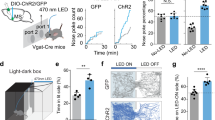
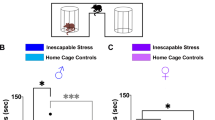
Abstract
Sensory perception is shaped by experience, giving stimuli behavioral significance. Basal forebrain (BF) cholinergic neurons in mice, which are crucial for arousal and motivation, also regulate sensory processing. Within BF nuclei, glutamatergic (vGlut2BF) neurons receive cholinergic input and modulate behaviors, but their roles in encoding sensory significance remain unclear. Using in vivo calcium imaging, we found that vGlut2BF neurons initially poorly encoded odor identity. However, their response to conditioned odors increased following associative learning, and their population activity more distinctly encoded paired stimuli, reflecting emergent value representation. Furthermore, pairing stimulation or inhibition of vGlut2BF neurons with specific odors altered odor preferences, suggesting that appropriately timed vGlut2BF neuronal activity is sufficient to influence valence assignment. Our findings reveal that vGlut2BF neurons transform sensory input into motivationally significant stimuli, positioning the BF as a key hub for linking sensory processing with motivational states and experience-driven plasticity.
Similar content being viewed by others
Data availability
Source data are provided with this paper. Additional datasets not included in the source-data Excel file are available at Zenodo: https://doi.org/10.5281/zenodo.17704682 Source data are provided with this paper.
Code availability
All original code has been deposited at Github https://github.com/julie200388/vGlut2BF_odor_learning.
References
Gilbert, C. D. & Sigman, M. Brain states: top-down influences in sensory processing. Neuron 54, 677–696 (2007).
Chun, M. M., Golomb, J. D. & Turk-Browne, N. B. A taxonomy of external and internal attention. Annu. Rev. Psychol. 62, 73–101 (2011).
Ananth, M. R., Rajebhosale, P., Kim, R., Talmage, D. A. & Role, L. W. Basal forebrain cholinergic signalling: development, connectivity and roles in cognition. Nat. Rev. Neurosci. 24, 233–251 (2023).
Ballinger, E. C., Ananth, M., Talmage, D. A. & Role, L. W. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 91, 1199–1218 (2016).
Nunez-Parra, A., Maurer, R. K., Krahe, K., Smith, R. S. & Araneda, R. C. Disruption of centrifugal inhibition to olfactory bulb granule cells impairs olfactory discrimination. Proc. Natl. Acad. Sci. USA 110, 14777–14782 (2013).
Gritton, H. J. et al. Cortical cholinergic signaling controls the detection of cues. Proc. Natl. Acad. Sci. USA 113, E1089–E1097 (2016).
Pinto, L. et al. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci. 16, 1857–1863 (2013).
Goard, M. & Dan, Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci. 12, 1444–1449 (2009).
Meir, I., Katz, Y. & Lampl, I. Membrane potential correlates of network decorrelation and improved SNR by cholinergic activation in the somatosensory cortex. J. Neurosci. 38, 10692–10708 (2018).
Minces, V., Pinto, L., Dan, Y. & Chiba, A. A. Cholinergic shaping of neural correlations. Proc. Natl. Acad. Sci. USA 114, 5725–5730 (2017).
Zhu, F., Elnozahy, S., Lawlor, J. & Kuchibhotla, K. V. The cholinergic basal forebrain provides a parallel channel for state-dependent sensory signaling to auditory cortex. Nat. Neurosci. 26, 810–819 (2023).
Chaudhury, D., Escanilla, O. & Linster, C. Bulbar acetylcholine enhances neural and perceptual odor discrimination. J. Neurosci. 29, 52–60 (2009).
Hu, R., Jin, S., He, X., Xu, F. & Hu, J. Whole-Brain Monosynaptic Afferent Inputs to Basal Forebrain Cholinergic System. Front. Neuroanat. 10, 98 (2016).
Xu, M. et al. Basal forebrain circuit for sleep-wake control. Nat. Neurosci. 18, 1641–1647 (2015).
Gielow, M. R. & Zaborszky, L. The Input-Output Relationship of the Cholinergic Basal Forebrain. Cell Rep. 18, 1817–1830 (2017).
Do, J. P. et al. Cell type-specific long-range connections of basal forebrain circuit. eLife 5, e13214 (2016).
Hangya, B., Ranade, S. P., Lorenc, M. & Kepecs, A. Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell 162, 1155–1168 (2015).
Harrison, T. C., Pinto, L., Brock, J. R. & Dan, Y. Calcium Imaging of Basal Forebrain Activity during Innate and Learned Behaviors. Front. Neural Circuits 10, 36 (2016).
Robert, B. et al. A functional topography within the cholinergic basal forebrain for encoding sensory cues and behavioral reinforcement outcomes. eLife 10, e69514 (2021).
Rajebhosale, P. et al. Functionally refined encoding of threat memory by distinct populations of basal forebrain cholinergic projection neurons. eLife 13, e86581 (2024).
Hegedüs, P., Sviatkó, K., Király, B., Martínez-Bellver, S. & Hangya, B. Cholinergic activity reflects reward expectations and predicts behavioral responses. iScience 26, 105814 (2023).
Stephenson-Jones, M. et al. Opposing contributions of gabaergic and glutamatergic ventral pallidal neurons to motivational behaviors. Neuron 105, 921–933.e5 (2020).
Kim, R., Ananth, M. R., Desai, N. S., Role, L. W. & Talmage, D. A. Distinct subpopulations of ventral pallidal cholinergic projection neurons encode valence of olfactory stimuli. Cell Rep. 43, 114009 (2024).
Gritti, I., Mainville, L., Mancia, M. & Jones, B. E. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J. Comp. Neurol. 383, 163–177 (1997).
Tingley, D., Alexander, A. S., Quinn, L. K., Chiba, A. A. & Nitz, D. A. Cell assemblies of the basal forebrain. J. Neurosci. 35, 2992–3000 (2015).
Gritti, I. et al. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience 143, 1051–1064 (2006).
Zou, Y. et al. Cell-type-specific optogenetic fMRI on basal forebrain reveals functional network basis of behavioral preference. Neuron 112, 1342–1357.e6 (2024).
Yang, C., Thankachan, S., McCarley, R. W. & Brown, R. E. The menagerie of the basal forebrain: how many (neural) species are there, what do they look like, how do they behave and who talks to whom?. Curr. Opin. Neurobiol. 44, 159–166 (2017).
Yang, C. et al. Cholinergic neurons excite cortically projecting basal forebrain GABAergic neurons. J. Neurosci. 34, 2832–2844 (2014).
Henny, P. & Jones, B. E. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur. J. Neurosci. 27, 654–670 (2008).
Lin, S.-C. & Nicolelis, M. A. L. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron 59, 138–149 (2008).
Zhou, P., Liu, P., Zhang, Y., Wang, D. & Li, A. The response dynamics and function of cholinergic and gabaergic neurons in the basal forebrain during olfactory learning. Front. Cell. Neurosci. 16, 911439 (2022).
Moss, E. H. et al. Distinct Patterns of PV and SST GABAergic Neuronal Activity in the Basal Forebrain during Olfactory-Guided Behavior in Mice. J. Neurosci. 45, e0200242025 (2025).
Patel, J. M. et al. Sensory perception drives food avoidance through excitatory basal forebrain circuits. eLife 8, e44548 (2019).
Swanson, J. L. et al. Activation of basal forebrain-to-lateral habenula circuitry drives reflexive aversion and suppresses feeding behavior. Sci. Rep. 12, 22044 (2022).
Cai, J. et al. An excitatory projection from the basal forebrain to the ventral tegmental area that underlies anorexia-like phenotypes. Neuron 112, 458–472.e6 (2024).
Cai, P. et al. A glutamatergic basal forebrain to midbrain circuit mediates wakefulness and defensive behavior. Neuropharmacology 208, 108979 (2022).
Fu, Y. The dual action of basal forebrain in feeding regulation. Nat. Metab. (2024) https://doi.org/10.1038/s42255-024-01111-x.
Golden, S. A. et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688–692 (2016).
Wang, J. et al. The basal forebrain to lateral habenula circuitry mediates social behavioral maladaptation. Nat. Commun. 15, 4013 (2024).
Peng, W. et al. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science 369, eabb0556 (2020).
Zheng, Y. et al. Different subgroups of cholinergic neurons in the basal forebrain are distinctly innervated by the olfactory regions and activated differentially in olfactory memory retrieval. Front. Neural Circuits 12, 99 (2018).
Li, X. et al. Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. USA 115, 415–420 (2018).
Rye, D. B., Wainer, B. H., Mesulam, M. M., Mufson, E. J. & Saper, C. B. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience 13, 627–643 (1984).
Niedworok, C. J. et al. Charting monosynaptic connectivity maps by two-color light-sheet fluorescence microscopy. Cell Rep. 2, 1375–1386 (2012).
Gracia-Llanes, F. J. et al. GABAergic basal forebrain afferents innervate selectively GABAergic targets in the main olfactory bulb. Neuroscience 170, 913–922 (2010).
Záborszky, L., Carlsen, J., Brashear, H. R. & Heimer, L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J. Comp. Neurol. 243, 488–509 (1986).
Smith, R. S. et al. Differential muscarinic modulation in the olfactory bulb. J. Neurosci. 35, 10773–10785 (2015).
Paolini, A. G. & McKenzie, J. S. Effects of lesions in the horizontal diagonal band nucleus on olfactory habituation in the rat. Neuroscience 57, 717–724 (1993).
Burton, S. D., Wipfel, M., Guo, M., Eiting, T. P. & Wachowiak, M. A novel olfactometer for efficient and flexible odorant delivery. Chem. Senses 44, 173–188 (2019).
Saraiva, L. R. et al. Combinatorial effects of odorants on mouse behavior. Proc. Natl. Acad. Sci. USA 113, E3300–E3306 (2016).
Li, Q. & Liberles, S. D. Aversion and attraction through olfaction. Curr. Biol. 25, R120–R129 (2015).
Dewan, A., Pacifico, R., Zhan, R., Rinberg, D. & Bozza, T. Non-redundant coding of aversive odours in the main olfactory pathway. Nature 497, 486–489 (2013).
Zheng, Y. et al. Basal Forebrain-Dorsal Hippocampus Cholinergic Circuit Regulates Olfactory Associative Learning. Int. J. Mol. Sci. 23, 8472 (2022).
Neyhart, E. et al. Cortical acetylcholine dynamics are predicted by cholinergic axon activity and behavior state. Cell Rep. 43, 114808 (2024).
Yang, C., McKenna, J. T. & Brown, R. E. Intrinsic membrane properties and cholinergic modulation of mouse basal forebrain glutamatergic neurons in vitro. Neuroscience 352, 249–261 (2017).
Liu, G. et al. An objective and reproducible test of olfactory learning and discrimination in mice. J. Vis. Exp. (2018) https://doi.org/10.3791/57142.
Stanislaw, H. & Todorov, N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 31, 137–149 (1999).
Messier, J. E., Chen, H., Cai, Z.-L. & Xue, M. Targeting light-gated chloride channels to neuronal somatodendritic domain reduces their excitatory effect in the axon. eLife 7, e38506 (2018).
Wietek, J. et al. An improved chloride-conducting channelrhodopsin for light-induced inhibition of neuronal activity in vivo. Sci. Rep. 5, 14807 (2015).
Hur, E. E. & Zaborszky, L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected]. J. Comp. Neurol. 483, 351–373 (2005).
Root, C. M., Denny, C. A., Hen, R. & Axel, R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature 515, 269–273 (2014).
Iurilli, G. & Datta, S. R. Population coding in an innately relevant olfactory area. Neuron 93, 1180–1197.e7 (2017).
Hanson, E., Brandel-Ankrapp, K. L. & Arenkiel, B. R. Dynamic Cholinergic Tone in the Basal Forebrain Reflects Reward-Seeking and Reinforcement During Olfactory Behavior. Front. Cell. Neurosci. 15, 635837 (2021).
Choi, G. B. et al. Driving opposing behaviors with ensembles of piriform neurons. Cell 146, 1004–1015 (2011).
Kobayakawa, K. et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature 450, 503–508 (2007).
Stettler, D. D. & Axel, R. Representations of odor in the piriform cortex. Neuron 63, 854–864 (2009).
Miyamichi, K. et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature 472, 191–196 (2011).
Ghosh, S. et al. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature 472, 217–220 (2011).
Wang, P. Y. et al. Transient and persistent representations of odor value in prefrontal cortex. Neuron 108, 209–224.e6 (2020).
Sosulski, D. L., Bloom, M. L., Cutforth, T., Axel, R. & Datta, S. R. Distinct representations of olfactory information in different cortical centres. Nature 472, 213–216 (2011).
Ortiz-Guzman, J. et al. Cholinergic basal forebrain connectivity to the basolateral amygdala modulates food intake. eNeuro 11, ENEURO.0369–23.2024 (2024).
Biane, J. S. et al. Neural dynamics underlying associative learning in the dorsal and ventral hippocampus. Nat. Neurosci. 26, 798–809 (2023).
Lin, S.-C., Brown, R. E., Hussain Shuler, M. G., Petersen, C. C. H. & Kepecs, A. Optogenetic dissection of the basal forebrain neuromodulatory control of cortical activation, plasticity, and cognition. J. Neurosci. 35, 13896–13903 (2015).
Avila, I. & Lin, S.-C. Motivational salience signal in the basal forebrain is coupled with faster and more precise decision speed. PLoS Biol. 12, e1001811 (2014).
McKenna, J. T. et al. Characterization of basal forebrain glutamate neurons suggests a role in control of arousal and avoidance behavior. Brain Struct. Funct. 226, 1755–1778 (2021).
Pearce, J. M. & Hall, G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol. Rev. 87, 532–552 (1980).
Qi, J. et al. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat. Neurosci. 19, 725–733 (2016).
Wu, H., Williams, J. & Nathans, J. Complete morphologies of basal forebrain cholinergic neurons in the mouse. eLife 3, e02444 (2014).
Nunez-Parra, A., Cea-Del Rio, C. A., Huntsman, M. M. & Restrepo, D. The basal forebrain modulates neuronal response in an active olfactory discrimination task. Front. Cell. Neurosci. 14, 141 (2020).
Zhou, Z. C. et al. Deep-brain optical recording of neural dynamics during behavior. Neuron 111, 3716–3738 (2023).
Morrison, F. G., Dias, B. G. & Ressler, K. J. Extinction reverses olfactory fear-conditioned increases in neuron number and glomerular size. Proc. Natl. Acad. Sci. USA 112, 12846–12851 (2015).
Papes, F., Nakahara, T. S. & Camargo, A. P. Behavioral assays in the study of olfaction: A practical guide. Methods Mol. Biol. 1820, 289–388 (2018).
Lyons-Warren, A. M. et al. Co-transmitting interneurons in the mouse olfactory bulb regulate olfactory detection and discrimination. Cell Rep. 42, 113471 (2023).
Acknowledgements
Special thanks to the Neurological Research Institute Viral Core (including Zihong Chen and Ying Wang) for production of AAVs used in these experiments. Additionally, thanks to members of the Arenkiel lab for their collaboration and help in editing this manuscript. We thank Inscopix field scientists Dr. Waylin Yu, Dr. Jing Liang-Guallpa, and Dr. Matthew Whitmire for their technical support with GRIN lens miniscope imaging. We also appreciate Dr. Elizabeth H. Moss, Dr. Matthew J. McGinley, Dr. Ariel Lyons-Warren, and Snigdha Srivastava for their valuable conceptual input during the writing of this manuscript. This work was supported by DoD award PR22135 to BRA and QT, R01DK109934 to BRA and QT, R01DK138518 to BRA, USDA project # 58-3092-5-008 to BRA, and P50HD103555.
Author information
Authors and Affiliations
Contributions
P.C.: conceptualization, experimental design, conducting experiments, collecting data, data analysis, manuscript writing and editing, and figure creation. Z.D.: experimental design, data analysis, manuscript writing. M.Y.K.: data collection, data analysis, figure creation, manuscript writing. S.S.: data collection. E.H.M.: data analysis, manuscript editing. Q.T.: conceptualization, mentorship, manuscript editing. B.R.A.: mentorship, conceptualization, experimental design, data analysis, and manuscript editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Min Xu and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chin, PS., Ding, Z., Kochukov, M. et al. Glutamatergic projection neurons in the basal forebrain underlie learned olfactory associational valence assignments. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68313-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68313-7