Abstract
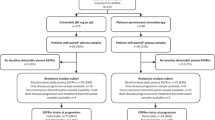
To investigate the efficacy and safety of osimertinib plus savolitinib for patients with advanced non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations and de novo MET aberrations, we conducted a randomized, multicenter, open-label, phase 2 study (ClinicalTrials.gov identifier: NCT05163249). Treatment-naïve patients with locally advanced or metastatic NSCLC harboring de novo MET amplification or overexpression and EGFR mutations were randomized to receive osimertinib monotherapy (cohort 1, 80 mg orally once daily) or combination therapy (cohort 2, osimertinib 80 mg orally once daily and savolitinib 300 mg orally twice daily). The primary endpoint was the confirmed objective response rate (ORR). A total of 44 patients were randomized to either cohort 1 (n = 23) or cohort 2 (n = 21). The pre-specified study endpoint was achieved. The confirmed ORR was 60.9% (95% confidence interval [CI]: 38.5–80.3) in cohort 1 and 90.5% (95% CI: 69.6–98.8) in cohort 2, with disease control rates of 87% (95% CI: 66.4–97.2) and 95.2% (95% CI: 76.2–99.9). Treatment-related adverse events of grade 3 or higher occurred in 2 patients (8.7%) in cohort 1 and 12 patients (57.1%) in cohort 2. Osimertinib plus savolitinib showed promising antitumor activity and manageable safety.
Similar content being viewed by others
Data availability
The deidentified patient data are not publicly available due to restrictions imposed by the institutional review board. Reasonable requests will be reviewed by the Guangdong Provincial People’s Hospital. Access is restricted to qualified researchers for non-commercial academic use and will be shared in a deidentified format for 10 years after publication. Individual patient-level raw data containing identifiable patient information cannot be shared. Source data are provided with this paper. The ctDNA data generated in this study have been deposited in the Genome Sequence Archive, an approved public repository, under accession code HRA014210 and are publicly available. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
References
Gijtenbeek, R. G. P. et al. Overall survival in advanced epidermal growth factor receptor mutated non-small cell lung cancer using different tyrosine kinase inhibitors in the Netherlands: a retrospective, nationwide registry study. Lancet Reg. Health Eu 27, 100592 (2023).
Shimamura, S. S. et al. Survival past five years with advanced, EGFR-mutated or ALK-rearranged non-small cell lung cancer—is there a “tail plateau” in the survival curve of these patients? BMC Cancer 22, 323 (2022).
Ramalingam, S. S. et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50 (2020).
Ferro, A. et al. The study of primary and acquired resistance to first-line osimertinib to improve the outcome of EGFR-mutated advanced Non-small cell lung cancer patients: the challenge is open for new therapeutic strategies. Crit. Rev. Oncol. Hematol. 196, 104295 (2024).
Vendrell, J. A. et al. EGFR-dependent mechanisms of resistance to osimertinib determined by ctDNA NGS analysis identify patients with better outcome. Transl. Lung Cancer R. 10, 4084 (2021).
Cooper, C. S. et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311, 29–33 (1984).
Erratum: clonal MET amplification as a determinant of tyrosine kinase inhibitor resistance in epidermal growth factor receptor–mutant non–small-cell lung cancer. J. Clin. Oncol. 42, 4356–4356 (2024).
Wang, Z. et al. Detection of EGFR mutations in plasma circulating tumour DNA as a selection criterion for first-line gefitinib treatment in patients with advanced lung adenocarcinoma (BENEFIT): a phase 2, single-arm, multicentre clinical trial. Lancet Resp. Med. 6, 681–690 (2018).
Wang, F. et al. Identification of genetic alterations associated with primary resistance to EGFR-TKIs in advanced non-small-cell lung cancer patients with EGFR sensitive mutations. Cancer Commun. 39, 1–15 (2019).
Lai, G. G. et al. Clonal MET amplification as a determinant of tyrosine kinase inhibitor resistance in epidermal growth factor receptor–mutant non–small-cell lung cancer. J. Clin. Oncol. 37, 876–884 (2019).
Yu, H. A. et al. Concurrent alterations in EGFR-mutant lung cancers associated with resistance to EGFR kinase inhibitors and characterization of MTOR as A Mediator Of Resistance. Clin. Cancer Res. 24, 3108–3118 (2018).
Mi, J. et al. Molecular characterization and clinical outcomes in EGFR-mutant de novo MET-overexpressed advanced non-small-cell lung cancer. ESMO Open 7 https://doi.org/10.1016/j.esmoop.2021.100347 (2022).
Ma, P. C., Maulik, G., Christensen, J. & Salgia, R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 22, 309–325 (2003).
Duan, J. et al. Refined stratification based on baseline concomitant mutations and longitudinal circulating tumor DNA monitoring in advanced EGFR-mutant lung adenocarcinoma under gefitinib treatment. J. Thorac. Oncol. 15, 1857–1870 (2020).
Tamiya, A. et al. Mechanisms of resistance and correlation between pre-treatment co-alterations and p-prognosis to osimertinib in chemo-naïve advanced non-small cell lung cancer. Lung Cancer 195, 107917 (2024).
Reis, H. et al. MET expression in advanced non-small-cell lung cancer: effect on clinical outcomes of chemotherapy, targeted therapy, and immunotherapy. Clin. Lung Cancer 19, e441–e463 (2018).
Scagliotti, G. et al. A randomized-controlled phase 2 study of the MET antibody emibetuzumab in combination with erlotinib as first-line treatment for EGFR mutation-positive NSCLC patients. J. Thorac. Oncol. 15, 80–90 (2020).
Planchard, D. et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. The New England journal of medicine 389, 1935–1948 (2023).
Cho, B. C. et al. LBA14 Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): primary results from MARIPOSA, a phase III, global, randomized, controlled trial. Ann. Oncol. 34 https://doi.org/10.1016/j.annonc.2023.10.062 (2023).
Brazel, D. & Nagasaka, M. MARIPOSA: can amivantamab and lazertinib replace osimertinib in the front-line setting? Lung Cancer Targets Ther. 12, 41–47 (2024).
Peng, K. C. et al. Clinical outcomes of EGFR+/MET amp+ vs. EGFR+/MET amp-untreated patients with advanced non-small cell lung cancer. Thorac. Cancer 13, 1619–1630 (2022).
Wang, H.-M. et al. Using patient-derived organoids to predict locally advanced or metastatic lung cancer tumor response: a real-world study. Cell Rep. Med. 4 https://doi.org/10.1016/j.xcrm.2022.100911 (2023).
Xiao, K., Chu, T., Zhu, Y., Yang, S. & Xiao, Y. EP.12A.40 savolitinib plus osimertinib for previously untreated NSCLC patients with EGFR mutations and met-amplification: a case series. J. Thorac. Oncol. 19, S637 (2024).
Wu, Y.-L. et al. Tepotinib plus osimertinib in patients with EGFR-mutated non-small-cell lung cancer with MET amplification following progression on first-line osimertinib (INSIGHT 2): A multicentre, open-label, phase 2 trial. Lancet Oncol. 25, 989–1002 (2024).
Passaro, A. et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann. Oncol. 35, 77–90 (2024).
McCoach, C. E. et al. Phase I/II study of capmatinib plus erlotinib in patients with MET-positive non-small-cell lung cancer. JCO Precis. Oncol. 1 https://doi.org/10.1200/po.20.00279 (2021).
Lou, N. N. et al. Response to tyrosine kinase inhibitors in advanced non-small-cell lung cancer with concomitant c-MET overexpression and EGFR mutation. J. Clin. Oncol. 34, 9054–9054 (2016).
Lou, N. N. The Impact of C-met Overexpression on Targeted Therapy in Advanced Lung Cancer With Driver Gene Positivity. Master's thesis, Southern Medical University, Guangzhou, China (2016). (in Chinese).
Ahn, M. J. et al. 2O: SAVANNAH: savolitinib (savo) + osimertinib (osi) in patients (pts) with EGFRm advanced NSCLC and METoverexpression (OverExp) and/or amplification (Amp) following progressive disease (PD) on osi. J. Thorac. Oncol. 20, S4–S5 (2025).
Li, A., Chen, H.-J. & Yang, J.-J. Design and rationale for a phase II, randomized, open-label, two-cohort multicenter interventional study of Osimertinib with or without savolitinib in de novo MET aberrant, EGFR-mutant patients with advanced non-small-cell lung cancer: the FLOWERS trial. Clin. Lung Cancer 24, 82–88 (2023).
Xiang, C. et al. Unraveling the significance of MET focal amplification in lung cancer: integrative NGS, FISH, and IHC investigation. Mod. Pathol. 37, 100451 (2024).
Hartmaier, R. J. et al. Osimertinib + savolitinib to overcome acquired MET-mediated resistance in epidermal growth factor receptor-mutated, MET-amplified non-small cell lung cancer: TATTON. Cancer Discov. 13, 98–113 (2023).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81972164 [J.-J.Y.] and 82003273 [A.L.]), the High-level Hospital Construction Project of Guangdong Provincial People’s Hospital (Grant No. DFJH201809 [J.-J.Y.]), the Natural Science Foundation of Guangdong Province (Grant No. 2019A1515010931 [J.-J.Y.]), the Guangdong Association of Clinical Trials Guangdong Association of Clinical Trials (GACT) /Chinese Thoracic Oncology Group (CTONG) (Grant No. CTONG-YC20220106 [J.-J.Y.]), Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer (2017B030314120 [Y.L.W.]), the Chinese Society of Clinical Oncology pilot MET aberrant Solid Tumor Research Project (Grant Nos. Y-2022METAZZD-0110 [J.-J.Y.] and Y-2022METAZQN-0122 [A.L.]), and AstraZeneca China [J.-J.Y.]. We extend our gratitude to the patients and their families who participated in the FLOWERS trial. We thank Xiaolong Cao from the Affiliated Panyu Central Hospital of Guangzhou Medical University for his efforts in carrying out this study. A professional medical writer funded by the sponsor, assisted in drafting this manuscript.
Author information
Authors and Affiliations
Contributions
J.-J.Y. and A.L. contributed to the conceptualization, writing—original draft, and funding acquisition. J.-J.Y., A.L., W.N.F., J.L., B.F.X., J.Z., Y.J., K.J.T., Y.S.L., C.Z.Z., Y.F., C.R.X., Y.L.S., and H.J.C. recruited and treated patients and gathered clinical data on efficacy and safety. J-JY and HHY contributed to formal analysis of the data. Z.K.S. performed the bioinformatics analysis. AstraZeneca contributed to resources for the study. All authors had access to all the data in the study, and participated in reviewing, editing, and approving the final paper. All authors had final responsibility for the decision to submit the manuscript for publication. J-JY and AL have accessed and verifed the data.
Corresponding author
Ethics declarations
Competing interests
J.J.Y. received grants or contracts outside the submitted work and consulting fees from AstraZeneca. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zhigang Zhang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, A., Feng, WN., Li, J. et al. Osimertinib with or without savolitinib as first-line treatment for MET-aberrant, EGFR-mutant NSCLC: randomized phase 2 trial (FLOWERS). Nat Commun (2026). https://doi.org/10.1038/s41467-025-67950-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-67950-8