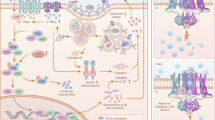
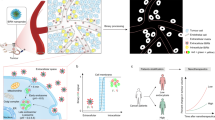
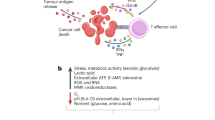
Abstract
Although disruptions in metal ion homeostasis leading to severe cellular damage and regulated cell death are a promising strategy for cancer immunotherapy, challenges in overloading these ions to tumor cells without premature release have limited their therapeutic applications. In this study, we develop binary mineral nanoparticles incorporating both Ca2+ and Na+ ions to enhance the cytotoxic effects of ion interference in cancer immunotherapy. Engineered using a microfluidic system for uniform size distribution and scalability, these nanoparticles exhibit pH-sensitive ion release. Systemically administered, they preferentially accumulate in tumors, elevating intracellular Ca2+ and Na+ levels and inducing immunogenic cell death without calcium channel activators or other small-molecule inducers. Our binary mineral nanoparticles significantly enhance antitumor immunity, especially when combined with an immune checkpoint inhibitor, leading to long-term immunity and inhibition of metastasis. This nanotechnology-enabled synergistic delivery of Ca²⁺ and Na⁺ ions represents a promising adjunct to existing metalloimmunotherapy strategies for cancer eradication.
Similar content being viewed by others
Data availability
All data presented in this study are available within the article, its Supplementary Information files. Source data are provided with this paper.
References
Nelson, N. Metal ion transporters and homeostasis. EMBO J. 18, 4361–4371 (1999).
Sun, X. et al. Strategies for the development of metalloimmunotherapies. Nat. Biomed. Eng. 8, 1073–1091 (2024).
Shen, F. et al. Metal ions and nanometallic materials in antitumor immunity: function, application, and perspective. J. Nanobiotechnol. 21, 20 (2023).
Sun, X. et al. Amplifying STING activation by cyclic dinucleotide–manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol. 16, 1260–1270 (2021).
Du, M. & Chen, Z. J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 361, 704–709 (2018).
Scambler, T. et al. ENaC-mediated sodium influx exacerbates NLRP3-dependent inflammation in cystic fibrosis. eLife 8, e49248 (2019).
Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Yu, S. P., Canzoniero, L. M. T. & Choi, D. W. Ion homeostasis and apoptosis. Curr. Opin. Cell Biol. 13, 405–411 (2001).
Blaustein, M. P. & Lederer, W. J. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79, 763–854 (1999).
Shi, X. et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature 493, 111–115 (2013).
Zheng, S., Wang, X., Zhao, D., Liu, H. & Hu, Y. Calcium homeostasis and cancer: insights from endoplasmic reticulum-centered organelle communications. Trends Cell Biol. 33, 312–323 (2023).
Yupeng Xiao, Z. Li, Alberto Bianco & B. M. Recent advances in calcium-based anticancer nanomaterials exploiting calcium overload. Adv. Funct. Mater. 33, 2209291 (2023).
Zhang, M. et al. Calcium-overload-mediated tumor therapy by calcium peroxide nanoparticles. Chem 5, 2171–2182 (2019).
Li, Y. et al. CaCO3 nanoparticles incorporated with KAE to enable amplified calcium overload cancer therapy. Biomaterials 277, 121080 (2021).
Wang, C. et al. Cancer-specific therapy by artificial modulation of intracellular calcium concentration. Adv. Healthc. Mater. 8, 1900501 (2019).
Zheng, P., Ding, B., Zhu, G., Li, C. & Lin, J. Biodegradable Ca2+ nanomodulators activate pyroptosis through mitochondrial Ca2+ overload for cancer immunotherapy. Angew. Chem. Int. Ed. 61, e202204904 (2022).
Zheng, L. et al. Potentiated calcium carbonate with enhanced calcium overload induction and acid neutralization capabilities to boost chemoimmunotherapy against liver cancer. ACS Nano, 18, 27597–27616 (2024).
Li, S. et al. Application of calcium overload-based ion interference therapy in tumor treatment: strategies, outcomes, and prospects. Front. Pharmacol. 15, 1352377 (2024).
Willebrand, R. et al. High salt inhibits tumor growth by enhancing anti-tumor immunity. Front. Immunol. 10, 1141 (2019).
He, W. et al. High-salt diet inhibits tumour growth in mice via regulating myeloid-derived suppressor cell differentiation. Nat. Commun. 11, 1732 (2020).
Barrett, T. et al. Quantification of total and intracellular sodium concentration in primary prostate cancer and adjacent normal prostate tissue with magnetic resonance imaging. Invest. Radiol. 53, 450–456 (2018).
Cameron, I. L., Smith, N. K. R., Pool, T. B. & Sparks, R. L. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 40, 1493–1500 (1980).
Jiang, W. et al. NaCl nanoparticles as a cancer therapeutic. Adv. Mater. 31, 1904058 (2019).
Liu, Y., Zhen, W., Wang, Y., Song, S. & Zhang, H. Na2S2O8 nanoparticles trigger antitumor immunotherapy through reactive oxygen species storm and surge of tumor osmolarity. J. Am. Chem. Soc. 142, 21751–21757 (2020).
Li, J. et al. Sodium citrate nanoparticles induce dual-path pyroptosis for enhanced antitumor immunotherapy through synergistic ion overload and metabolic disturbance. Nano Lett. 23, 10034–10043 (2023).
Richardson, J. J. et al. Innovation in Layer-by-Layer Assembly. Chem. Rev. 116, 14828–14867 (2016).
Pires, I. S., Gordon, E., Suh, H., Irvine, D. J. & Hammond, P. T. High-Throughput Microfluidic-Mediated Assembly of Layer-By-Layer Nanoparticles. Adv. Funct. Mater. 35, 2503965 (2025).
Kim, J. et al. Microfluidic Platform Enables Shearless Aerosolization of Lipid Nanoparticles for mRNA Inhalation. ACS Nano 18, 11335–11348 (2024).
Chuzeville, L. et al. Eco-friendly processes for the synthesis of amorphous calcium carbonate nanoparticles in ethanol and their stabilisation in aqueous media. Green. Chem. 24, 1270–1284 (2022).
Seo, K.-S., Han, C., Wee, J.-H., Park, J.-K. & Ahn, J.-W. Synthesis of calcium carbonate in a pure ethanol and aqueous ethanol solution as the solvent. J. Cryst. Growth 276, 680–687 (2005).
Dong, Z. et al. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials 110, 60–70 (2016).
Thapa, R. K. & Kim, J. O. Nanomedicine-based commercial formulations: current developments and future prospects. J. Pharm. Investig. 53, 19–33 (2023).
Shinn, J., Kwon, N., Lee, S. A. & Lee, Y. Smart pH-responsive nanomedicines for disease therapy. J. Pharm. Investig. 52, 427–441 (2022).
Hao, N. et al. Microfluidic continuous flow synthesis of functional hollow spherical silica with hierarchical sponge-like large porous shell. Chem. Eng. J. 366, 433–438 (2019).
Monteith, G. R., Prevarskaya, N. & Roberts-Thomson, S. J. The calcium–cancer signalling nexus. Nat. Rev. Cancer 17, 373–380 (2017).
Verkhratsky, A., Trebak, M., Perocchi, F., Khananshvili, D. & Sekler, I. Crosslink between calcium and sodium signalling. Exp. Physiol. 103, 157–169 (2018).
Romero-Garcia, S. & Prado-Garcia, H. Mitochondrial calcium: transport and modulation of cellular processes in homeostasis and cancer (Review). Int. J. Oncol. 54, 1155–1167 (2019).
Villalobos, C., Gutiérrez, L. G., Hernández-Morales, M., Del Bosque, D. & Núñez, L. Mitochondrial control of store-operated Ca2+ channels in cancer: pharmacological implications. Pharmacol. Res. 135, 136–143 (2018).
Giorgi, C., Marchi, S. & Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 19, 713–730 (2018).
Dong, Z., Saikumar, P., Weinberg, J. M. & Venkatachalam, M. A. Calcium in cell injury and death. Annu. Rev. Pathol. Mech. Dis. 1, 405–434 (2006).
Yang, W. et al. Calcium nanoparticles target and activate T cells to enhance anti-tumor function. Nat. Commun. 15, 10095 (2024).
Soll, D. et al. Sodium chloride in the tumor microenvironment enhances T cell metabolic fitness and cytotoxicity. Nat. Immunol. 25, 1830–1844 (2024).
Kim, J., Eygeris, Y., Ryals, R. C., Jozić, A. & Sahay, G. Strategies for non-viral vectors targeting organs beyond the liver. Nat. Nanotechnol. 19, 428–447 (2024).
Iwamoto, T., Watanabe, Y., Kita, S. & Blaustein, M. Na+/Ca2+ exchange inhibitors: a new class of calcium regulators. Cardiovasc. Hematol. Disord. -Drug Targets 7, 188–198 (2007).
Miyashita, M. et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res. 17, 124 (2015).
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Fathman, C. G. & Lineberry, N. B. Molecular mechanisms of CD4+ T-cell anergy. Nat. Rev. Immunol. 7, 599–609 (2007).
Macián, F. et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109, 719–731 (2002).
Martinez, G. J. et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity 42, 265–278 (2015).
Chen, R., Kang, R. & Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 54, 91–102 (2022).
Shim, N., Cho, H., Jeon, S. I. & Kim, K. Recent developments in chemodrug-loaded nanomedicines and their application in combination cancer immunotherapy. J. Pharm. Investig. 54, 13–36 (2024).
Görlach, A., Bertram, K., Hudecova, S. & Krizanova, O. Calcium and ROS: a mutual interplay. Redox Biol. 6, 260–271 (2015).
Bezu, L. et al. eIF2α phosphorylation is pathognomonic for immunogenic cell death. Cell Death Differ. 25, 1375–1393 (2018).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011).
Martins, I. et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 21, 79–91 (2014).
Nguyen, B. L. et al. Liposomal co-delivery of toll-like receptors 3 and 7 agonists induce a hot triple-negative breast cancer immune environment. J. Controlled Release 361, 443–454 (2023).
Phung, C. D. et al. Shaping the “hot” immunogenic tumor microenvironment by nanoparticles co-delivering oncolytic peptide and TGF-β1 siRNA for boosting checkpoint blockade therapy. Bioeng. Transl. Med. 8, e10392 (2023).
Nguyen, B. L. et al. Reigniting the cancer-immunity cycle with nanoparticles for simultaneous delivery of oncolytic peptides and a TLR agonist. Nano Today 55, 102179 (2024).
Fucikova, J. et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 11, 1013 (2020).
Xu, J., Lamouille, S. & Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 19, 156–172 (2009).
Liu, X. et al. Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source of metastasis. Sci. Adv. 5, eaav4275 (2019).
Su, S., Du, F.-S. & Li, Z.-C. Synthesis and pH-dependent hydrolysis profiles of mono- and dialkyl substituted maleamic acids. Org. Biomol. Chem. 15, 8384–8392 (2017).
Le, X. T. et al. Peroxidase-mimicking iron-based single-atom upconversion photocatalyst for enhancing chemodynamic therapy. Adv. Funct. Mater. 34, 2401893 (2024).
Acknowledgements
This work was supported by the National Research Foundation (NRF) of Korea Grant funded by the Korean Government (Grant No. RS-2022-NR070844 to J.K. and J.O.K., RS-2024-00343765 to J.K., RS-2025-02303064 and RS-2025-14292970 to J.O.K.). The authors thank the Core Research Support Center for Natural Products and Medical Materials (CRCNM, Yeungnam University) for technical support.
Author information
Authors and Affiliations
Contributions
J.K. and J.O.K. conceived the idea. B.L.N., J.K. and J.O.K. designed the experiments. B.L.N., N.D.L., T.O.O.N., D.A., B.R.P., B.K., J.M.L., and S.K.K. performed the experiments and analyzed the data. C.D.P., D-V.P., J.H., and J-H.C. helped the data interpretation. J.H., J-H.C., and S.K.K. provided constructive advice for data analysis and manuscript writing. B.L.N., J.K., and J.O.K. wrote the manuscript with feedback from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yumiao Zhang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nguyen, B.L., Le, N.D., Nguyen, T.O.O. et al. Binary mineral nanoparticles enable intravascular delivery of metal ions to tumors for metalloimmunotherapy. Nat Commun (2026). https://doi.org/10.1038/s41467-025-68279-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-68279-y