Abstract
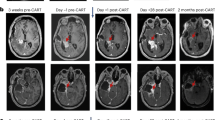
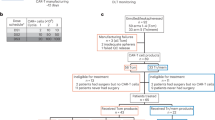
Patients with recurrent high-grade glioblastoma have a median survival of 6-8 months, with limited therapeutic options. In recent years, interest has grown in applying chimeric antigen receptor T (CAR-T) cells to solid cancers, including advanced gliomas. Here we generated off-the-shelf CRISPR-Cas9–edited IL-13Rα2-specific allogeneic universal CAR-T cells (MT026) by disrupting the endogenous TCR to prevent graft-versus-host disease and knocking out HLA class I molecules to mitigate the host-versus-graft response, and observed minimal NK-cell–mediated rejection in preclinical studies. In a first-in-human, single-center, open-label investigator-initiated trial (ChiCTR2000028801) in patients with high-grade glioma with prior therapy failure and short life expectancy, intrathecal injection of MT026 via lumbar puncture (1.0-3.0×10^7 cells per dose) demonstrated favorable tolerability and safety (primary outcome), pharmacokinetic characteristics, and preliminary clinical activity (secondary outcomes). Among the five patients enrolled, one achieved a complete response and three achieved partial responses. No grade ≥3 adverse events were observed; the predominant treatment-related toxicities were grade 1-2 pyrexia, hypoxia, and vomiting. Trial enrolment was halted after enrolment of the first five patients, however these preliminary clinical data support the potential benefit of locally administered allogeneic universal CAR-T cell therapy for recurrent glioblastoma.
Similar content being viewed by others
Data availability
The off-target detection datasets—comprising GUIDE-seq, PEM-seq, AID-seq, and amplicon sequencing—have been deposited in the Genome Sequence Archive in the National Genomics Data Center, China National Center for Bioinformation, under accession code HRA015321. To protect donor privacy concerning HLA genetic sequences, these data are under controlled access for two years following publication. During this two-year controlled-access period, qualified researchers may request access through the GSA controlled-access system or by contacting the corresponding author, Yulun Huang (Y.H.), for legitimate scientific purposes. Additionally, since the sequencing primers used in the data analysis methods implicate donor HLA privacy, all inquiries concerning data analysis methods must be addressed directly to the corresponding author. A copy of the study protocol is available in the Supplementary Information file. Individual de-identified participant data, including clinical information, imaging data, and treatment responses, are provided in the manuscript and/or the Supplementary Information. Additional de-identified participant-level data may be made available for academic, non-commercial research purposes upon reasonable request to the corresponding author Yulun Huang (Y.H.), subject to institutional approvals and a data-sharing agreement. All other data supporting the findings of this study are available in the article, its supplementary files and source data and/or from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 20, iv1–iv86 (2018).
Horbinski, C. et al. NCCN guidelines(R) insights: central nervous system cancers, Version 2.2022. J. Natl. Compr. Canc Netw. 21, 12–20 (2023).
Stupp, R. et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318, 2306–2316 (2017).
Walbert, T. & Mikkelsen, T. Recurrent high-grade glioma: a diagnostic and therapeutic challenge. Expert Rev. Neurother. 11, 509–518 (2011).
Bagley, S. J. et al. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 20, 1429–1438 (2018).
Lin, Y. J., Mashouf, L. A. & Lim, M. CAR T cell therapy in primary brain tumors: current investigations and the future. Front Immunol. 13, 817296 (2022).
Taraseviciute, A. et al. Chimeric antigen receptor T cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 8, 750–763 (2018).
Xu, S. et al. Immunotherapy for glioma: current management and future application. Cancer Lett. 476, 1–12 (2020).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med 375, 2561–2569 (2016).
Lombardi, G. et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 20, 110–119 (2019).
Stupp, R. et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur. J. Cancer 48, 2192–2202 (2012).
Braud, V. M. et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391, 795–799 (1998).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Cox, D. B., Platt, R. J. & Zhang, F. Therapeutic genome editing: prospects and challenges. Nat. Med 21, 121–131 (2015).
Choo, S. Y. The HLA system: genetics, immunology, clinical testing, and clinical implications. Yonsei Med J. 48, 11–23 (2007).
Xu, H. et al. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell 24, 566–578 e7 (2019).
Kitano, Y. et al. Generation of hypoimmunogenic induced pluripotent stem cells by CRISPR-Cas9 system and detailed evaluation for clinical application. Mol. Ther. Methods Clin. Dev. 26, 15–25 (2022).
Torikai, H. et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood 122, 1341–1349 (2013).
Torikai, H. et al. Genetic editing of HLA expression in hematopoietic stem cells to broaden their human application. Sci. Rep. 6, 21757 (2016).
Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res 23, 2255–2266 (2017).
Depil, S. et al. Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020).
Duygu, B. et al. HLA Class I molecules as immune checkpoints for NK cell alloreactivity and anti-viral immunity in kidney transplantation. Front Immunol. 12, 680480 (2021).
Sun, W. et al. Universal chimeric antigen receptor T cell therapy - The future of cell therapy: A review providing clinical evidence. Cancer Treat. Res Commun. 33, 100638 (2022).
Meril, S. et al. Targeting glycosylated antigens on cancer cells using siglec-7/9-based CAR T-cells. Mol. Carcinog. 59, 713–723 (2020).
Ouyang, H. et al. Baseline and early changes in the neutrophil-lymphocyte ratio (NLR) predict survival outcomes in advanced colorectal cancer patients treated with immunotherapy. Int Immunopharmacol. 123, 110703 (2023).
Rui, Y. & Green, J. J. Overcoming delivery barriers in immunotherapy for glioblastoma. Drug Deliv. Transl. Res 11, 2302–2316 (2021).
Choi, B. D. et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N. Engl. J. Med 390, 1290–1298 (2024).
Bagley, S. J. et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13Ralpha2 in recurrent glioblastoma: phase 1 trial interim results. Nat. Med 30, 1320–1329 (2024).
Brown, C. E. et al. Locoregional delivery of IL-13Ralpha2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial. Nat. Med 30, 1001–1012 (2024).
Brown, C. E. et al. Bioactivity and safety of IL13Ralpha2-Redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res 21, 4062–4072 (2015).
Haque, W. et al. Patterns of management and outcomes of unifocal versus multifocal glioblastoma. J. Clin. Neurosci. 74, 155–159 (2020).
Chamberlain, M. C. et al. Pharmacokinetics of intralumbar DTC-101 for the treatment of leptomeningeal metastases. Arch. Neurol. 52, 912–917 (1995).
Mackensen, A. et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial. Nat. Med 29, 2844–2853 (2023).
Kalbasi, A. et al. Potentiating adoptive cell therapy using synthetic IL-9 receptors. Nature 607, 360–365 (2022).
O’Rourke, D. M., et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Brown, M. P., Ebert, L. M. & Gargett, T. Erratum: clinical chimeric antigen receptor T-cell therapy: a new and promising treatment modality for glioblastoma. Clin. Transl. Immunol. 10, e1331 (2021).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med 6, 224ra225 (2014).
Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022).
Waxman, E. S. & Gerber, D. L. Pseudoprogression and immunotherapy phenomena. J. Adv. Pr. Oncol. 11, 723–731 (2020).
Brandes, A. A. et al. Temozolomide in patients with glioblastoma at second relapse after first line nitrosourea-procarbazine failure: a phase II study. Oncology 63, 38–41 (2002).
Chang, S. M. et al. Temozolomide in the treatment of recurrent malignant glioma. Cancer 100, 605–611 (2004).
Brada, M. et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann. Oncol. 12, 259–266 (2001).
Yung, W. K. et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br. J. Cancer 83, 588–593 (2000).
Moen, M. D. Bevacizumab: in previously treated glioblastoma. Drugs 70, 181–189 (2010).
Kim, M. M., Umemura, Y. & Leung, D. Bevacizumab and glioblastoma: past, present, and future directions. Cancer J. 24, 180–186 (2018).
Chen, Z., Hu, Y. & Mei, H. Advances in CAR-engineered immune cell generation: engineering approaches and sourcing strategies. Adv. Sci. (Weinh.) 10, e2303215 (2023).
Martinez, M. & Moon, E. K. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 10, 128 (2019).
Acknowledgements
This work was supported by foundation of Chongqing International Institute for Immunology (2021YZH01 to X-Y. S), National Natural Science Foundation of China (No. 82173279, 82472981 to Y-L.H), and National Science and Technology Resource Sharing Service Platform (YCZYPT[2020]06-1 to Y-L.H), Suzhou Medical and Health Innovation Project (CXYJ2024A05 to Y-L.H), Suzhou Industrial Park Healthcare Talent Support Initiative (2024-54 to Y-L.H), Gusu Talent Program(2024-105 to Y-L.H), and the National Natural Science Foundation of China (82350112 to H-B.L.). These funders provided support for study design, data collection and analysis, and manuscript preparation. We thank the patients who participated in this trial and their families for making this trial possible; the study team, caregivers, and other personnel for their individual professional assistance; and the staff of the department of technology, T-MAXIMUM Pharmaceuticals (Suzhou) Co., Ltd., for technical support. We also extend our gratitude to Yijin Li and Xiu Zhao from T-MAXIMUM Pharmaceuticals for their valuable insights and fruitful discussions that significantly contributed to the development of this study.
Author information
Authors and Affiliations
Contributions
Y.H., X.S., and Y.W. conceived the clinical trial concept. ZhongW., X.L, ZhiminW., J. L., X. Y., H.Z. and Z.X. enrolled patients in the clinical trial, X.Z., X.R., Y.L., X.L., J.S., Z.B., and L.H. evaluated toxicity and participated in critical discussions, as well as manuscript writing and editing. W.G. performed the pathology assessments. H.L. and L.W. performed the immunological assays. X.J., YangZ., and J.C. conducted formal analysis, data visualization, writing—original draft and writing—review and editing. X.S., YuZ., J.G., X.M., and Y.Wang obtained resources, managed data, performed project administration, and performed writing—review and editing. Y.W. and Y.H. contributed to writing—original draft, writing—review and editing, funding acquisition, formal analysis, and data curation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Shang, X., Liu, J. et al. Intrathecal CRISPR-edited allogeneic IL-13Rα2 CAR T Cells for recurrent high-grade Glioma: preclinical characterization and phase I trial. Nat Commun (2026). https://doi.org/10.1038/s41467-025-68112-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-68112-6