Abstract
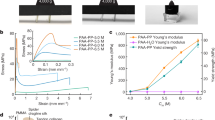
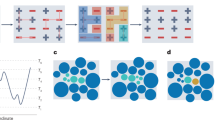
Supercooled liquids undergo a rapid change in dynamics as they are cooled to their glass transition temperature and turn from a flowing liquid into an amorphous solid. Depending on how steeply the viscosity changes with temperature around the glass transition, glass formers are classified as strong or fragile. An empirical relation exists between the fragility of the liquid and the non-exponentiality of its α-relaxation. However, the microscopic origins of this correlation remain unclear and its generality has been debated. Here, we demonstrate that this relationship is inverted in organic materials with ionic interactions. We introduce a class of materials consisting of highly charged hydrophobic polymers cross-linked via moderated ionic interactions, and show that these combine a strong glass transition with an unusually stretched mechanical relaxation spectrum. By surveying a large variety of ionic liquids, polymerized ionic liquids, and ionomers, we show that all these charged materials follow a trend between fragility and non-exponential relaxation that is opposite to that of non-charged materials. This finding suggests a special role of long-ranged ionic interactions in vitrification and opens up a route toward developing new materials that combine the processability of strong glass formers with the mechanical dissipation of polymers.
Similar content being viewed by others
Data availability
All relevant data are included in this article and its Supplementary Information files. Source data are provided with this paper.
Code availability
Scripts for the simulations are available on https://github.com/giuntoli-group/ionic-glass-formers94
References
Angell, C. A. Formation of glasses from liquids and biopolymers. Science 267, 1924–1935 (1995).
Angell, C. A. Relaxation in liquids, polymers and plastic crystals—strong/fragile patterns and problems. J. Non-Cryst. Solids 131-133, 13–31 (1991).
Montarnal, D., Capelot, M., Tournilhac, F. & Leibler, L. Silica-like malleable materials from permanent organic networks. Science 334, 965–968 (2011).
Denissen, W., Winne, J. M. & Du Prez, F. E. Vitrimers: permanent organic networks with glass-like fluidity. Chem. Sci. 7, 30–38 (2016).
Ciarella, S., Biezemans, R. A. & Janssen, L. M. C. Understanding, predicting, and tuning the fragility of vitrimeric polymers. Proc. Natl. Acad. Sci. USA 116, 25013–25022 (2019).
Dalle-Ferrier, C. et al. Why many polymers are so fragile: a new perspective. J. Chem. Phys. 145, 154901 (2016).
Saika-Voivod, I., Poole, P. H. & Sciortino, F. Fragile-to-strong transition and polyamorphism in the energy landscape of liquid silica. Nature 412, 514–517 (2001).
Debenedetti, P. G. & Stillinger, F. H. Supercooled liquids and the glass transition. Nature 410, 259–267 (2001).
Sastry, S., Debenedetti, P. G. & Stillinger, F. H. Signatures of distinct dynamical regimes in the energy landscape of a glass-forming liquid. Nature 393, 554–557 (1998).
Hall, R. W. & Wolynes, P. G. Microscopic theory of network glasses. Phys. Rev. Lett. 90, 085505 (2003).
Ikeda, M. & Aniya, M. Correlation between fragility and cooperativity in bulk metallic glass-forming liquids. Intermetallics 18, 1796–1799 (2010).
Ruocco, G., Sciortino, F., Zamponi, F., De Michele, C. & Scopigno, T. Landscapes and fragilities. J. Chem. Phys. 120, 10666–10680 (2004).
Betancourt, P. B. A., Starr, F. W. & Douglas, J. F. String-like collective motion in the α-and β-relaxation of a coarse-grained polymer melt. J. Chem. Phys. 148, 104508 (2018).
Furukawa, A. & Tanaka, H. Significant difference in the dynamics between strong and fragile glass formers. Phys. Rev. E 94, 052607 (2016).
Betancourt, B. A. P., Douglas, J. F. & Starr, F. W. Fragility and cooperative motion in a glass-forming polymer–nanoparticle composite. Soft Matter 9, 241–254 (2013).
Vilgis, T. A. Strong and fragile glasses: a powerful classification and its consequences. Phys. Rev. B 47, 2882 (1993).
Krausser, J., Samwer, K. H. & Zaccone, A. Interatomic repulsion softness directly controls the fragility of supercooled metallic melts. Proc. Natl. Acad. Sci. USA 112, 13762–13767 (2015).
Kohlrausch, R. Theorie des elektrischen Rückstandes in der Leidener Flasche. Ann. Phys. 167, 179–214 (1854).
Williams, G. & Watts, D. C. Non-symmetrical dielectric relaxation behaviour arising from a simple empirical decay function. Trans. Faraday Soc. 66, 80–85 (1970).
Boehmer, R., Ngai, K. L., Angell, C. A. & Plazek, D. J. Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. 99, 4201 (1993).
Plazek, D. J. & Ngai, K. L. Correlation of polymer segmental chain dynamics with temperature-dependent time-scale shifts. Macromolecules 24, 1222–1224 (1991).
Zhang, D., Sun, D. & Gong, X. Angell plot from the potential energy landscape perspective. Phys. Rev. E 106, 064129 (2022).
Gupta, P. K. & Mauro, J. C. Two factors governing fragility: stretching exponent and configurational entropy. Phys. Rev. E—Stat., Nonlinear, Soft Matter Phys. 78, 062501 (2008).
Xia, X. & Wolynes, P. G. Microscopic theory of heterogeneity and nonexponential relaxations in supercooled liquids. Phys. Rev. Lett. 86, 5526 (2001).
Hong, L., Novikov, V. & Sokolov, A. P. Is there a connection between fragility of glass forming systems and dynamic heterogeneity/cooperativity? J. Non-Cryst. Solids 357, 351–356 (2011).
Heuer, A. Exploring the potential energy landscape of glass-forming systems: from inherent structures via metabasins to macroscopic transport. J. Phys.: Condens. Matter 20, 373101 (2008).
Dyre, J. C. Ten themes of viscous liquid dynamics. J. Phys.: Condens. Matter 19, 205105 (2007).
Ojovan, M. I. & Tournier, R. F. On structural rearrangements near the glass transition temperature in amorphous silica. Materials (Basel) 14, 5235 (2021).
Yang, Y. et al. Detecting topology freezing transition temperature of vitrimers by AIE luminogens. Nat. Commun. 10, 3165 (2019).
Nakamura, K., Fukao, K. & Inoue, T. Viscoelastic behavior of polymerized ionic liquids with various charge densities. Nihon Reoroji Gakkaishi 41, 21–27 (2013).
Ueno, K., Zhao, Z., Watanabe, M. & Angell, C. A. Protic ionic liquids based on decahydroisoquinoline: lost superfragility and ionicity-fragility correlation. J. Phys. Chem. B 116, 63–70 (2012).
Michaels, A. S. Polyelectrolyte complexes. Ind. Eng. Chem. 57, 32–40 (1965).
Wang, Q. & Schlenoff, J. B. The polyelectrolyte complex/coacervate continuum. Macromolecules 47, 3108–3116 (2014).
Zhang, Y., Li, F., Valenzuela, L. D., Sammalkorpi, M. & Lutkenhaus, J. L. Effect of water on the thermal transition observed in poly(allylamine hydrochloride)-poly(acrylic acid) complexes. Macromolecules 49, 7563–7570 (2016).
van Lange, S. G. M. et al. Moderated ionic bonding for water-free recyclable polyelectrolyte complex materials. Sci. Adv. 10, 3606 (2024).
Gebbie, M. A. et al. Long range electrostatic forces in ionic liquids. Chem. Commun. 53, 1214–1224 (2017).
van Lange, S. G. M. et al. Unraveling the temperature-dependent relaxation dynamics of ionic liquid-plasticized compleximers. Macromolecules 58, 7522 (2025).
Wu, J., Huang, G., Qu, L. & Zheng, J. Correlations between dynamic fragility and dynamic mechanical properties of several amorphous polymers. J. Non-Cryst. Solids 355, 1755–1759 (2009).
Spruijt, E., Cohen Stuart, M. A. & van der Gucht, J. Linear viscoelasticity of polyelectrolyte complex coacervates. Macromolecules 46, 1633–1641 (2013).
Ferry, J. D. Viscoelastic Properties of Polymers (John Wiley and Sons, New York, 1980).
Zaccone, A., Winter, H., Siebenbürger, M. & Ballauff, M. Linking self-assembly, rheology, and gel transition in attractive colloids. J. Rheol. 58, 1219–1244 (2014).
Urbain, G., Bottinga, Y. & Richet, P. Viscosity of liquid silica, silicates and alumino-silicates. Geochim. Cosmochim. Acta 46, 1061–1072 (1982).
Huang, D. & McKenna, G. B. New insights into the fragility dilemma in liquids. J. Chem. Phys. 114, 5621–5630 (2001).
Ljubić, D., Stamenović, M., Smithson, C., Nujkić, M., Medo, B. & Putić, S. Time-temperature superposition principle: application of WLF equation in polymer analysis and composites. Zaštita Mater. 55, 395–400 (2014).
Shi, R., Russo, J. & Tanaka, H. Origin of the emergent fragile-to-strong transition in supercooled water. Proc. Natl. Acad. Sci. USA 115, 9949 (2018).
Zhang, H., Wang, X., Yu, H.-B. & Douglas, J. F. Dynamic heterogeneity, cooperative motion, and Johari-Goldstein β-relaxation in a metallic glass-forming material exhibiting a fragile-to-strong transition. Eur. Phys. J. E 44, 56 (2021).
Dixon, P. K. Specific-heat spectroscopy and dielectric susceptibility measurements of salol at the glass transition. Phys. Rev. B 42, 8179–8186 (1990).
Meng, F., Saed, M. O. & Terentjev, E. M. Rheology of vitrimers. Nat. Commun. 13, 5753 (2022).
Elling, B. R. & Dichtel, W. R. Reprocessable cross-linked polymer networks: are associative exchange mechanisms desirable? ACS Cent. Sci. 6, 1488 (2020).
Roig, A., Agizza, M., Serra, A. & Flor, S. Disulfide vitrimeric materials based on cystamine and diepoxy eugenol as bio-based monomers. Eur. Polym. J. 194, 112185 (2023).
Zhu, Y. et al. Catalyst-free cardanol-based epoxy vitrimers for self-healing, shape memory, and recyclable materials. Polymers 16, 307 (2024).
Hong, Y. & Goh, M. Vitrimer nanocomposites for highly thermal conducting materials with sustainability. Polym. (Basel) 16, 365 (2024).
Cywar, R. M. et al. Elastomeric vitrimers from designer polyhydroxyalkanoates with recyclability and biodegradability. Sci. Adv. 9, 1735 (2023).
Denissen, W., Rivero, G., Nicolaÿ, R., Leibler, L., Winne, J. M. & Du Prez, F. E. Vinylogous urethane vitrimers. Adv. Funct. Mater. 25, 2451–2457 (2015).
Bin Rusayyis, M. A. & Torkelson, J. M. Reprocessable covalent adaptable networks with excellent elevated-temperature creep resistance: facilitation by dynamic, dissociative bis(hindered amino) disulfide bonds. Polym. Chem. 12, 2760–2771 (2021).
Qiao, J., Casalini, R., Pelletier, J.-M. & Yao, Y. Dynamics of the strong metallic glass Zn38Mg12Ca32Yb18. J. Non-Cryst. Solids 447, 85–90 (2016).
Chen, Q., Tudryn, G. J. & Colby, R. H. Ionomer dynamics and the sticky Rouse model. J. Rheol. 57, 1441–1462 (2013).
Weiss, R., Fitzgerald, J. & Kim, D. Viscoelastic behavior of lightly sulfonated polystyrene ionomers. Macromolecules 24, 1071–1076 (1991).
Tao, R. & Simon, S. L. Rheology of imidazolium-based ionic liquids with aromatic functionality. J. Phys. Chem. B 119, 11953–11959 (2015).
Shamim, N. & McKenna, G. B. Glass dynamics and anomalous aging in a family of ionic liquids above the glass transition temperature. J. Phys. Chem. B 114, 15742–15752 (2010).
Pogodina, N. et al. Molecular dynamics of ionic liquids as probed by rheology. J. Rheol. 55, 241–256 (2011).
Yokokoji, A. et al. Viscoelastic relaxation of polymerized ionic liquid and lithium salt mixtures: effect of salt concentration. Polymers 13, 1772 (2021).
Shi, G., Zheng, X. & Liu, Y. Unusual nonlinearity and nonexponentiality in glass-forming polymers governed by ionic interactions. J. Phys. Chem. B 129, 9537–9550 (2025).
Lunkenheimer, P., Loidl, A., Riechers, B., Zaccone, A. & Samwer, K. Thermal expansion and the glass transition. Nat. Phys. 19, 694–699 (2023).
MacDonald, D. & Roy, S. Vibrational anharmonicity and lattice thermal properties. ii. Phys. Rev. 97, 673 (1955).
Dyre, J. C. Source of non-Arrhenius average relaxation time in glass-forming liquids. J. Non-Cryst. Solids 235, 142–149 (1998).
Hecksher, T. & Dyre, J. C. A review of experiments testing the shoving model. J. Non-Cryst. Solids 407, 14–22 (2015).
Dyre, J. C. & Wang, W. H. The instantaneous shear modulus in the shoving model. J. Chem. Phys. 136, 224108 (2012).
Puosi, F. & Leporini, D. Communication: correlation of the instantaneous and the intermediate-time elasticity with the structural relaxation in glassforming systems. J. Chem. Phys. 136, 041104 (2012).
Yuan, Q.-L., Xu, X., Douglas, J. F. & Xu, W.-S. Understanding relaxation in the Kob-Andersen liquid based on entropy, string, shoving, localization, and parabolic models. J. Phys. Chem. B 128, 10999 (2024).
Dudowicz, J., Freed, K. F. & Douglas, J. F. Generalized entropy theory of polymer glass formation. Adv. Chem. Phys. 137, 125 (2008).
Xu, W.-S., Douglas, J. F. & Freed, K. F. Entropy theory of polymer glass-formation in variable spatial dimension. Adv. Chem. Phys. 161, 443 (2016).
Stukalin, E. B., Douglas, J. F. & Freed, K. F. Application of the entropy theory of glass formation to poly(α-olefins). J. Chem. Phys. 131, 114905 (2009).
Xu X., Douglas, J. F. & Xu, W.-S. Generalized entropy theory investigation of the relatively high segmental fragility of many glass-forming polymers. Soft Matter 21, 2664 (2025).
Yuan, Q.-L., Xu, X., Douglas, J. F. & Xu, W.-S. Physical origin of the mass dependence of glass transition temperature and fragility of polymer liquids. Macromolecules 58, 9528 (2025).
Ediger, M. D. Spatially heterogeneous dynamics in supercooled liquids. Annu. Rev. Phys. Chem. 51, 99–128 (2000).
Berthier, L. Dynamic heterogeneity in amorphous materials. Physics 4, 42 (2011).
Arbe, A., Colmenero, J., Monkenbusch, D. M. & Richter, D. Dynamics of glass-forming polymers: “homogeneous” versus “heterogeneous” scenario. Phys. Rev. Lett. 81, 590 (1998).
Russell, E. V. & Israeloff, N. E. Direct observation of molecular cooperativity near the glass transition. Nature 408, 695 (2000).
Ediger, M. D., Angell, C. A. & Nagel, S. R. Supercooled liquids and glasses. J. Phys. Chem. 100, 13200–13212 (1996).
Kob, W., Donati, C., Plimpton, S. J., Poole, P. H. & Glotzer, S. S. Dynamical heterogeneities in a supercooled Lennard-Jones liquid. Phys. Rev. Lett. 79, 2827 (1997).
Starr, F. W., Douglas, J. F. & Sastry, S. The relationship of dynamical heterogeneity to the Adam-Gibbs and random first-order transition theories of glass formation. J. Chem. Phys. 138, 12–541 (2013).
Milkus, R., Ness, C., Palyulin, V. V., Weber, J., Lapkin, A. & Zaccone, A. Interpretation of the vibrational spectra of glassy polymers using coarse-grained simulations. Macromolecules 51, 1559–1572 (2018).
Giuntoli, A. & Leporini, D. Boson peak decouples from elasticity in glasses with low connectivity. Phys. Rev. Lett. 121, 185502 (2018).
Zaccone, A. Relaxation and vibrational properties in metal alloys and other disordered systems. J. Phys.: Condens. Matter 32, 203001 (2020).
Cui, B., Milkus, R. & Zaccone, A. The relation between stretched-exponential relaxation and the vibrational density of states in glassy disordered systems. Phys. Lett. A 381, 446–451 (2017).
Wrede, M., Ganza, V., Bucher, J. & Straub, B. F. Polyelectrolyte gels comprising a lipophilic, cost-effective aluminate as fluorine-free absorbents for chlorinated hydrocarbons and diesel fuel. ACS Appl. Mater. Interfaces 4, 3453–3458 (2012).
Kammakakam, I. et al. Tailored CO2-philic anionic poly (ionic liquid) composite membranes: synthesis, characterization, and gas transport properties. ACS Sustain. Chem. Eng. 8, 5954–5965 (2020).
Ghostine, R. A., Shamoun, R. F. & Schlenoff, J. B. Doping and diffusion in an extruded saloplastic polyelectrolyte complex. Macromolecules 46, 4089–4094 (2013).
Yang, Z., Xu, X. & Xu, W.-S. Influence of ionic interaction strength on glass formation of an ion-containing polymer melt. Macromolecules 54, 9587–9601 (2021).
Thompson, A. P. et al. LAMMPS - a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 271, 108171 (2022).
Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the open visualization tool. Model. Simul. Mater. Sci. Eng. 18, 015012 (2010).
Liu, A. Y., Emamy, H., Douglas, J. F. & Starr, F. W. Effects of chain length on the structure and dynamics of semidilute nanoparticle–polymer composites. Macromolecules 54, 3041–3051 (2021).
Vengallur, N., van Lange, S. G. M., van der Gucht, J. & Giuntoli, A. giuntoli-group/ionic-glass-formers https://doi.org/10.5281/zenodo.17900465 (2025).
Matsuda, Y. et al. Calorimetric study of the glass transition dynamics in lithium borate glasses over a wide composition range by modulated dsc. Fluid Phase Equilib. 256, 127–131 (2007).
Kodama, M. & Kojima, S. Anharmonicity and fragility in lithium borate glasses. J. Therm. Anal. Calorim. 69, 961–970 (2002).
Acknowledgements
This work was supported by the Dutch Research Council (NWO) OCENW.KLEIN.326 (J.v.d.G.).
Author information
Authors and Affiliations
Contributions
S.v.L., J.S., and J.v.d.G. conceived the project. S.v.L., J.v.d.G., D.t.B., A.G., and N.V. developed the methodology. S.v.L., D.t.B., E.B., J.P., and M.v.N. performed synthesis and characterization. S.v.L., E.B., M.v.N., and N.V. curated the data. S.v.L., E.B., M.v.N., and J.v.d.G. carried out the data analysis. N.V. and A.G. performed the programming and code testing; S.v.L. and J.v.d.G. validated modeling data with help of A.G. and A.Z. S.v.L. created the visualizations. S.v.L. and J.v.d.G. wrote the original draft. All authors (S.v.L., D.t.B., E.G., J.P., M.v.N., N.V., A.Z., A.G., J.S., and J.v.d.G.) reviewed and edited the manuscript. J.v.d.G. and J.S. supervised the project. J.v.d.G. acquired the funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jack Douglas and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
van Lange, S.G.M., te Brake, D.W., Brink, E.F. et al. Ionic glass formers show an inverted relation between fragility and non-exponential alpha-relaxation. Nat Commun (2026). https://doi.org/10.1038/s41467-025-68124-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-68124-2