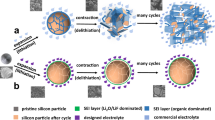
Abstract
Silicon-based solid-state batteries are promising next-generation high-energy-density technologies. However, poor (electro)chemical compatibility between silicon negative electrodes and solid electrolytes (e.g., Li6PS5Cl) plus sluggish interfacial kinetics severely limits their reversibility and Coulombic efficiency. Here, we propose a surface halogenation strategy that transforms the native amorphous SiO2 passivation layer on silicon particles into a functional Al(Si)OCl composite surface via controlled reaction with AlCl3. This artificial interphase reconciles interfacial incompatibility and enables fast ionic/electronic transport, suppressing irreversible lithium loss. The optimized negative electrode achieves a high initial Coulombic efficiency of 94.3% in half-cells and 85.6% initial Coulombic efficiency (86.6% with pre-lithiation) in full cells paired with LiNi0.88Co0.09Mn0.03O2. Enhanced reversibility further delivers long-term cyclability. The optimized negative electrode delivers 86% capacity retention and 99.998% average Coulombic efficiency over 200 cycles. Even at high-loading ( > 10 mAh cm-2, and no adhesives/conductive carbon/electrolyte), it retains 72% capacity after 500 cycles. The full cells maintain 80% capacity after 200 cycles at 1 C, with an average Coulombic efficiency exceeding 99.95%. The versatility of this halogenation strategy underscores halide chemistry’s broad potential in advancing high-performance, reversible silicon-based solid-state batteries.
Similar content being viewed by others
Data availability
The authors declare that all the relevant data are available within the paper and its Supplementary Information file or from the corresponding author upon request. Source data are provided with this paper.
References
Janek, J. & Zeier, W. G. Challenges in speeding up solid-state battery development. Nat. Energy 8, 230–240 (2023).
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Huo, H. et al. Chemo-mechanical failure mechanisms of the silicon anode in solid-state batteries. Nat. Mater. 23, 543–551 (2024).
Yan, W. et al. Hard-carbon-stabilized Li–Si anodes for high-performance all-solid-state Li-ion batteries. Nat. Energy 8, 800–813 (2023).
Tan, D. H. S. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 373, 1494–1499 (2021).
Ham, S.-Y. et al. Overcoming low initial coulombic efficiencies of Si anodes through prelithiation in all-solid-state batteries. Nat. Commun. 15, 2991 (2024).
Pan, H. et al. A solid-state lithium-ion battery with micron-sized silicon anode operating free from external pressure. Nat. Commun. 15, 2263 (2024).
Cao, D. et al. Long-cycling sulfide-based all-solid-state batteries enabled by electrochemo-mechanically stable electrodes. Adv. Mater. 34, 2200401 (2022).
Zhang, D., Yu, P., Zhang, Y., Zhao, X. & Yu, J. Vertical graphene sheet-encapsulated silicon nanoparticles for anodes of polymer-based all-solid-state batteries. ACS Appl. Energy Mater. 7, 726–734 (2024).
Yamamoto, M., Terauchi, Y., Sakuda, A. & Takahashi, M. Slurry mixing for fabricating silicon-composite electrodes in all-solid-state batteries with high areal capacity and cycling stability. J. Power Sources 402, 506–512 (2018).
Cangaz, S. et al. Enabling high-energy solid-state batteries with stable anode interphase by the use of columnar silicon anodes. Adv. Energy Mater. 10, 2001320 (2020).
Huo, H. et al. Decoupling the effects of interface chemical degradation and mechanical cracking in solid-state batteries with a silicon electrode. Adv. Mater. 37, 2415006 (2024).
Huang, Y., Shao, B., Wang, Y. & Han, F. Solid-state silicon anode with extremely high initial coulombic efficiency. Energy Environ. Sci. 16, 1569–1580 (2023).
Zhang, Z. et al. Silicon-based all-solid-state batteries operating free from external pressure. Nat. Commun. 16, 1013 (2025).
Li, Y. et al. Unveiling the mechanisms into Li-trapping induced (ir)reversible capacity loss for silicon anode. Energy Storage Mater. 55, 660–668 (2023).
Cao, D. et al. Unveiling the mechanical and electrochemical evolution of nanosilicon composite anodes in sulfide-based all-solid-state batteries. Adv. Energy Mater. 13, 2203969 (2023).
Lee, J. et al. Dry pre-lithiation for a graphite-silicon diffusion-dependent electrode for an all-solid-state battery. Adv. Energy Mater. 13, 23oo172 (2023).
Ye, L., Lu, Y., Wang, Y., Li, J. & Li, X. Fast cycling of lithium metal in solid-state batteries by constriction-susceptible anode materials. Nat. Mater. 23, 244–251 (2024).
Iwamura, S. et al. Li-Rich Li-Si Alloy as a Lithium-containing negative electrode material towards high-energy Lithium-ion batteries. Sci. Rep. 5, 8085 (2015).
Xiao, J. et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 5, 561–568 (2020).
Zhao, X., Zhao-Karger, Z., Fichtner, M. & Shen, X. Halide-based materials and chemistry for rechargeable batteries. Angew. Chem. Int. Ed. 59, 5902–5949 (2020).
Su, H. et al. A scalable Li-Al-Cl stratified structure for stable all-solid-state lithium metal batteries. Nat. Commun. 15, 4202 (2024).
Flores-González, N. et al. Mechanochemical synthesis and structure of Lithium tetrahaloaluminates, LiAlX4 (X = Cl, Br, I): a family of Li-ion conducting ternary halides. ACS Mater. Lett. 3, 652–657 (2021).
Cai, M. et al. Zincothermic reduction of silica to silicon: make the impossible possible. J. Mater. Chem. A 9, 21323–21331 (2021).
Ghita, R. V. et al. In Crystalline Silicon-Properties and Uses (ed. Sukumar B.) (IntechOpen, 2011).
Zhang, S. et al. A universal self-propagating synthesis of aluminum-based oxyhalide solid-state electrolytes. Angew. Chem. Int Ed. Engl. 63, e202401373 (2024).
Je, M. et al. Metal-mediated chlorine transfer for molten salt-driven thermodynamic change on silicon production. Adv. Sci. 12, 2412239 (2025).
Kaur, A., Chahal, P. & Hogan, T. Selective fabrication of SiC/Si diodes by excimer laser under ambient conditions. IEEE Electron Device Lett. 37, 142–145 (2016).
Dai, T. et al. Inorganic glass electrolytes with polymer-like viscoelasticity. Nat. Energy 8, 1221–1228 (2023).
Duan, H. et al. Amorphous AlOCl compounds enable nanocrystalline LiCl with abnormally high ionic conductivity. J. Am. Chem. Soc. 146, 29335–29343 (2024).
Yu, J. et al. A low temperature MgH2-AlCl3-SiO2 system to synthesize nano-silicon for high-performance Li-ion batteries. Chem. Eng. J. 406, 126805 (2021).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Yang, Z. et al. Surface passivated LixSi with improved storage stability as a prelithiation reagent in anodes. Electrochem. Commun. 138, 107272 (2022).
Fang, R. et al. Reaction mechanism optimization of solid-state Li–S batteries with a PEO-based electrolyte. Adv. Funct. Mater. 31, 2001812 (2021).
Wood, K. N. et al. Operando X-ray photoelectron spectroscopy of solid electrolyte interphase formation and evolution in Li2S-P2S5 solid-state electrolytes. Nat. Commun. 9, 2490 (2018).
Lu, Y., Zhao, C.-Z., Huang, J.-Q. & Zhang, Q. The timescale identification decoupling complicated kinetic processes in lithium batteries. Joule 6, 1172–1198 (2022).
Jiang, Y., Offer, G., Jiang, J., Marinescu, M. & Wang, H. Voltage hysteresis model for silicon electrodes for lithium ion batteries, including multi-step phase transformations, crystallization and amorphization. J. Electrochem. Soc. 167, 130533 (2020).
Jin, L. et al. Pre-lithiation strategies for next-generation practical lithium-ion batteries. Adv. Sci. 8, 2005031 (2021).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22075268, 22409173, 22472079, W2441017), the Key R&D Program of Zhejiang (2024SSYS0050), the Zhejiang Provincial Natural Science Foundation of China (LQ24B030015), and Zhejiang Provincial Postdoctoral Science Foundation (ZJ2024026).
Author information
Authors and Affiliations
Contributions
H.L. conceived and designed the experimental work and prepared the manuscript. Y.L. revised the Figures. G.H. and Y.L. provided technical guidance for solid-state battery assembly processes. C.X. and L.Z. performed NDP characterization. H.H. revised the manuscript. H.Z. supplemented the interfacial reaction calculation. W.X. and N.L. supervised the overall project and revised the manuscript. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Daxian Cao, Gemeng Liang and Jongwoo Lim for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Li, Y., Hu, G. et al. Surface halogenation engineering for reversible silicon-based solid-state batteries. Nat Commun (2025). https://doi.org/10.1038/s41467-025-67985-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-67985-x