasis on the morphology regulation of individual particles at the nanoscale usually overlooks the crucial impact of carbon particle stacking at larger scales on MT/ET and site accessibility. Moreover, the involvement of harsh experimental conditions and complex template removal processes poses significant challenges for large-scale production. To this end, enhancing the Usite of SAs by concurrently optimizing the micro- and macrostructures of nanocarbon particles through a simple and scalable strategy is greatly important but challenging.
Herein, we developed a surfactant-assisted freeze-casting (SAFC) strategy to reconfigure conventional MNCs into single-layer two-dimensional (2D) superstructures with abundant mesopores. During rapid freezing, sodium dodecyl sulfate (SDS)-modified Fe/ZIF-8 colloidal particles are confined within ice crystal channels. By precisely tuning SDS concentration, interparticle spacing and surface liquid film thickness can be well controlled to achieve the construction of single-layer 2D Fe/ZIF-8 superstructures. Upon pyrolysis, the synergistic dual-stress effects from the SDS shell and dense packing of Fe/ZIF-8 particles within the 2D planes induce their anisotropic contraction and micropore expansion, yielding concave carbon structures enriched with mesopores. Consequently, 2D FeNC exhibits enhanced MT/ET capability, achieving nearly 100% Usite of Fe SAs during oxygen reduction reaction (ORR). Furthermore, the tailored carbon matrix with rich defects and edges optimizes the local microenvironment of Fe SAs, enhancing their intrinsic catalytic activity. 2D FeNC demonstrates a half-wave potential (E1/2) of 0.958 V vs. a reversible hydrogen electrode (RHE) for ORR in alkaline media, much better than the SAFC strategy-free counterpart (3D FeNC, 0.914 V vs. RHE. The structural versatility of ZIF-8 toward diverse metal dopants and the controllability of the freeze casting collectively underscore the broad applicability of the proposed SAFC strategy across various 2D MNCs, demonstrating its universal effectiveness in boosting electrocatalytic performance.
Results
Structure and composition characterizations
Fe/ZIF-8 with an average size of ~50 nm was synthesized according to the previous report6. Then, Fe/ZIF-8 suspension was mixed with SDS aqueous solution to obtain the colloidal dispersion (Supplementary Fig. 1a). During rapid freezing in liquid nitrogen (N2), SDS-modified Fe/ZIF-8 particles are expelled by the advancing ice front, forming ordered arrays between ice crystals (Fig. 1a and Supplementary Fig. 1b)10. The ice is then removed in situ upon subsequent freeze-drying, yielding the 2D orderly arrangement of Fe/ZIF-8 superstructures (2D Fe/ZIF-8). Compared to the Fe/ZIF-8 obtained by direct drying (3D Fe/ZIF-8), 2D Fe/ZIF-8 with the equivalent mass exhibits a larger volume and distinct super-assembly characteristics (Supplementary Figs. 1-2). Scanning electron microscopy (SEM) images show that 2D Fe/ZIF-8 exhibits sheet-like morphology with particles assembled in a well-organized fashion. Then, 2D Fe/ZIF-8 was subjected to high-temperature pyrolysis at 1100 °C to obtain 2D FeNC. Low-magnification SEM images in Fig. 1b and Supplementary Fig. 3 show that millimeter-scale aligned carbon superstructures were retained during carbonization. Carbon particles are arranged in a single-layer feature with an interlayer spacing resulting from the ice template removal11. More importantly, periodic stripe-like patterns were observed in the 2D plane, which is attributed to the stress generated by ice crystal growth and particle shrinkage during annealing (Fig. 1c, d). Further observations through transmission electron microscopy (TEM) images reveal that individual particles contain abundant concave and hollow structures (Fig. 1e, f). This can be attributed to the dual outward shrinkage stresses from the close packing of Fe/ZIF-8 particles and rigid SDS-derived interfacial layer, which accelerates the micropore expansion and carbon particle deformation (Supplementary Fig. 4)12. Note that the interlayer spacing, periodic ‘strip’, and optimized carbon architecture provide hierarchical transport networks, enabling rapid MT/ET and improved active site accessibility during electrocatalysis.
a Schematic illustration of 2D FeNC synthesis. b, c Low- and d high-magnification SEM images of 2D FeNC. e TEM image, f, g HADDF-STEM images, and h elemental mapping images of g (overlay, Fe, N, and C) of 2D FeNC. i AC-HAADF-STEM image with 3D atomic Gaussian fitting overlapping of 2D FeNC. j FT-EXAFS spectra at the Fe K-edge for 2D FeNC, 3D FeNC, and reference compounds. k R-space FT-EXAFS spectra (magnitude and real part) with fitting curves for 2D FeNC. l Normalized Fe K-edge XANES spectra of 2D FeNC, 3D FeNC, and standards.
Crystalline Fe species are excluded in 2D FeNC through high-angle annular dark-field scanning TEM (HAADF-STEM) images (Fig. 1g) and X-ray diffraction analysis (XRD) (Supplementary Fig. 5). Elemental mapping images (Fig. 1h) verify a homogeneous distribution of Fe, N, and C elements over 2D FeNC. Aberration-corrected (AC)-HAADF-STEM image combined with 3D Gaussian fitting analysis identifies the dominant presence of densely distributed Fe SAs (Fig. 1i). The Fe contents of both 2D FeNC and 3D FeNC were determined to be 1.01 wt% by inductively coupled plasma optical emission spectrometry (ICP-OES). Raman spectroscopy (Supplementary Fig. 6a) reveals a higher density of defects in 2D FeNC, further evidenced by its reduced contents of pyridinic N species because of the promoted micropore expansion and carbon decomposition (Supplementary Fig. 6b-f and Supplementary Note 1)13,14,15. Note that these defects and edges are anticipated to optimize the electronic structure of Fe SAs14.
Various methods were employed to elucidate the detailed structural characteristics of catalysts. Fourier-transformed (FT) extended X-ray absorption fine structure (EXAFS) analysis reveals that the dominant peak in both 2D and 3D FeNC corresponds to the Fe-N scattering path, with coordination numbers of approximately 4 based on the fitting results (Fig. 1j, k, Supplementary Figs. 7-8, Supplementary Table 1 and Supplementary Note 2). X-ray absorption near-edge structure (XANES) spectra (Supplementary Fig. 1l) show a positive shift in the Fe K-edge position for 2D FeNC relative to 3D FeNC. Given the identical FeN4 configuration, the lower charge density at the Fe sites in 2D FeNC compared to 3D FeNC highlights the impact of the defects and edges on the electronic structure of the FeN4 moieties16. By this token, the carbon substrate with optimized porous and concave structures facilitates electron delocalization at the Fe sites for 2D FeNC, which is expected to mitigate their excessive adsorption of oxygenated intermediates, thereby endowing 2D FeNC with enhanced catalytic activity17.
SAFC mechanism and condition optimization
To systematically evaluate the SAFC strategy’s effectiveness, we further investigated how surfactant concentration and type govern the structural evolution of both Fe/ZIF-8 precursors and derived FeNC (Fig. 2a). As shown in Supplementary Fig. 9, SDS-free assemblies (denoted as 2D Fe/ZIF-8_S0, where the subscript represents relative SDS concentration to 2D Fe/ZIF-8) exhibit multilayered aggregates with reduced sizes. This aligns with the intrinsic difficulty in ice-templating small colloidal particles because their limited size prevents effective confinement within inter-ice channels18. Simultaneously, assembling them into large-scale 2D arrays remains problematic. The thermodynamic prerequisite for successful freeze-casting requires the free energy (γ) to satisfy ∆γ0 = γsp-(γlp+γsl) > 0 (Fig. 2b, Supplementary Fig. 10 and Supplementary Note 3), where γsp, γlp and γsl represent interface free energy between solid and particle, liquid and particle, and solid and liquid, respectively10. However, small colloidal particles with high specific surface area generally exhibit elevated γlp, promoting random aggregation over directional alignment along ice crystals during freezing (Fig. 2c). Moreover, the equilibrium between these interfacial energies governs the repulsive (FR = 2πr∆γ0(α0/d)n) and attractive (FA = 6πηνr2/d) forces between the particles and ice front, where r is the particle radius, ν the ice-front velocity, η the liquid’s dynamic viscosity, α0 the average intermolecular distance in the liquid phase, d the liquid-layer thickness at the solid-liquid interface, and n an empirical correction factor (1 ≤ n ≤ 4). Surfactant-free Fe/ZIF-8 particles display small α0 and d values due to their low surface charge (Fig. 2d, Supplementary Fig. 11 and Supplementary Note 4), making them susceptible to be captured by ice crystals because of strong FA (Fig. 2e). While introducing SDS (2D Fe/ZIF-8_S0.5) can elevate α0 and d through electrostatic repulsion, enabling particle alignment along ice-crystal interfaces (Supplementary Fig. 12). Increasing SDS concentration further optimizes α0 and d, which allows the production of the expanded 2D sheet-like superstructures for 2D Fe/ZIF-819. Moreover, the strengthened electrostatic repulsion facilitates single-particle confinement within ice channels, producing single-layer 2D superstructures (Supplementary Fig. S1). Although higher SDS concentrations (2D Fe/ZIF-8_S2) preserve the 2D morphology, the bilayer adsorption of SDS after its saturated adsorption on the Fe/ZIF-8 surface decreases α0 and d, ultimately causing multilayer stacking (Supplementary Fig. 13).
a Schematic representations of FeNC carbon particles synthesized under different conditions. b Force analysis diagram of a suspended Fe/ZIF-8 particle near an advancing ice front. c Controlled assembly of Fe/ZIF-8 particles during freeze-casting modulated by SDS concentration or surfactant type. d Zeta potentials of Fe/ZIF-8 colloids with different SDS concentrations and surfactants. e Structural evolution of 2D Fe/ZIF-8 assemblies showing layer number dependence. f Stress evolution during pyrolysis (top) and corresponding pore/morphology changes in derived carbon particles (bottom). g–l SEM and m–r HAADF-STEM images of 2D FeNC_S0, 2D FeNC_S0.5, 2D FeNC, 2D FeNC_S2, 2D FeNC_CTAB, and 2D FeNC_PVP, respectively.
Furthermore, cationic cetyltrimethylammonium bromide (CTAB) and nonionic polyvinylpyrrolidone (PVP) were investigated as alternative surfactants (Supplementary Figs. S14-15). CTAB enhances Fe/ZIF-8 surface charge through hydrophobic interactions, enabling 2D Fe/ZIF-8_CTAB assembly. However, its lower critical micelle concentration (vs. SDS) induces premature micelle/bilayer formation at reduced concentrations (1.62 mM), decreasing α0 and d values and yielding multilayer architectures. In contrast, PVP with weaker charge modulation produces smaller, disordered arrays. These results unequivocally demonstrate the pivotal role of surfactant in controlling Fe/ZIF-8 alignment during freeze-casting.
The surfactant-mediated carbonization mechanism was further elucidated (Fig. 2f). With SDS integration, carbon particles evolve from well-defined rhombic dodecahedrons (2D FeNC_S0) to roof-like concave architectures (2D FeNC_S0.5 and 2D FeNC) (Fig. 2g-i and Supplementary Figs. 16-17). In SDS-free systems, ligand decomposition and gas release generate inward stresses that drive particle shrinkage20,21. Conversely, SDS-coated Fe/ZIF-8 undergoes anisotropic deformation through two concurrent outward stress effects. The first stress (Foutward1) arises from the differential shrinkage coefficients between the SDS shell and Fe/ZIF-8 core, with vertex regions experiencing amplified stress due to force superposition from adjacent SDS layers. A secondary stress (Foutward2) originates from interparticle compression within the 2D planes during freezing22. The synergistic action of Foutward1 and Foutward2 promotes anisotropic contraction and micropore expansion, ultimately yielding the characteristic concave mesoporous structures observed in 2D FeNC_S0.5 and 2D FeNC. While moderate SDS concentrations can optimize the morphology of 2D FeNC (Fig. 2i), excessive SDS (2D FeNC_S2) markedly augments Foutward1, accelerating ligand depletion and gas evolution. This triggers the structural collapse and Fe aggregation into nanoparticle (NP) in 2D FeNC_S2 (Fig. 2j and Supplementary Fig. 18). Similarly, the high decomposition temperatures of CTAB and PVP in comparison to SDS enable stronger and more prolonged Foutward1, also inducing structural collapse and NP formation (Supplementary Figs. 19-21 and Supplementary Note 5). Taken together, the microscopic morphology, porous structure, and macroscopic alignment of FeNC can be rationally governed through surfactant selection and concentration tuning, demonstrating the effectiveness of the SAFC strategy in the structural optimization of FeNC.
Catalytic performance and quantitative analysis
Taking ORR as a model reaction, the electrocatalytic performance of the as-prepared catalysts was evaluated in 0.1 M KOH (aq) solutions. Notably, when the 2D FeNC is prepared as an ink, it retains a relatively intact 2D sheet-like structure, which not only facilitates MT and ET but also promotes the dispersion of ionomers on the catalyst surface (Supplementary Fig. 22). As illustrated in Figs. 3a, 3D FeNC demonstrates markedly improved ORR activity compared to metal-free NC, demonstrating the primary role of Fe SAs23. Notably, 2D FeNC achieves a E1/2 of 0.958 V vs. RHE for ORR, surpassing both 3D FeNC (0.914 V vs. RHE) and commercial Pt/C (0.906 V vs. RHE), further verified by its high kinetic current density (JK, 66.2 mA/cm2 at 0.93 V vs. RHE) and low Tafel slope (45.6 mV/dec) (Supplementary Fig. 23). Rotating ring disk electrode (RRDE) and Koutecký-Levich analyses verify a dominant 4-electron ORR pathway for all catalysts (Supplementary Figs. 24-25). Among all 2D FeNC_Sx variants, 2D FeNC delivers the best performance, attributable to its ideal particle arrangement and porous architecture (Fig. 3b). Control experiments reveal that 3D FeNC/S derived from the physical mixture of Fe/ZIF-8 and SDS without freeze-casting fails to enhance ORR activity, underscoring the indispensable role of freeze-casting in structural optimization (Supplementary Fig. 26). Both 2D FeNC_CTAB and 2D FeNC_PVP exhibit degraded activity due to structural collapse and Fe NP formation. Remarkably, 2D FeNC shows low E1/2 loss (4 mV) after 30,000 cyclic voltammetry (CV) cycles and maintains structural integrity without Fe NP generation (Supplementary Figs. 27-29). These results collectively establish 2D FeNC as a top-tier ORR catalyst and make it competitive with most reported analogues in both activity and durability (Supplementary Tables 2-3).
a ORR polarization curves of Pt/C, NC, 3D FeNC, and 2D FeNC and b 2D FeNC_Sx with different SDS contents. c ORR polarization curves and d CV curves of 2D FeNC and 3D FeNC during nitrite stripping protocol (unpoisoned, poisoned, and recovered states). Insets in (d): charge quantification for nitrite reduction on Fe SAs. e SDmass and Usite comparisons across catalysts. f Comparison of TOF and SDmass across catalysts. g Schematic of SECM setup. h, i SECM mapping at 0.4 V vs. RHE for (h) 2D FeNC and (i) 3D FeNC. j Tip-recorded charges and SI-SECM-derived SDmass for 2D FeNC and 3D FeNC.
In situ nitrite stripping tests were carried out to uncover the origin of the enhanced ORR performance, as detailed in the Experimental Section (Supplementary Fig. 30)24. Upon nitrite poisoning, all catalysts show reduced activity, with 2D FeNC exhibiting the most significant decline, reflecting its higher density of accessible active sites (Fig. 3c). Accordingly, 2D FeNC yields a 4-fold greater stripping charge (52.2 vs. 13.1 C/g for 3D FeNC). Based on the stripping charge, the SDmass can be calculated (Fig. 3d and Supplementary Figs. 31–33). Instead of the five-electron reduction of nitrosyl to ammonia as previously reported24, we assumed a three-electron pathway to hydroxylamine at FeN4 sites (Supplementary Note 5)5,25. This analysis reveals the SDmass of 6.52 × 1019 sites/g for 2D FeNC, four times that of 3D FeNC (1.63 × 1019 sites/g) (Fig. 3e). Given the identical Fe content across all catalysts, this difference demonstrates the higher accessibility of SAs in 2D FeNC during electrocatalysis. Importantly, the Usite is calculated to be 99.4% for 2D FeNC using Fe content determined by ICP-OES, demonstrating that 2D FeNC enables nearly all Fe SAs to participate in the catalytic process. Furthermore, the TOF is also estimated based on the nitrite stripping results. To exclude ORR contributions from non-Fe sites such as N-doped carbon, the JK used for TOF calculation is defined as the difference between JK values before and after nitrite poisoning (i.e., JK unpoisoned - JK poisoned)5. Fig. 3f shows that 2D FeNC also exhibits a significant enhancement in TOF values, which can be ascribed to the optimization of electronic structures for Fe SAs driven by defect and edge generation under dual-stress effects14. Leveraging the significantly improved Usite and intrinsic activity of 2D FeNC over 3D FeNC, we evaluated its ORR performance at reduced loadings (Supplementary Fig. 34). Even at one-fourth the loading of 3D FeNC, 2D FeNC (0.15 mg/cm2) still delivers superior performance. This is crucial for practical applications, as excessive loading of non-precious metal catalysts often leads to MT limitations26. The enhancement of Usite and intrinsic activity in 2D FeNC offers an effective solution to this challenge.
Scanning electrochemical microscopy (SECM) technique with low catalyst loading and minimized MT/ET barriers provides a highly sensitive signal feedback compared to the nitrite stripping method27. To corroborate our findings, we further employed in situ SECM and surface-interrogation (SI)-SECM to quantify SDmass and TOF (Fig. 3g). In tip-generation/substrate-collection (TG/SC) mode, oxygen generated at the Pt tip undergoes ORR at the substrate electrode, generating a feedback current28. Accordingly, 2D FeNC delivers high feedback currents compared to 3D FeNC, further confirming its superior ORR activity (Fig. 3h, i and Supplementary Figs. 35-37). Subsequently, the Smass and TOF were determined by the SI-SECM method, where the catalytic centers can be quantified based on the charge obtained from the Fe(II)/Fe(III) redox cycling between ferrocenemethanol (Fc) and Fe catalytic centers in catalysts (Supplementary Note 6)29. The tip current for 2D FeNC roses at a higher substrate Es (1.05 V vs. RHE) than 3D FeNC (0.95 V vs. RHE), and attains a balance at Es of ~0.5 V vs. RHE because the redox cycle at the catalytic site reaches saturation (Fig. 3j and Supplementary Figs. 38–40). Time-current integration obtains Smass values of 6.57 × 1019 (2D FeNC) and 1.64×1019 sites/g (3D FeNC), aligning with nitrite stripping results. Especially, 2D FeNC’s Smass from both methods matches its ICP-OES-derived Fe content, confirming near-100% Usite of Fe SAs. Besides, the TOF of both 2D FeNC (113.8 e site−1 s−1) and 3D FeNC (105.24 e site−1 s−1) was also obtained through the SI-SECM method, further suggesting the enhanced intrinsic activity of 2D FeNC compared to 3D FeNC. This explains its comparable ORR activity despite lower Fe loading to literature benchmarks (Supplementary Table 4, and Supplementary Note 7).
Origin exploration
Generally, both Usite and TOF critically depend on MT and ET efficiency during electrocatalysis (Fig. 4a). Brunauer-Emmett-Teller (BET) measurements reveal that 2D FeNC possesses the highest surface area at 1637.7 m2/g (Supplementary Figs. 41-42 and Supplementary Table 5). Non-local density functional theory (NLDFT) calculations show that 2D FeNC exhibits a significantly increased mesopore ratio of 80.3% compared to 3D FeNC (41.1%), 3D FeNC_S (65.4%), 2D FeNC_CTAB (59.1%) and 2D FeNC_PVP (65.2%) while its micropore ratio decreases to 15.4% from 46.8% (3D FeNC) and 24.3% (3D FeNC_S), respectively (Fig. 4b)30. This pore optimization induced by the dual outward stress effects overcomes the inherent MT/ET limitations of microporous ZIF-8-derived carbons, making it possible to expose more active sites to participate in the reaction26.
a Schematic comparison of O2 diffusion pathways in 3D FeNC vs. 2D FeNC. The green ball-and-stick model represents the O2 molecules, and the red arrow indicates the direction of MT. b Pore volume distribution and c Cdl_CV, Cdl_EIS, Cdl_CV/Cdl_EIS for catalysts. d Potential-dependent Rct (left) and Rmt (right) of 3D FeNC and 2D FeNC from operando hydrodynamic EIS. e DRT analysis of 3D FeNC and 2D FeNC at different potentials. f FEM-simulated O2 concentration profiles across 3D FeNC (top) and 2D FeNC (bottom) models (1–15 ms timeframe). g In situ ATR-FTIR spectra of 3D FeNC (top) and 2D FeNC (bottom) under applied potentials.
The electrochemical performance of the catalysts was evaluated after loading onto electrodes. Accordingly, the double-layer capacitances (Cdl_EIS) determined through electrochemical impedance spectroscopy (EIS) at open circuit potential (OCP) and CV curves in the non-Faradaic region (Cdl_CV) correspond to the electrochemically wettable surface area (EWSA) and electrochemically active surface area (ECSA), respectively31. As displayed in Fig. 4c, 3D FeNC demonstrates limited EWSA and ECSA due to the poor pore structure and disordered stacking (Supplementary Figs. 43 and 44a). In contrast, 2D FeNC shows significantly improved Cdl_EIS, Cdl_CV and a higher Cdl_CV/Cdl_EIS ratio, confirming that its ordered architecture and tailored porosity simultaneously enhance electrolyte infiltration and electrochemical responsiveness. A progressive EWSA increase across 3D FeNC, 3D FeNC/S, 2D FeNC_S0, and 2D FeNC directly correlates with structural evolution toward single-layer ordered arrays with multiple channels, highlighting their essential role in optimizing MT/ET. This is further supported by 2D FeNC’s superior hydroxide ion diffusion coefficient (DOH) (Supplementary Fig. 44b and Supplementary Note 8)32.
Operando EIS analysis was conducted to investigate MT and ET processes during the ORR. By fitting an equivalent circuit model (Supplementary Fig. 45), we extracted potential-dependent charge transfer resistance (Rct) and mass transport resistance (Rmt) across the ORR potential range (Fig. 4d and Supplementary Figs. 46–50)33. With increasing overpotential, the ORR process is progressively governed by Rct, mixed kinetic, and Rmt. For 3D FeNC, mixed kinetic control initiates at 0.88 V vs. RHE, while Rct rebounds below 0.74 V vs. RHE, caused by the difficulty in the timely diffusion of products or intermediates on the active sites. In stark contrast, 2D FeNC exhibits consistently lower Rmt and Rct. Its monotonically decreasing Rct indicates a rapid and sustained ET process, demonstrating the superiority of its single-layer 2D architecture in enhancing MT and ET.
The distribution of relaxation times (DRT) analysis serves as a model-free method to evaluate electrochemical processes34,35. As shown in Fig. 4e, both 2D and 3D FeNC exhibit three characteristic peaks in the high-, mid-, and low-frequency regions, corresponding to ion diffusion, ORR kinetics, and gas diffusion, respectively. Notably, ion diffusion impedance is much lower than that of ORR and gas diffusion. At 0.76 V vs. RHE, 2D FeNC reaches its lowest ORR kinetic resistance and maintains this level, in contrast to 3D FeNC, which exhibits persistently higher resistance. As the overpotential increased, the gas diffusion peaks of both catalysts moved toward lower frequencies, indicating faster O2 consumption and greater concentration gradients between the solution and electrode interface. Importantly, 2D FeNC demonstrates significantly reduced gas diffusion resistance compared to 3D FeNC, a result of its improved transport pathways. These findings were further corroborated by finite element method (FEM) simulations of O2/OH- transport within the catalyst layers8. Supplementary Fig. 51 shows the models to simulate 2D FeNC and 3D FeNC. The time-dependent O2/OH-concentration-time profiles reveal that 2D FeNC maintains significantly higher O2/OH- concentration levels than 3D FeNC across both horizontal and vertical dimensions under identical time gradients (Fig. 4e and Supplementary Figs. 52–57). This is attributed to the large-scale ordered arrangement of carbon particles in 2D FeNC, which promotes efficient O2/OH- transport, while the disordered stacking of 3D FeNC increases the O2/OH- diffusion pathways, thereby hindering its transport.
In situ attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy measurements were performed to further investigate the ORR catalytic mechanism36,37,38. The four-electron ORR process proceeds via two fundamental pathways, as illustrated in Supplementary Fig. 58. The first is the associative mechanism, in which *O2 undergoes protonation to form the *OOH intermediate. The second is the dissociative mechanism, where *O-O* directly cleaves into two *OH species, followed by sequential protonation39,40,41. As shown in Fig. 4g, the peak at ~1050 cm−1 can be attributed to the *O-O* intermediate associated with the dissociative pathway, while the peak at ~1220 cm−1 corresponds to the *OOH intermediate from the associative pathway. Compared with 3D FeNC, the *OOH signal on 2D FeNC decreases significantly with decreasing potential, indicating its rapid consumption. In contrast, the *O-O* intermediate shows relatively minor changes. These results suggest that both catalysts preferentially follow the associative pathway, with 2D FeNC exhibiting higher ORR efficiency. Notably, the *OH adsorption peak emerges at more negative potentials for 2D FeNC, demonstrating weaker oxygenate binding on Fe SAs and highlighting its enhancement in intrinsic activity42.
To demonstrate practical applicability, we fabricated ZABs using 2D FeNC as the air cathode (Supplementary Figs. 59–60 and Supplementary Notes 9–10)43,44,45. In addition to an robust performance in liquid-based ZABs, the 2D FeNC+RuO2-based flexible ZABs deliver a much higher discharge current and maximum power density than ZABs that use benchmark Pt/C + RuO2 as a catalyst (93.5 mA/cm2 and 48.4 mW/cm2 vs. 42.5 mA/cm2 and 21.0 mW/cm2, respectively). Furthermore, the assembled flexible ZABs successfully powered digital timers and smartphones, demonstrating their reliability and practical potential for real-world applications.
Despite differences in the optimal metal species across catalytic systems, maximizing the accessibility and Usite of SAs remains a common demand for efficient electrocatalysis7,46. As shown in Supplementary Figs. 61-63, the ORR activities of 2D CoNC, 2D NiNC, 2D MnNC and 2D CuNC are superior to those of their 3D MNC counterparts. Furthermore, the SAFC strategy was extended to the synthesis of 2D IrNC and 2D PtNC, which were then applied to formic acid oxidation and methanol oxidation reaction systems, respectively (Supplementary Fig. 64). Despite their larger size compared to O2, the optimized 2D MNCs significantly enhance MT/ET, thereby improving the accessibility of SAs and ultimately boosting catalytic activity.
Discussion
We present a general SAFC strategy for constructing 2D MNC superstructures with highly accessible and maximal Usite of SAs. The adsorption of SDS on Fe/ZIF-8 particles modulates their surface charge, enabling precise control over interparticle interactions and liquid film thickness, thereby guiding the assembly of particles along ice-crystal interfaces. The dense packing of Fe/ZIF-8 particles and SDS shell serves as the dual-stress effect to induce anisotropic shrinkage of Fe/ZIF-8 particles upon pyrolysis, generating porous and concave FeNC structures. This approach yields single-layer 2D FeNC architectures featuring long-range 2D ordering at the macroscopic level and enhanced mesoporosity at the microscopic level. 2D FeNC enables multi-channels for efficient ET/MT, achieving nearly 100% Usite of Fe SAs and delivering desirable ORR performance. This method is proven to be universal in the synthesis of various 2D MNCs and the enhancement of electrocatalytic performance for various reactions.
Methods
Synthesis of 2D Fe/ZIF-8
Fe/ZIF-8 was prepared according to the reference6. Fe/ZIF-8 crystals were synthesized by mixing 300 mL methanol solution containing 3.94 g 2-methylimidazole and 300 mL methanol solution containing 3.39 g Zn(NO3)2·6H2O and 100 mg Fe(NO3)3·9H2O, followed by crystallization at 60 °C for 24 h. The obtained solids were collected by centrifugation, thoroughly washed with ethanol, and dried under vacuum for 30 min. ZIF-8 was synthesized with the same procedure as Fe/ZIF-8, except for the addition of Fe(NO3)3·9H2O. The as-prepared Fe/ZIF-8 was dispersed into 1.62 mM SDS aqueous solutions and kept the concentration of Fe/ZIF-8 at 10 mg/mL, which was ultrasonicated for 10 min, followed by stirring for another 2 hours to obtain the colloidal solutions. Then, 30 mL colloidal solutions were frozen in liquid N2 for 3 min using paper cup molds, followed by 24 h freeze-drying (−50 °C condenser temperature, 20 Pa vacuum) to yield 2D Fe/ZIF-8 superstructures. By adjusting SDS concentrations to 0, 0.81, 1.62, and 3.24 mM, we obtained 2D Fe/ZIF-8_S0, 2D Fe/ZIF-8_S0.5, 2D Fe/ZIF-8, and 2D Fe/ZIF-8_S2 variants, respectively. Parallel experiments used CTAB (1.62 mM) or PVP (5 mg/mL) to prepare 2D Fe/ZIF-8_CTAB and 2D Fe/ZIF-8_PVP controls.
Synthesis of 2D FeNC
The as-prepared 2D Fe/ZIF-8 powders were transferred to a porcelain boat and subjected to thermal annealing at 1100 °C for 2 hours under N2 atmosphere with a heating rate of 5 °C/min to produce the final 2D FeNC series.
Synthesis of other 2D MNCs and 3D MNCs
To verify the universality of the SAFC strategy proposed in this work, we synthesized different types of 2D MNCs and evaluated their electrocatalytic performance. Meanwhile, to simplify the synthetic procedure and reduce the cost, we adopted a universal host-guest strategy reported in the literature to prepare the M/ZIF-8 precursors47. Although the M/ZIF-8 obtained by this strategy exhibited larger particle sizes compared with Fe/ZIF-8, the resulting 2D MNCs still demonstrated well-defined 2D array structures and excellent electrocatalytic performance.
Physical characterization
SEM analysis employed a Zeiss GeminiSEM 560 system operating at 2 kV acceleration voltage. TEM images were acquired using a JEM-2100 Electron Microscope, while AC-HAADF-STEM characterization was conducted on a Titan G2-600 instrument. XRD patterns were collected on a Bruker D8 ADVANCE diffractometer with Cu Kα radiation (λ = 0.15406 nm) at 40 kV and 40 mA, scanning 2θ angles from 10° to 80°. Raman spectroscopy measurements utilized a Thermo Fisher Scientific DXR spectrometer with 780 nm laser excitation in backscattering geometry. The BET surface areas of all samples were determined by N2 adsorption-desorption measurements at 77 K using an AUTOSORB system. Prior to analysis, the samples were degassed at 200 °C for 12 h under vacuum. The pore size distribution was obtained using the NLDFT method. ICP-OES analysis was performed on a PerkinElmer Optima 8000 system for metal content quantification. XPS data were collected on a Thermo ESCALAB 250Xi instrument with Al Kα radiation, with all binding energies referenced to the C 1 s peak at 284.6 eV after Shirley background subtraction and peak deconvolution using XPSPEAK software. EXAFS measurements were carried out at beamline 21 A of the Taiwan Photon Source, National Synchrotron Radiation Research Center.
Electrochemical measurements and ORR performance evaluation
All RRDE measurements were performed using a three-electrode system with an ALS RRDE-3A rotating electrode setup at room temperature. An Ag/AgCl (KCl-saturated) electrode and a graphite rod were used as the reference and counter electrodes, respectively. The reference electrode was calibrated against RHE before testing. Catalyst inks were prepared by ultrasonically dispersing 4 mg of catalyst in 1 mL of water/ethanol/Nafion (49:50:1 volume ratio) solution for 2 h. A glassy carbon RRDE coated with catalyst ink with a controlled loading of 0.6 mg cm−2 was the working electrode. A commercial 20 wt% Pt/C catalyst (Alfa Aesar) with a loading of 20 μgPt/cm2 was used as a reference. ORR activity was recorded in O2-saturated 0.1 M KOH (aq) solution by Linear Sweep Voltammetry (LSV, 1.0-0.2 V vs. RHE, 10 mV/s, 1600 rpm) curves. 4-electron selectivity and H2O2 yield were determined by using RRDE with a constant Pt ring potential (1.20 V vs. RHE). The collection efficiency is determined to be 0.37. ORR results were background-corrected using N2-saturated electrolyte measurements. E1/2 is defined as the middle point of a certain region of LSV curves from the reduction current that starts increasing sharply to the reduction current that gets saturated. During the accelerated durability testing, the catalyst was examined in N2-saturated 0.1 M KOH (aq) electrolyte over a potential cycling window of 0.6 V to 1.0 V vs. RHE at a scan rate of 100 mV/s.
In situ nitrite stripping test
The nitrite stripping was conducted with 0.2 mg/cm2 catalyst loading. To ensure that nitrite can be adequately reduced, 0.5 M sodium acetate buffer (aq) with pH = 5.2 was chosen as an electrolyte25. The experimental process includes cleaning, measurement (unpoisoned), electrode poisoning, measurement (poisoned and stripped), and measurement (recovered), depicted in Supplementary Fig. 305,24. Based on the stripping charge derived from the CV curves, Smass could be calculated by the following equation:
where Qstrip represents the nitrite reductive stripping charge derived from the stripping peak. NA and F are Avogadro’s constant (mol-1) and Faraday’s constant (C mol-1), respectively. nstrip is the number of electrons for the nitrosyl ligand reduction. Then TOF can be calculated in the following equation:
where \({J}_{{\mathrm{kin mass}}}^{{\mathrm{unpoisoned}}}\) (A/g) and \({J}_{{\mathrm{kin mass}}}^{{\mathrm{poisoned}}}\)(A/g) represent the kinetic mass-specific activity obtained from in situ nitrite stripping test.
SECM and SI-SECM measurements
The SECM experiments were performed using a Model 920D system (CH Instruments, Inc.), equipped with a bi-potentiostat and a high-resolution 3D electrode positioner. A Pt UME (CHI116, 25 μm tip diameter) was utilized as the probe, while a custom-made concave gold electrode (25 μm recessed microdisk, ~7 μm depth) served as the substrate27. The reference and counter electrodes were an Ag/AgCl and a Pt wire, respectively. The substrate was processed by inserting a 25 μm gold wire into a glass capillary sealed using an oxy-hydrogen flame. The capillary tip was polished with successive grit sandpapers, followed by electrochemical etching in a CaCl2 solution to create a cavity, the depth and sealing of which were verified using an optical microscope.
The catalyst was loaded by pressing the concave electrode onto the catalyst powder placed on a glass slide, ensuring flatness and thorough filling via optical microscopy. The positioning of the SECM probe along the z-axis was fine-tuned using the probe approach curve technique in an N2-purged 0.1 M KOH (aq) solution with 0.5 mM Fc, with the probe biased at 0.50 V vs. Ag/AgCl for Fc oxidation. The platform was adjusted using the three-point fix method to prevent crashes and stabilize current readings. The SECM scanning was performed by applying 1.00 V vs. RHE (probe) and -0.56 V vs. RHE (substrate), starting with a 500 × 500 μm rough scan, followed by a 100 × 100 μm fine scan to locate the catalyst region. Images in the TG/SC mode were captured under various substrate potentials under the N2-purged 0.1 M KOH (aq) solution.
Subsequent SI-SECM experiments involved measuring the Cdl by CV at scan rates between 5 and 50 mV/s to determine the ECSA of the UME (ECSAsubstrate). The resulting feedback current at the probe (Itip) was recorded, and the integrated charge (Qtip) was calculated after background subtraction. The SDECSA was then determined based on the following equation:
where F represents Faraday’s constant (96485 C mol-¹), and NA is Avogadro’s constant (6.02 × 1023 mol-¹). Then the TOF was determined using the following equation:
where jm (A/g) represents the kinetic mass-specific activity obtained from prior electrochemical measurements, which were calculated as 1198.36 A/g and 276.62 A/g for 2D FeNC and 3D FeNC, respectively. While SDmass refers to the site density per gram of catalyst, which can be calculated using the following equation:
Cdl test and operando EIS analysis
The Cdl_CV was obtained through CV curves conducted within a non-Faradaic potential range (1.00-1.10 V vs. RHE) at scan rates varying between 1 and 50 mV/s. And ECSA is estimated using the following equation:
where Cs represents the specific capacitance of the total double layer (0.04 mF/cm2)48. Also, EIS was performed at OCP without rotating with an amplitude of 10 mV between 100 kHz and 10 mHz to obtain Cdl_EIS. Specifically, Cdl_EIS can be obtained from the slope of the linear relationship between the imaginary part of the impedance \(({Z}^{{\prime\prime} })\) and the reciprocal of the angular frequency (ω) in the low-frequency region31:
Rct and Rmt were calculated from operando EIS data using an SP-300 potentiostat (BioLogic Science Instruments, France) with an O2-purged 0.1 M KOH (aq) solution. The impedance data were collected by varying the frequency range from 1 MHz to 10 mHz at a series of potentials with 11 data points recorded per decade, using a 5 mV sinusoidal AC amplitude. A single-sine mode was employed for all measurements. The spectra were first analyzed using complex non-linear least squares (CNLS) fitting using “Zview” software, which is a series circuit composed of solution resistance (Rs) and the parallel circuits of Rct/CPEdl (Constant phase element) and Rmass/CPEmass (Supplementary Fig. 45).
DRT analysis was then performed, which is a model-free analysis and visualization strategy designed for complex electrochemical systems34,35:
where R∞ represents the ohmic resistance, while Rpol corresponds to the polarization resistance. The DRT function g characterizes the time relaxation behavior of the electrochemical system under investigation. τ and f denote the relaxation time and frequency, respectively. The DRT function of the measured spectra is obtained using a Tikhonov regularization approach. The regularization parameter λ, which balances the residual noise and the solution norm, is crucial for accurately extracting the pseudo-continuous DRT function from discrete impedance data. Based on Heinzmann’s study, a regularization parameter λ of 10-3 was selected, as it offers an optimal compromise between low residuals, high selectivity, and minimal oscillations. The DRT function was calculated using the Matlab® software DRTtools, developed by the Ciucci group35.
In situ ATR-FTIR tests
In situ ATR-FTIR was performed with a Thermo iS50 FT-IR spectrometer, equipped with a liquid N2-cooled mercury-cadmium-telluride detector. A gold-plated silicon crystal modified with the catalyst served as the working electrode, while an Ag/AgCl (KCl-saturated) electrode and a graphite rod were used as the reference and counter electrodes, respectively. All measurements were performed in 0.1 M KOH (aq) solution, with the applied potential being stepped from 1.1 V to 0.2 V vs. RHE in 500 mV intervals.
FEM simulations
FEM simulations were conducted using the COMSOL Multiphysics finite-element solver22. Two modules were employed to develop an integrated chemistry and MT model for both 2D FeNC and 3D FeNC. This approach enables a detailed analysis of the transport phenomena and electrochemical reactions within the system. Based on the SEM images, the rhombic dodecahedron was simplified to a regular hexagon to simulate carbon particles in 3D FeNC catalysts, while an indented hexagon containing an internal hole structure was chosen for the carbon particles in 2D FeNC catalysts. Two parallel arrays of 20 × 20 particles were used to simulate 2D FeNC catalysts, where the blue regions represent solid particles and the gray areas correspond to the pore structure. For the 3D FeNC catalyst, the random packing of particles results in significant dead volume.
Rechargeable liquid and flexible ZABs tests
Liquid ZABs were constructed in a two-electrode configuration using ~99.9% pure zinc foil as the anode and a catalyst-coated gas diffusion layer (TORAY-YLS 30 T carbon paper) as the cathode49. Catalyst ink was dried at 80 °C, ensuring a catalyst loading of 1.0 mg cm−2. The electrolyte consisted of 6 M KOH and 0.2 M zinc acetate (aq), purged continuously with O2 at 40 mL min−1. Before testing, the electrolyte was purged with O2 for 30 min to ensure saturation. OCP, and discharge-charge polarization curves were recorded using a CHI760E electrochemical workstation. The rate performance of the assembled ZABs was assessed by monitoring the voltage profiles during galvanostatic discharge at varying current densities (0–50 mA cm−2). The constant current discharge-charge cycles and specific capacity were measured at room temperature using a Land 3001 A battery test system. Each galvanostatic cycle involved 150 s discharge and 150 s charge periods at current densities of 10 mA cm−2. The specific capacity and energy density were calculated using the following equations:
where I represents the applied current (A), t denotes the operation time (s), V is the average discharge voltage (V), and wZn refers to the mass of zinc consumed (g).
The flexible solid-state ZABs comprise an air electrode, a polyacrylic acid (PAA) gel film as the solid electrolyte, and zinc foil as the anode. The air electrode consists of nickel foam as the current collector, a gas diffusion layer in the middle, and a carbon cloth coated with catalysts (1.0 mg cm−2) on the electrolyte-facing side. The PAA gel electrolyte is prepared by combining acrylic acid, KOH, and zinc acetate, followed by cross-linking with N, N-methylene diacrylamide, and initiated by K2S2O8. Polarization curves were measured using LSV on a CHI 760E electrochemical workstation. ZABs underwent galvanostatic cycling with 150-second discharge and charge intervals at current densities of 1 mA cm−2.
Data availability
The data supporting the findings of this study are included in the published article and its Supplementary Information or available from the corresponding authors on request. Source data are provided with this paper.
References
Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Wei, X. et al. Tuning the spin state of Fe single atoms by Pd nanoclusters enables robust oxygen reduction with dissociative pathway. Chem 9, 181–197 (2023).
Li, J. et al. Identification of durable and non-durable FeNx sites in Fe–N–C materials for proton exchange membrane fuel cells. Nat. Catal. 4, 10–19 (2021).
Rao, P. et al. Movable type printing method to synthesize high-entropy single-atom catalysts. Nat. Commun. 13, 5071 (2022).
Jiao, L. et al. Chemical vapour deposition of Fe-N-C oxygen reduction catalysts with full utilization of dense Fe–N4 sites. Nat. Mater. 20, 1385–1391 (2021).
Zhang, H. et al. Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation. J. Am. Chem. Soc. 139, 14143–14149 (2017).
Wu, Z.-Y. et al. A general synthesis of single atom catalysts with controllable atomic and mesoporous structures. Nat. Synth. 1, 658–667 (2022).
Li, J. et al. Selective etching of metal–organic frameworks for open porous structures: mass-efficient catalysts with enhanced oxygen reduction reaction for fuel cells. J. Am. Chem. Soc. 145, 27262–27272 (2023).
Wang, X. et al. Atomically dispersed pentacoordinated-zirconium catalyst with axial oxygen ligand for oxygen reduction reaction. Angew. Chem. Int. Ed. 61, e202209746 (2022).
Shao, G., Hanaor, D. A. H., Shen, X. & Gurlo, A. Freeze casting: from low-dimensional building blocks to aligned porous structures-a review of novel materials, methods, and applications. Adv. Mater. 32, 1907176 (2020).
Song, Y. et al. Two-dimensional metal–organic framework superstructures from ice-templated self-assembly. J. Am. Chem. Soc. 144, 17457–17467 (2022).
Wan, X. et al. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2, 259–268 (2019).
Gong, K., Du, F., Xia, Z., Durstock, M. & Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 323, 760–764 (2009).
Jiang, R. et al. Edge-site engineering of atomically dispersed Fe–N4 by selective C–N bond cleavage for enhanced oxygen reduction reaction activities. J. Am. Chem. Soc. 140, 11594–11598 (2018).
Zhou, H. et al. Negative pressure pyrolysis induced highly accessible single sites dispersed on 3D graphene frameworks for enhanced oxygen reduction. Angew. Chem. Int. Ed. 59, 20465–20469 (2020).
Yue, C. et al. Secondary coordination sphere engineering of single-Sn-atom catalyst via P doping for efficient CO2 electroreduction. Adv. Energy Mater. 14, 2401448 (2024).
Peng, Y., Lu, B. & Chen, S. Carbon-supported single atom catalysts for electrochemical energy conversion and storage. Adv. Mater. 30, 1801995 (2018).
Wegst, U. G. K., Kamm, P. H., Yin, K. & García-Moreno, F. Freeze casting. Nat. Rev. Methods Prim. 4, 28 (2024).
Shen, X. et al. Assembly of colloidal nanoparticles directed by the microstructures of polycrystalline ice. ACS Nano 5, 8426–8433 (2011).
Chen, G. et al. Hierarchically porous carbons with highly curved surfaces for hosting single metal FeN4 sites as outstanding oxygen reduction catalysts. Adv. Mater. 35, 2300907 (2023).
He, Y. et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: carbon-shell confinement strategy. Energy Environ. Sci. 12, 250–260 (2019).
Song, X. et al. 2D arrays of hollow carbon nanoboxes: outward contraction-induced hollowing mechanism in Fe–N–C catalysts. Chem. Sci. 15, 10110–10120 (2024).
Wei, X. et al. Recent advances in synergistically enhanced single-atomic site catalysts for boosted oxygen reduction reaction. Nano Energy 84, 105817 (2021).
Malko, D., Kucernak, A. & Lopes, T. In situ electrochemical quantification of active sites in Fe–N/C non-precious metal catalysts. Nat. Commun. 7, 13285 (2016).
Kim, D. H. et al. Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst. Nat. Commun. 12, 1856 (2021).
Wu, G. & Zelenay, P. Activity versus stability of atomically dispersed transition-metal electrocatalysts. Nat. Rev. Mater. 9, 643–656 (2024).
Jin, Z., Li, P., Fang, Z. & Yu, G. Emerging electrochemical techniques for probing site behavior in single-atom electrocatalysts. Acc. Chem. Res. 55, 759–769 (2022).
Tang, X. et al. Carbon nanocage with maximum utilization of atomically dispersed iron as efficient oxygen electroreduction nanoreactor. Adv. Mater. 35, 2208942 (2023).
Huang, B. et al. Electron-donors–acceptors interaction enhancing electrocatalytic activity of metal-organic polymers for oxygen reduction. Angew. Chem. Int. Ed. 62, e202306667 (2023).
Han, J. et al. “Hard” emulsion-induced interface super-assembly: a general strategy for two-dimensional hierarchically porous metal–organic framework nanoarchitectures. J. Am. Chem. Soc. 146, 18979–18988 (2024).
Kim, M. et al. Strategic design of Fe and N co-doped hierarchically porous carbon as superior ORR catalyst: from the perspective of nanoarchitectonics. Chem. Sci. 13, 10836–10845 (2022).
Kim, M. et al. End-to-end pierced carbon nanosheets with meso-holes. Adv. Sci. 12, 2409546 (2025).
Fang, Q. et al. Single-atom platinum promotes Cu(I)/Cu(II) redox loop of Cu aerogels for efficient glucose electrooxidation. Nano Energy 131, 110217 (2024).
Zhao, Y. et al. Deciphering mesopore-augmented CO2 electroreduction over atomically dispersed Fe–N-doped carbon catalysts. ACS Catal. 14, 3926–3932 (2024).
Meyer, Q., Liu, S., Li, Y. & Zhao, C. Operando detection of oxygen reduction reaction kinetics of Fe–N–C catalysts in proton exchange membrane fuel cells. J. Power Sources 533, 231058 (2022).
Wei, X. et al. Pt nanoparticle–Mn single-atom pairs for enhanced oxygen reduction. ACS Nano 18, 4308–4319 (2024).
Zhao, Y. et al. Vacancy defects inductive effect of asymmetrically coordinated single-atom Fe-N3S1 active sites for robust electrocatalytic oxygen reduction with high turnover frequency and mass activity. Adv. Mater. 36, 2308243 (2024).
Pan, Y. et al. Construction of N, P Co-doped carbon frames anchored with fe single atoms and Fe2P nanoparticles as a robust coupling catalyst for electrocatalytic oxygen reduction. Adv. Mater. 34, 2203621 (2022).
Qiu, Y. et al. Boosting oxygen reduction performances in Pd-based metallenes by co-confining interstitial H and p-block single atoms. Nat. Commun. 16, 5262 (2025).
Luo, J. et al. Oxygen-coordinated cr single-atom catalyst for oxygen reduction reaction in proton exchange membrane fuel cells. Angew. Chem. Int. Ed. 64, e202500500 (2025).
Zhao, S. et al. Cascade synthesis of Fe-N2-Fe dual-atom catalysts for superior oxygen catalysis. Angew. Chem. Int. Ed. 63, e202408914 (2024).
Xu, W. et al. Bioinspired single-atom sites enable efficient oxygen activation for switching anodic/cathodic electrochemiluminescence. Angew. Chem. Int. Ed. 62, e202304625 (2023).
Liu, J. et al. Edge-hosted Fe-N3 sites on a multiscale porous carbon framework combining high intrinsic activity with efficient mass transport for oxygen reduction. Chem. Catal. 1, 1291–1307 (2021).
Qin, J. et al. Altering ligand fields in single-atom sites through second-shell anion modulation boosts the oxygen reduction reaction. J. Am. Chem. Soc. 144, 2197–2207 (2022).
Yan, Y. et al. Orienting electron fillings in d orbitals of cobalt single atoms for effective zinc–air battery at a subzero temperature. Adv. Func. Mater. 34, 2316100 (2024).
Jiang, Z. et al. Atomic interface effect of a single atom copper catalyst for enhanced oxygen reduction reactions. Energy Environ. Sci. 12, 3508–3514 (2019).
Li, Z. et al. Iridium single-atom catalyst on nitrogen-doped carbon for formic acid oxidation synthesized using a general host–guest strategy. Nat. Catal. 12, 764–772 (2020).
Tang, X. et al. Structural and chemical origin of dual-atom sites for enhanced oxygen electroreduction. ACS Catal. 14, 13065–13080 (2024).
Wei, J. et al. Superstructured carbon with enhanced kinetics for zinc-air battery and self-powered overall water splitting. Small 20, 2308956 (2024).
Acknowledgements
This work originated from a series of studies conducted as part of the Knowledge Hub Aichi, whose support is gratefully acknowledged. The authors also acknowledge the financial support from the ES Program (via Nagoya University), a start-up fund of Central China Normal University, and the National Natural Science Foundation of China (No. 22375078, No. 52376063). We also thank beamline 07A1 of Taiwan Light Source (TLS) of the National Synchrotron Radiation Research Center (NSRRC) for X-ray absorption spectroscopy measurements.
Author information
Authors and Affiliations
Contributions
X.W. conceived the idea, designed the experiments, conducted the measurements, and wrote the manuscript. Y.Y. and C.Z. supervised the entire project and contributed to the conceptual development. X.S. contributed to the experimental design and conceptual development. M.A. and P.Y. performed the FEM simulations. X.T. conducted the SECM measurements. J.W. carried out the Zn-air battery tests. Y.Q. performed the in situ ATR-FTIR tests. D.J. conducted the TEM characterizations. Y. A. and T.A. assisted with data analysis. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Mingkai Liu, Yaqiong Su, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, X., An, M., Tang, X. et al. Near-100% site utilization of single atoms for efficient electrocatalysis. Nat Commun 17, 1013 (2026). https://doi.org/10.1038/s41467-025-67756-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-67756-8