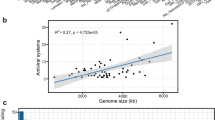
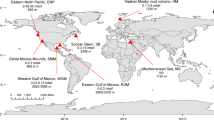
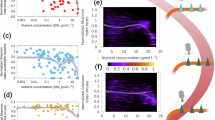
Abstract
Cold seeps host diverse microbes and viruses with numerous unexplored defense and anti-defense systems. Analysis of 3813 microbial and 13,336 viral genomes from 191 metagenomes across 17 cold seep sites reveals extensive microbial defense repertoires, with over 60% representing candidate systems. Experimental validation confirms that several candidates protect against viral infection. These defense systems frequently co-occur, suggesting potential synergistic interactions, and are broadly distributed across sediments. In response, viruses have evolved diverse anti-defense genes, and the concurrent presence of multiple viral and microbial systems highlights intricate coevolution. Functionally critical lineages, such as anaerobic methanotrophic archaea, sulfate-reducing bacteria, and diazotrophs, appear to modify their defensive strategies under ecological and environmental pressures; for example, sulfate-reducing bacteria harbor multiple Gabija systems while corresponding viruses carry anti-Gabija genes, illustrating specific coevolutionary adaptations. Overall, these findings underscore the critical role of virus-microbe interactions in shaping microbial metabolic functions and environmental adaptation in deep-sea ecosystems.
Similar content being viewed by others
Data availability
The metagenomic data analyzed in this study are available from our previous publication65 and the NCBI BioProject ID PRJNA1169195 (Supplementary Data 17). The metatranscriptomic raw reads are available under NCBI BioProject IDs PRJNA738468, PRJNA739005, and PRJNA831433 (Supplementary Data 18). The non-redundant MAG catalogue and viral genomes are available under NCBI BioProject PRJNA950938. Accession numbers of the protein sequences used to construct the custom database for identifying anti-defence genes are provided in Supplementary Data 19. Protein sequences corresponding to defence systems, candidate defence systems, and anti-defence systems have been deposited in the GSA database under BioProject PRJCA052411105,106. Phylogenetic trees of GajAB based on amino acid sequences and protein structural analyses generated in this study are available on Figshare at (https://doi.org/10.6084/m9.figshare.27004438). All additional data supporting the findings of this study are provided within the article and its Supplementary Information files. Source data are provided with this paper.
Code availability
The present study did not generate codes, and mentioned tools used for the data analysis were applied with default parameters unless specified otherwise.
References
Dong, X. et al. Metabolic potential of uncultured bacteria and archaea associated with petroleum seepage in deep-sea sediments. Nat. Commun. 10, 1816 (2019).
Joye, S. B. The geology and biogeochemistry of hydrocarbon seeps. Annu. Rev. Earth Planet. Sci. 48, 205–231 (2020).
Dong, X. et al. Thermogenic hydrocarbon biodegradation by diverse depth-stratified microbial populations at a Scotian Basin cold seep. Nat. Commun. 11, 5825 (2020).
Kleindienst, S. et al. Diverse sulfate-reducing bacteria of the Desulfosarcina/Desulfococcus clade are the key alkane degraders at marine seeps. ISME J. 8, 2029–2044 (2014).
Yang, X. Y., Shen, Z., Lin, Q. & Fu, T. M. Molecular basis of Gabija anti-phage supramolecular assemblies. Nature 15, 836 (2023).
Li, Z. et al. Deep sea sediments associated with cold seeps are a subsurface reservoir of viral diversity. ISME J. 15, 2366–2378 (2021).
Doron, S. et al. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359, eaar4120 (2018).
Millman, A. et al. An expanded arsenal of immune systems that protect bacteria from phages. Cell Host Microbe 30, 1556–1569 (2022).
Beavogui, A. et al. The defensome of complex bacterial communities. Nat. Commun. 15, 2146 (2024).
Peng, Y. et al. Viruses in deep-sea cold seep sediments harbor diverse survival mechanisms and remain genetically conserved within species. ISME J. 17, 1774–1784 (2023).
Georjon, H. & Bernheim, A. The highly diverse antiphage defence systems of bacteria. Nat. Rev. Microbiol. 21, 686–700 (2023).
Tock, M. R. & Dryden, D. T. F. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 8, 466–472 (2005).
Hille, F. et al. The biology of CRISPR-Cas: backward and forward. Cell 172, 1239–1259 (2018).
Cheng, R. et al. Prokaryotic Gabija complex senses and executes nucleotide depletion and DNA cleavage for antiviral defense. Cell Host Microbe 31, 1331–1344 (2023).
Rousset, F. et al. A conserved family of immune effectors cleaves cellular ATP upon viral infection. Cell 186, 3619–3631 (2023).
Lopatina, A., Tal, N. & Sorek, R. Abortive infection: bacterial suicide as an antiviral immune strategy. Annu. Rev. Virol. 7, 371–384 (2020).
Saenz, J. S., Seifert, J. & Rios-Galicia, B. Antiviral defense systems in the rumen microbiome. mSystems 10, e01521–e01524 (2025).
Piel, D. et al. Phage–host coevolution in natural populations. Nat. Microbiol. 7, 1075–1086 (2022).
Tesson, F. et al. Systematic and quantitative view of the antiviral arsenal of prokaryotes. Nat. Commun. 13, 2561 (2022).
Bernheim, A. & Sorek, R. The pan-immune system of bacteria: antiviral defence as a community resource. Nat. Rev. Microbiol. 18, 113–119 (2020).
Rocha, E. P. C. & Bikard, D. Microbial defenses against mobile genetic elements and viruses: Who defends whom from what? PLoS Biol. 20, e3001514 (2022).
Picton, D. M. et al. The phage defence island of a multidrug resistant plasmid uses both BREX and type IV restriction for complementary protection from viruses. Nucleic Acids Res. 49, 11257–11273 (2021).
Wu, Y. et al. Bacterial defense systems exhibit synergistic anti-phage activity. Cell Host Microbe 32, 557–572.e6 (2024).
Mayo-Muñoz, D., Pinilla-Redondo, R., Camara-Wilpert, S., Birkholz, N. & Fineran, P. C. Inhibitors of bacterial immune systems: discovery, mechanisms and applications. Nat. Rev. Genet. 25, 237–254 (2024).
Hampton, H. G., Watson, B. N. J. & Fineran, P. C. The arms race between bacteria and their phage foes. Nature 577, 327–336 (2020).
Duan, N., Hand, E., Pheko, M., Sharma, S. & Emiola, A. Structure-guided discovery of anti-CRISPR and anti-phage defense proteins. Nat. Commun. 15, 649 (2024).
Gussow, A. B. et al. Machine-learning approach expands the repertoire of anti-CRISPR protein families. Nat. Commun. 11, 3784 (2020).
Serfiotis-Mitsa, D. et al. The structure of the KlcA and ArdB proteins reveals a novel fold and antirestriction activity against Type I DNA restriction systems in vivo but not in vitro. Nucleic Acids Res. 38, 1723–1737 (2010).
Hobbs, S. J. et al. Phage anti-CBASS and anti-Pycsar nucleases subvert bacterial immunity. Nature 605, 522–526 (2022).
Antine, S. P. et al. Structural basis of Gabija anti-phage defence and viral immune evasion. Nature 625, 360–365 (2024).
An, L. et al. Global diversity and ecological functions of viruses inhabiting oil reservoirs. Nat. Commun. 15, 6789 (2024).
Zhong, Z.-P. et al. Viral potential to modulate microbial methane metabolism varies by habitat. Nat. Commun. 15, 1857 (2024).
Payne, L. J. et al. Identification and classification of antiviral defence systems in bacteria and archaea with PADLOC reveals new system types. Nucleic Acids Res. 49, 10868–10878 (2021).
Payne,L. J., Hughes, T.C.D.,Fineran, P.C. & Jackson, S. A. New antiviral defences are genetically embedded within prokaryotic immune systems.bioRxivhttps://doi.org/10.1101/2024.01.29.577857 (2024).
Mayo-Munoz, D., Pinilla-Redondo, R., Birkholz, N. & Fineran, P. C. A host of armor: Prokaryotic immune strategies against mobile genetic elements. Cell Rep. 42, 112672 (2023).
Tesson, F. et al. A comprehensive resource for exploring antiphage defense: DefenseFinder webservice, wiki and databases. Nucleic Acids Res. 53, 13 (2025).
Leão, P. et al. Asgard archaea defense systems and their roles in the origin of eukaryotic immunity. Nat. Commun. 15, 6386 (2024).
Liu, Y., Botelho, J. & Iranzo, J. Timescales and genetic linkage explain the variable impact of defense systems on horizontal gene transfer. Genome Res. 35, 268–278 (2025).
Kogay, R., Wolf, Y. I. & Koonin, E. V. Defence systems and horizontal gene transfer in bacteria. Environ. Microbiol. 26, e16630 (2024).
Li, M. et al. Toxin-antitoxin RNA pairs safeguard CRISPR-Cas systems. Science 372, eabe5601 (2021).
Dupuis, M. -È, Villion, M., Magadán, A. H. & Moineau, S. CRISPR-Cas and restriction–modification systems are compatible and increase phage resistance. Nat. Commun. 4, 2087 (2013).
Maguin, P., Varble, A., Modell, J. W. & Marraffini, L. A. Cleavage of viral DNA by restriction endonucleases stimulates the type II CRISPR-Cas immune response. Mol. Cell 82, 907–919.e7 (2022).
Dong, X. et al. Evolutionary ecology of microbial populations inhabiting deep sea sediments associated with cold seeps. Nat. Commun. 14, 1127 (2023).
Dong, X. et al. A vast repertoire of secondary metabolites potentially influences community dynamics and biogeochemical processes in cold seeps. Sci. Adv. 10, eadl2281 (2024).
Kieft, K., Zhou, Z. & Anantharaman, K. VIBRANT: automated recovery, annotation and curation of microbial viruses, and evaluation of viral community function from genomic sequences. Microbiome 8, 1–23 (2020).
Guo, J. et al. VirSorter2: a multi-classifier, expert-guided approach to detect diverse DNA and RNA viruses. Microbiome 9, 1–13 (2021).
Camargo, A. P. et al. Identification of mobile genetic elements with geNomad. Nat. Biotechnol. 42, 1303–1312 (2023).
Roux, S. et al. Minimum Information about an Uncultivated Virus Genome (MIUViG). Nat. Biotechnol. 37, 29–37 (2019).
Roux, S. et al. iPHoP: An integrated machine learning framework to maximize host prediction for metagenome-derived viruses of archaea and bacteria. PLoS Biol. 21, e3002083 (2023).
Zhou, K. et al. Genomic and transcriptomic insights into complex virus-prokaryote interactions in marine biofilms. ISME J. 17, 2303–2312 (2023).
Silas, S. et al. Activation of bacterial programmed cell death by phage inhibitors of host immunity. Mol. Cell 85, 1838–1851.e10 (2025).
Yirmiya, E. et al. Phages overcome bacterial immunity via diverse anti-defence proteins. Nature 625, 352–359 (2024).
Yang, L. et al. Insights into the inhibition of type I-F CRISPR-Cas system by a multifunctional anti-CRISPR protein AcrIF24. Nat. Commun. 13, 1931 (2022).
Suresh, S. K., Murugan, K. & Sashital, D. G. Enzymatic anti-CRISPRs improve the bacteriophage arsenal. Nat. Struct. Mol. Biol. 26, 250–251 (2019).
Knott, G. J. et al. Broad-spectrum enzymatic inhibition of CRISPR-Cas12a. Nat. Struct. Mol. Biol. 26, 315–321 (2019).
Müller, A. L., Kjeldsen, K. U., Rattei, T., Pester, M. & Loy, A. Phylogenetic and environmental diversity of DsrAB-type dissimilatory (bi)sulfite reductases. ISME J. 9, 1152–1165 (2015).
Gu, Y. et al. Bacterial Shedu immune nucleases share a common enzymatic core regulated by diverse sensor domains. Mol. Cell 85, 523–536 (2024).
Dong, X. et al. Phylogenetically and catabolically diverse diazotrophs reside in deep-sea cold seep sediments. Nat. Commun. 13, 4885 (2022).
Sheng, Y. et al. Insertion sequence transposition inactivates CRISPR-Cas immunity. Nat. Commun. 14, 4366 (2023).
Koonin, E. V., Makarova, K. S. & Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 37, 67–78 (2017).
Martínez Arbas, S. et al. Roles of bacteriophages, plasmids and CRISPR immunity in microbial community dynamics revealed using time-series integrated meta-omics. Nat. Microbiol. 6, 123–135 (2021).
Sun, C. L., Thomas, B. C., Barrangou, R. & Banfield, J. F. Metagenomic reconstructions of bacterial CRISPR loci constrain population histories. ISME J. 10, 858–870 (2016).
Guerrero, L. D. et al. Long-run bacteria-phage coexistence dynamics under natural habitat conditions in an environmental biotechnology system. ISME J. 15, 636–648 (2021).
Huo, Y. et al. Structural and biochemical insights into the mechanism of the Gabija bacterial immunity system. Nat. Commun. 15, 836 (2024).
Han, Y. et al. A comprehensive genomic catalog from global cold seeps. Sci. Data 10, 596 (2023).
Olm, M. R., Brown, C. T., Brooks, B. & Banfield, J. F. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 11, 2864–2868 (2017).
Parks, D. H. et al. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 50, D785–D794 (2022).
Nguyen, L.-T., Schmidt, H. A., Von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evolution 32, 268–274 (2015).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Breitwieser, F. P. & Salzberg, S. L. Pavian: interactive analysis of metagenomics data for microbiome studies and pathogen identification. Bioinformatics 36, 1303–1304 (2020).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinforma. 11, 1–11 (2010).
Zhang, C. et al. The majority of microorganisms in gas hydrate-bearing subseafloor sediments ferment macromolecules. Microbiome 11, 37 (2023).
Xiao, X. et al. Metal-driven anaerobic oxidation of methane as an important methane sink in methanic cold seep sediments. Microbiol. Spectr. 11, e05337–05322 (2023).
Li, W. L. et al. Microbial ecology of sulfur cycling near the sulfate–methane transition of deep-sea cold seep sediments. Environ. Microbiol. 23, 6844–6858 (2021).
Uritskiy, G. V., DiRuggiero, J. & Taylor, J. MetaWRAP—a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome 6, 1–13 (2018).
Kopylova, E., Noé, L. & Touzet, H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 28, 3211–3217 (2012).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Nayfach, S. et al. CheckV assesses the quality and completeness of metagenome-assembled viral genomes. Nat. Biotechnol. 39, 578–585 (2021).
Shaffer, M. et al. DRAM for distilling microbial metabolism to automate the curation of microbiome function. Nucleic Acids Res. 48, 8883–8900 (2020).
Ye, J., McGinnis, S. & Madden, T. L. BLAST: improvements for better sequence analysis. Nucleic Acids Res. 34, W6–W9 (2006).
Coutinho, F. H. et al. RaFAH: Host prediction for viruses of Bacteria and Archaea based on protein content. Patterns 2, 100274 (2021).
Galiez, C., Siebert, M., Enault, F., Vincent, J. & Söding, J. WIsH: who is the host? Predicting prokaryotic hosts from metagenomic phage contigs. Bioinformatics 33, 3113–3114 (2017).
Wang, W. et al. A network-based integrated framework for predicting virus–prokaryote interactions. NAR Genomics Bioinforma. 2, lqaa044 (2020).
Lu, C. et al. Prokaryotic virus host predictor: a Gaussian model for host prediction of prokaryotic viruses in metagenomics. BMC Biol. 19, 5 (2021).
Couvin, D. et al. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 46, W246–W251 (2018).
Grandjean, M. et al. Gephi: Introduction To Network Analysis And Visualisation. International AAAI Conference on Weblogs and Social Media (2015).
Yan, Y., Zheng, J., Zhang, X. & Yin, Y. dbAPIS: a database of anti-prokaryotic immune system genes. Nucleic Acids Res. 52, 419–425 (2023).
Buchfink, B., Reuter, K. & Drost, H.-G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nature Methods 18, 366–368 (2021).
Mazzocco, A., Waddell, T.E., Lingohr, E. & Johnson, R.P. Enumeration of Bacteriophages Using The Small Drop Plaque Assay System. Bacteriophages: methods and protocols,1,isolation, characterization, and interactions, 81–85 (2009).
Khedkar, S. et al. Landscape of mobile genetic elements and their antibiotic resistance cargo in prokaryotic genomes. Nucleic Acids Res. 50, 3155–3168 (2022).
Neukirchen, S. & Sousa, F. L. DiSCo: a sequence-based type-specific predictor of Dsr-dependent dissimilatory sulphur metabolism in microbial data. Microb. Genomics 7, 000603 (2021).
Zhou, Z. et al. METABOLIC: high-throughput profiling of microbial genomes for functional traits, metabolism, biogeochemistry, and community-scale functional networks. Microbiome 10, 33 (2022).
Tu, Q., Lin, L., Cheng, L., Deng, Y. & He, Z. NCycDB: a curated integrative database for fast and accurate metagenomic profiling of nitrogen cycling genes. Bioinformatics 35, 1040–1048 (2019).
Nakamura, T., Yamada, K. D., Tomii, K. & Katoh, K. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics 34, 2490–2492 (2018).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Chen, C. et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 16, 1733–1742 (2023).
Paysan-Lafosse, T. et al. InterPro in 2022. Nucleic Acids Res. 51, D418–D427 (2023).
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Mirdita, M., Steinegger, M. & Söding, J. MMseqs2 desktop and local web server app for fast, interactive sequence searches. Bioinformatics 35, 2856–2858 (2019).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Van Kempen, M. et al. Fast and accurate protein structure search with Foldseek. Nat. Biotechnol. 42, 243–246 (2024).
DeLano, W. L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr 40, 82–92 (2002).
Members, C.-N. Partners database resources of the National Genomics Data Center, China National Center for Bioinformation in 2025. Nucleic Acids Res. 53, D30–D44 (2025).
Zhang, S. et al. The GSA family in 2025: a broadened sharing platform for multi-omics and multimodal data. Genomics, Proteom. Bioinforma. 23, qzaf072 (2025).
Acknowledgements
The work was supported by National Key R&D Program of China (No. 2024YFC2816200 to X.D.), National Science Foundation of China (No. 42376115 to X.D., No. 42406109 to Y.H., No. 32470036 to R.C. and No. 32100025 to R.C.), Natural Science Foundation Project of Xiamen City (No. 3502Z202373076 to X.D.), Natural Science Foundation of Fujian Province (No. 2023J06042 to X.D.), Scientific Research Foundation of Third Institute of Oceanography, MNR (No. 2022025 to X.D., No. 2023022 to X.D. and No. 2025013 to Y.H.), Open Funding Project of State Key Laboratory of Microbial Metabolism (No. MMLKF23-05 to X.D.) and Fundamental Research Funds for the Central Universities (No. 2662025SYPY003 to R.C.).
Author information
Authors and Affiliations
Contributions
X.D. conceived and designed the study. Y.H. and J.L. performed the omics analyses. C.L. contributed to viral identification and classification. R.C. designed and conducted the experimental implementations. X.L. participated in the analysis of some metagenomic data. C.L., F.X., J.P. and W.X. actively participated in discussions and data interpretation. F.W. and H.J. provided valuable suggestions for manuscript revision. Y.H., J.L. and X.D. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.”
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, Y., Liao, J., Li, C. et al. Co-occurrence of diverse defense systems shapes complex microbe-virus relationships in deep-sea cold seeps. Nat Commun (2026). https://doi.org/10.1038/s41467-025-68174-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-68174-6