Abstract
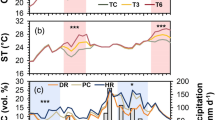
Elevated temperatures pose challenges to stomatal conductance, which regulates transpiration and photosynthesis. However, the coupling of stomatal conductance, transpiration and photosynthesis may shift with warming. Here, we synthesize evidence from a meta-analysis of 207 studies to assess leaf physiological responses to warming. On average, the responses of stomatal conductance are highly variable, exhibiting no consistent directional trend, whereas transpiration increases and photosynthesis decreases, reflecting a shift towards transpirational cooling. Stomatal conductance declines until temperatures exceed 5 °C above ambient, whereas transpiration remains stable under warming <3 °C. Beyond these two thresholds, both stomatal conductance and transpiration increase with further warming. The sensitivity of stomatal conductance, photosynthesis, and water-use efficiency to warming varies substantially among plant functional types, with distinct responses across life forms, phylogenetic groups, and photosynthetic pathways. Higher mean annual temperature amplifies the positive responses of stomatal conductance and transpiration to warming, whereas greater mean annual precipitation mitigates the warming-induced declines in photosynthesis. Elevated CO2 exacerbates warming-induced declines in photosynthesis, while drought constrains transpirational cooling. Collectively, these findings highlight a progressive decoupling of stomatal conductance, transpiration and photosynthesis with warming, revealing complex trade-offs between plant water use, thermal regulation, and carbon assimilation.
Similar content being viewed by others
Data availability
The data used in this study are available at https://doi.org/10.6084/m9.figshare.30685220. Source data are provided with this paper.
Code availability
All analyses were conducted using R software, and the codes that generate all results are available at https://doi.org/10.6084/m9.figshare.30685220.
References
Buckley, T. N. The control of stomata by water balance. N. Phytol. 168, 275–292 (2005).
Medlyn, B. E. et al. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob. Change Biol. 17, 2134–2144 (2011).
Wang, Z. & Wang, C. Responses of tree leaf gas exchange to elevated CO2 combined with changes in temperature and water availability: a global synthesis. Glob. Ecol. Biogeogr. 30, 2500–2512 (2021).
Marchin, R. M., Broadhead, A. A., Bostic, L. E., Dunn, R. R. & Hoffmann, Wi. A. Stomatal acclimation to vapour pressure deficit doubles transpiration of small tree seedlings with warming. Plant Cell Environ. 39, 2221–2234 (2016).
Aparecido, L. M. T., Woo, S., Suazo, C., Hultine, K. R. & Blonder, B. High water use in desert plants exposed to extreme heat. Ecol. Lett. 23, 1189–1200 (2020).
Perkins, S. E., Alexander, L. V. & Nairn, J. R. Increasing frequency, intensity and duration of observed global heatwaves and warm spells. Geophys. Res. Lett. 39, L20714 (2012).
Kirschbaum, M. U. F. & McMillan, A. M. S. Warming and elevated CO2 have opposing influences on transpiration. Which is more Important? Curr. Rep. 4, 51–71 (2018).
De Boeck, H. J., Kimball, B. A., Miglietta, F. & Nijs, I. Quantification of excess water loss in plant canopies warmed with infrared heating. Glob. Change Biol. 18, 2860–2868 (2012).
Grossiord, C. et al. Plant responses to rising vapor pressure deficit. N. Phytol. 226, 1550–1566 (2020).
Slot, M., Rifai, S. W., Eze, C. E. & Winter, K. The stomatal response to vapor pressure deficit drives the apparent temperature response of photosynthesis in tropical forests. N. Phytol. 244, 1238–1249 (2024).
Marchin, R. M., Medlyn, B. E., Tjoelker, M. G. & Ellsworth, D. S. Decoupling between stomatal conductance and photosynthesis occurs under extreme heat in broadleaf tree species regardless of water access. Glob. Change Biol. 29, 6319–6335 (2023).
Urban, J., Ingwers, M. W., McGuire, M. A. & Teskey, R. O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 68, 1757–1767 (2017).
Evans, M. E. K., Hu, J. & Michaletz, S. T. Scaling plant responses to heat: from molecules to the biosphere. Science 388, 1167–1173 (2025).
Garen, J. C. & Michaletz, S. T. Temperature governs the relative contributions of cuticle and stomata to leaf minimum conductance. N. Phytol. 245, 1911–1923 (2025).
Garen, J. C. et al. Gas exchange analysers exhibit large measurement error driven by internal thermal gradients. N. Phytol. 236, 369–384 (2022).
Slot, M. et al. Large differences in leaf cuticle conductance and its temperature response among 24 tropical tree species from across a rainfall gradient. N. Phytol. 232, 1618–1631 (2021).
Blonder, B. W. et al. Plant water use theory should incorporate hypotheses about extreme environments, population ecology, and community ecology. N. Phytol. 238, 2271–2283 (2023).
Wright, I. J. et al. Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 14, 411–421 (2005).
Michaletz, S. T. et al. The energetic and carbon economic origins of leaf thermoregulation. Nat. Plants 2, 16129 (2016).
Matsuo, T. et al. Herbaceous species and dry forest species have more acquisitive leaf traits than woody species and wet forest species. Funct. Ecol. 38, 194–205 (2024).
Zhou, L. et al. Global systematic review with meta-analysis shows that warming effects on terrestrial plant biomass allocation are influenced by precipitation and mycorrhizal association. Nat. Commun. 13, 4914 (2022).
Taylor, S. H. et al. Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment. N. Phytol. 185, 780–791 (2010).
Zeppel, M. J. B., Wilks, J. V. & Lewis, J. D. Impacts of extreme precipitation and seasonal changes in precipitation on plants. Biogeosciences 11, 3083–3093 (2014).
Díaz, S. et al. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol. Evol. 3, 2958–2975 (2013).
Muñoz-Gálvez, F. J. et al. Trait coordination and trade-offs constrain the diversity of water use strategies in Mediterranean woody plants. Nat. Commun. 16, 4103 (2025).
León-Sánchez, L. et al. Altered leaf elemental composition with climate change is linked to reductions in photosynthesis, growth and survival in a semi-arid shrubland. J. Ecol. 108, 47–60 (2020).
Grossiord, C. et al. Prolonged warming and drought modify belowground interactions for water among coexisting plants. Tree Physiol. 39, 55–63 (2019).
Querejeta, J. I., Ren, W. & Prieto, I. Vertical decoupling of soil nutrients and water under climate warming reduces plant cumulative nutrient uptake, water-use efficiency and productivity. N. Phytol. 230, 1378–1393 (2021).
He, P. et al. Growing-season temperature and precipitation are independent drivers of global variation in xylem hydraulic conductivity. Glob. Change Biol. 26, 1833–1841 (2020).
Wu, T. et al. Leaf photosynthetic and respiratory thermal acclimation in terrestrial plants in response to warming: a global synthesis. Glob. Change Biol. 31, 1833–1841 (2025).
Dusenge, M. E., Duarte, A. G. & Way, D. A. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. N. Phytol. 221, 32–49 (2019).
Hasper, T. B. et al. Water use by Swedish boreal forests in a changing climate. Funct. Ecol. 30, 690–699 (2016).
Reich, P. B. et al. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 562, 263–267 (2018).
Teskey, R. et al. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 38, 1699–1712 (2015).
Marchin, R. M. et al. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Change Biol. 28, 1133–1146 (2022).
Slot, M. & Kitajima, K. General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia 177, 885–900 (2015).
Liang, X. et al. Stomatal responses of terrestrial plants to global change. Nat. Commun. 14, 2188 (2023).
Zhang, J. et al. The effects of elevated CO2, elevated O3, elevated temperature, and drought on plant leaf gas exchanges: a global meta-analysis of experimental studies. Environ. Sci. Pollut. Res. 28, 15274–15289 (2021).
Crous, K. Y., Uddling, J. & De Kauwe, M. G. Temperature responses of photosynthesis and respiration in evergreen trees from boreal to tropical latitudes. N. Phytol. 234, 353–374 (2022).
Wang, D., Heckathorn, S. A., Wang, X. & Philpott, S. M. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169, 1–13 (2012).
Sadok, W., Lopez, J. R. & Smith, K. P. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 44, 2102–2116 (2021).
Drake, J. E. et al. Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Glob. Change Biol. 24, 2390–2402 (2018).
Sperry, J. S. Coordinating stomatal and xylem functioning–an evolutionary perspective. N. Phytol. 162, 568–570 (2004).
Hüve, K., Bichele, I., Rasulov, B. & Niinemets, Ü. When it is too hot for photosynthesis: heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ. 34, 113–126 (2011).
Wujeska-Klause, A., Bossinger, G. & Tausz, M. Responses to heatwaves of gas exchange, chlorophyll fluorescence and antioxidants ascorbic acid and glutathione in congeneric pairs of Acacia and Eucalyptus species from relatively cooler and warmer climates. Trees 29, 1929–1941 (2015).
Lamba, S. et al. Physiological acclimation dampens initial effects of elevated temperature and atmospheric CO2 concentration in mature boreal Norway spruce. Plant Cell Environ. 41, 300–313 (2018).
Wertin, T. M., Belnap, J. & Reed, S. C. Experimental warming in a dryland community reduced plant photosynthesis and soil CO2 efflux although the relationship between the fluxes remained unchanged. Funct. Ecol. 31, 297–305 (2017).
Slot, M., Garcia, M. N. & Winter, K. Temperature response of CO2 exchange in three tropical tree species. Funct. Plant Biol. 43, 468–478 (2016).
Teuling, A. J. et al. Contrasting response of European forest and grassland energy exchange to heatwaves. Nat. Geosci. 3, 722–727 (2010).
Scafaro, A. P., Posch, B. C., Evans, J. R., Farquhar, G. D. & Atkin, O. K. Rubisco deactivation and chloroplast electron transport rates co-limit photosynthesis above optimal leaf temperature in terrestrial plants. Nat. Commun. 14, 2820 (2023).
Busch, F., Huner, N. & Ensminger, I. Increased air temperature during simulated autumn conditions impairs photosynthetic electron transport between photosystem II and photosystem I. Plant Physiol. 147, 402–414 (2008).
Schultz, H. R. & Matthews, M. A. High vapour pressure deficit exacerbates xylem cavitation and photoinhibition in shade-grown Piper auritum H.B. & K. during prolonged sunflecks. Oecologia 110, 312–319 (1997).
Still, C. J. et al. No evidence of canopy-scale leaf thermoregulation to cool leaves below air temperature across a range of forest ecosystems. Proc. Natl. Acad. Sci. USA 119, e2205682119 (2022).
Lin, H., Chen, Y., Zhang, H., Fu, P. & Fan, Z. Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 31, 2202–2211 (2017).
Wright, I. J. et al. Global climatic drivers of leaf size. Science 357, 917–921 (2017).
Yates, M. J., Anthony Verboom, G., Rebelo, A. G. & Cramer, M. D. Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic Region. Funct. Ecol. 24, 485–492 (2010).
He, Y., Gao, J., Guo, N. & Guo, Y. Variations of leaf cuticular waxes among C3 and C4 gramineae herbs. Chem. Biodivers. 13, 1460–1468 (2016).
Grünhofer, P. et al. Changes in wax composition but not amount enhance cuticular transpiration. Plant Cell Environ. 47, 91–105 (2024).
Berry, J. & Björkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Biol. 31, 491–543 (1980).
Sage, R. F., Sage, T. L. & Kocacinar, F. Photorespiration and the evolution of C4 photosynthesis. Annu. Rev. Plant Biol. 63, 19–47 (2012).
Grossiord, C. et al. Tree water dynamics in a drying and warming world. Plant Cell Environ. 40, 1861–1873 (2017).
Peng, S. et al. Asymmetric effects of daytime and night-time warming on northern hemisphere vegetation. Nature 501, 88–92 (2013).
Turnbull, M. H., Murthy, R. & Griffin, K. L. The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant Cell Environ. 25, 1729–1737 (2002).
Querejeta, J. I. et al. Higher leaf nitrogen content is linked to tighter stomatal regulation of transpiration and more efficient water use across dryland trees. N. Phytol. 235, 1351–1364 (2022).
Novick, K. A. et al. The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nat. Clim. Change 6, 1023–1027 (2016).
Barton, C. V. M. et al. Effects of elevated atmospheric [CO2] on instantaneous transpiration efficiency at leaf and canopy scales in Eucalyptus saligna. Glob. Change Biol. 18, 585–595 (2012).
Duursma, R. A. et al. The peaked response of transpiration rate to vapour pressure deficit in field conditions can be explained by the temperature optimum of photosynthesis. Agric. Meteorol. 189-190, 2–10 (2014).
Wang, Z., Wang, C. & Liu, S. Elevated CO2 alleviates adverse effects of drought on plant water relations and photosynthesis: a global meta-analysis. J. Ecol. 110, 2836–2849 (2022).
Dusenge, M. E., Madhavji, S. & Way, D. A. Contrasting acclimation responses to elevated CO2 and warming between an evergreen and a deciduous boreal conifer. Glob. Change Biol. 26, 3639–3657 (2020).
Wujeska-Klause, A., Crous, K., Ghannoum, O. & Ellsworth, D. Lower photorespiration in elevated CO2 reduces leaf N concentrations in mature Eucalyptus trees in the field. Glob. Change Biol. 25, 1282–1295 (2019).
Karger, D. N. et al. Climatologies at high resolution for the Earth’s land surface areas. Sci. Data 4, 170122 (2017).
Wang, Z. & Wang, C. Interactive effects of elevated temperature and drought on plant carbon metabolism: a meta-analysis. Glob. Change Biol. 29, 2824–2835 (2023).
Hedges, L. V., Gurevitch, J. & Curtis, P. S. The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 (1999).
Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010).
Vega-Trejo, R., De Boer, R. A., Fitzpatrick, J. L. & Kotrschal, A. Sex-specific inbreeding depression: a meta-analysis. Ecol. Lett. 25, 1009–1026 (2022).
Acknowledgements
We are grateful to all the researchers whose data contributed to this meta-analysis. We also sincerely appreciate the editors and reviewers for their insightful comments and constructive recommendations. This work was financially supported by the National Natural Science Foundation of China (32371657), China Postdoctoral Science Foundation (2023M730531), Heilongjiang Postdoctoral Fund (LBH-Z22063) and the National Key Research, Development Program of China (2021YFD2200401).
Author information
Authors and Affiliations
Contributions
Z.W. and C.W. designed the study. Z.W. collected and analyzed the data and drafted the manuscript. M.S. and C.W. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks José Ignacio Querejeta and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Z., Slot, M. & Wang, C. Decoupling of stomatal conductance, transpiration and photosynthesis in terrestrial plants under elevated temperature: a meta-analysis. Nat Commun (2026). https://doi.org/10.1038/s41467-025-68250-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-68250-x