Abstract
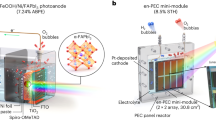
Perovskite-based solar water splitting systems are promising candidates for addressing environmental challenges, exceeding the commercialization efficiency of solar-to-hydrogen (STH) at >10%. However, the operational stability remains suboptimal due to insufficient in situ/operando insights into charge carrier dynamics. Here, we investigate the role of charge accumulation on operational stability through operando catalytic modulation via near-infrared (NIR) toggling on a photothermal catalyst. Electrochemical analyses under operando NIR toggling demonstrate enhanced hydrogen evolution reaction kinetics and reduced charge recombination. In situ analyses confirm that reduced charge accumulation suppresses ion migration in the perovskite layer, thereby enhancing operational stability. The NIR-irradiated cathode delivers a photocurrent density of 25.48 mA cm–2, maintaining 90% of its initial photocurrent density at 0 VRHE for 310 h. A parallelly-illuminated coplanar system with NIR-irradiated perovskite-based water splitting cathode operates without bias, achieving a STH efficiency of 15.18%, retaining 70% of their initial performance for 115 h.
Similar content being viewed by others
Data availability
All data generated in this study are provided in the Source Data file. Source data are provided with this paper.
References
Lewis, N. S. et al. Research opportunities to advance solar energy utilization. Science 351, 6271 (2016).
Yang, W., Prabhakar, R. R., Tan, J., Tilley, S. D. & Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 48, 4979–5015 (2019).
Burschka, J. et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499, 316–319 (2013).
Park, N. G. Organometal perovskite light absorbers toward a 20% efficiency low-cost solid-state mesoscopic solar cell. J. Phys. Chem. Lett. 4, 2423–2429 (2013).
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050 (2009).
Liu, S. W. et al. Buried interface molecular hybrid for inverted perovskite solar cells. Nature 632, 536–542 (2024).
Suo, J. J. et al. Multifunctional sulfonium-based treatment for perovskite solar cells with less than 1% efficiency loss over 4,500-h operational stability tests. Nat. Energy 9, 143–153 (2024).
Azmi, R. et al. Double-side 2D/3D heterojunctions for inverted perovskite solar cells. Nature 628, 93–98 (2024).
Yun, J. et al. Conductive passivator and dipole layer mixture enabling high-performance stable perovskite photoelectrode-based solar water splitting. Adv. Energy Mater. 13, 2301693 (2023).
Andrei, V. et al. Scalable triple cation mixed halide perovskite-bivo4 tandems for bias-free water splitting. Adv. Energy Mater. 8, 1801403 (2018).
Yang, H. et al. Monolithic FAPbBr3 photoanode for photoelectrochemical water oxidation with low onset-potential and enhanced stability. Nat. Commun. 14, 5486 (2023).
Mubarok, M. A. et al. Efficient and stable tin-lead perovskite photoconversion devices using dual-functional cathode interlayer. Adv. Energy Mater. 14, 2302555 (2024).
Kim, J. H. et al. Efficient and stable perovskite-based photocathode for photoelectrochemical hydrogen production. Adv. Func. Mater. 31, 2008277 (2021).
Park, J. et al. Hybrid perovskite-based wireless integrated device exceeding a solar to hydrogen conversion efficiency of 11%. Small 19, 2300174 (2023).
Chen, H. J. et al. Integrating low-cost earth-abundant co-catalysts with encapsulated perovskite solar cells for efficient and stable overall solar water splitting. Adv. Func. Mater. 31, 202008245 (2021).
Choi, H. et al. Organometal halide perovskite-based photoelectrochemical module systems for scalable unassisted solar water splitting. Adv. Sci. 10, 202303106 (2023).
Fehr, A. M. K. et al. Integrated halide perovskite photoelectrochemical cells with solar-driven water-splitting efficiency of 20.8%. Nat. Commun. 14, 3797 (2023).
Kim, J. H., Hansora, D., Sharma, P., Jang, J. W. & Lee, J. S. Toward practical solar hydrogen production - an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 48, 1908–1971 (2019).
Jeong, W. et al. Large-area all-perovskite-based coplanar photoelectrodes for scaled-up solar hydrogen production. Energy Environ. Sci. 17, 3604–3617 (2024).
Hansora, D. et al. All-perovskite-based unassisted photoelectrochemical water splitting system for efficient, stable and scalable solar hydrogen production. Nat. Energy 9, 272–284 (2024).
Yun, J. et al. Efficient and ultrastable iodide oxidation reaction over defect-passivated perovskite photoanode for unassisted solar fuel production. Adv. Energy Mater. 14, 2401055 (2024).
Li, N. X., Niu, X. X., Chen, Q. & Zhou, H. P. Towards commercialization: the operational stability of perovskite solar cells. Chem. Soc. Rev. 49, 8235–8286 (2020).
Kim, J. Y., Lee, J. W., Jung, H. S., Shin, H. & Park, N. G. High-efficiency perovskite solar cells. Chem. Rev. 120, 7867–7918 (2020).
Shi, J. J. et al. From ultrafast to ultraslow: charge-carrier dynamics of perovskite solar cells. Joule 2, 879–901 (2018).
Choi, J. et al. Understanding charge carrier dynamics in organic photocatalysts for hydrogen evolution. Energy Environ. Sci. 17, 7999–8018 (2024).
Tan, J. et al. Fullerene as a photoelectron transfer promoter enabling stable TiO2-Protected Sb2Se3 photocathodes for photoelectrochemical water splitting. Adv. Energy Mater. 9, 1900179 (2019).
Liu, T. Y. et al. Low catalyst loading enhances charge accumulation for photoelectrochemical water splitting. Angew. Chem. Int. 62, e202307909 (2023).
Lee, C. U. et al. Enhanced stability of spin-dependent chiral 2D perovskite embedded PV-biased anode via cross-linking strategy. Acs. Energy Lett. 9, 4032–4043 (2024).
Lee, H. et al. A dual spin-controlled chiral two-/three-dimensional perovskite artificial leaf for efficient overall photoelectrochemical water splitting. Nat. Commun. 15, 4672 (2024).
Kim, T. G. et al. Monolithic lead halide perovskite photoelectrochemical cell with 9.16% applied bias photon-to-current efficiency. Acs. Energy Lett. 7, 320–327 (2022).
Rhee, R. et al. Unassisted overall water splitting with a solar-to-hydrogen efficiency of over 10% by coupled lead halide perovskite photoelectrodes. Carbon Energy 5, e232 (2023).
Poli, I. et al. Graphite-protected CsPbBr3 perovskite photoanodes functionalised with water oxidation catalyst for oxygen evolution in water. Nat. Commun. 10, 2097 (2019).
Choi, H. et al. An organometal halide perovskite photocathode integrated with a MoS2 catalyst for efficient and stable photoelectrochemical water splitting. J. Mater. Chem. A. 11, 993–993 (2023).
Zhu, Z. H. et al. Ultrastable halide perovskite CsPbBr3 photoanodes achieved with electrocatalytic glassy-carbon and boron-doped diamond sheets. Nat. Commun. 15, 2791 (2024).
Choi, H. et al. Suppression of Undesired Losses in Organometal Halide Perovskite-Based Photoanodes for Efficient Photoelectrochemical Water Splitting. Adv. Energy Mater. 13, 2300951 (2023).
Daboczi, M., Cui, J. Y., Temerov, F. & Eslava, S. Scalable All-Inorganic Halide Perovskite Photoanodes with >100 h Operational Stability Containing Earth-Abundant Materials. Adv. Mater. 35, 2304350 (2023).
Kim, H. et al. Thermal Effect on Photoelectrochemical Water Splitting Toward Highly Solar to Hydrogen Efficiency. Chemsuschem 16, e202202017 (2023).
Tang, S. T. et al. Harvesting of Infrared Part of Sunlight to Enhance Polaron Transport and Solar Water Splitting. Adv. Func. Mater. 32, 2110284 (2022).
Lu, J. L. et al. Photothermal effect of carbon dots for boosted photothermal-assisted photocatalytic water/seawater splitting into hydrogen. Chem. Eng. J. 453, 139834 (2023)
Li, X. J. et al. Coupling hydrothermal and photothermal single-atom catalysis toward excellent water splitting to hydrogen. Appl. Catal. B. Environ. 283, 119660 (2021)
Pornrungroj, C. et al. Hybrid photothermal–photocatalyst sheets for solar-driven overall water splitting coupled to water purification. Nat. Water 1, 952–960 (2023).
He, B. et al. General and robust photothermal-heating-enabled high-efficiency photoelectrochemical water splitting. Adv. Mater. 33, 2004406 (2021).
Shinagawa, T. et al. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 5, 12801 (2015).
van der Heijden, O. et al. Temperature-dependent kinetic parameters for the alkaline oxygen evolution reaction on NiFeOOH. ACS Energy Lett. 10, 3040–3049 (2025).
Wang, X. S., Xu, C. C., Jaroniec, M., Zheng, Y. & Qiao, S. Z. Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions. Nat. Commun. 10, 4876 (2019).
Niaura, G. & Jakubenas, R. The alkali metal cation effect on the surface-enhanced Raman spectra of phosphate anions adsorbed at silver electrodes. J. Electroanal. Chem. 510, 50–58 (2001).
Yang, W., Moehl, T., Service, E. & Tilley, S. D. Operando analysis of semiconductor junctions in multi-layered photocathodes for solar water splitting by impedance spectroscopy. Adv. Energy Mater. 11, 2003569 (2021).
Lee, H. et al. Crystal facet-controlled efficient SnS photocathodes for high performance bias-free solar water splitting. Adv. Sci. 8, 2102458 (2021).
Le Formal, F. et al. Back electron-hole recombination in hematite photoanodes for water splitting. J. Am. Chem. Soc. 136, 2564–2574 (2014).
Le Formal, F., Sivula, K. & Grätzel, M. The transient photocurrent and photovoltage behavior of a hematite photoanode under working conditions and the influence of surface treatments. J. Phys. Chem. C. 116, 26707–26720 (2012).
Thorne, J. E. et al. Understanding the origin of photoelectrode performance enhancement by probing surface kinetics. Chem. Sci. 7, 3347 (2016).
Chen, X. Q., Shirai, Y., Yanagida, M. & Miyano, K. Photocarrier dynamics in perovskite-based solar cells revealed by intensity-modulated photovoltage spectroscopy. Phys. Chem. Chem. Phys. 20, 17918–17926 (2018).
Tan, J. et al. Surface restoration of polycrystalline Sb2Se3 thin films by conjugated molecules enabling high-performance photocathodes for photoelectrochemical water splitting. Appl. Catal. B. Environ. 286, 119890 (2021)
Wang, Z. B. et al. One-Step Hydrothermal Synthesis of Sn-Doped Sb2Se3 for Solar Hydrogen Production. Acs. Catal. 14, 9877–9886 (2024).
Li, H. et al. Sequential vacuum-evaporated perovskite solar cells with more than 24% efficiency. Sci. Adv. 8, eabo7422 (2022).
Zhang, F. et al. Comprehensive passivation strategy for achieving inverted perovskite solar cells with efficiency exceeding 23% by trap passivation and ion constraint. Nano Energy 89, 106370 (2021).
Eames, C. et al. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 6, 7497 (2015).
Zhao, Y. P. et al. Suppressing ion migration in metal halide perovskite via interstitial doping with a trace amount of multivalent cations. Nat. Mater. 21, 1396–1402 (2022).
Lin, Y. Z. et al. Excess charge-carrier induced instability of hybrid perovskites. Nat. Commun. 9, 4981 (2018).
Berhe, T. A. et al. Organometal halide perovskite solar cells: degradation and stability. Energy Environ. Sci. 9, 323–356 (2016).
Di Girolamo, D. et al. Ion migration-induced amorphization and phase segregation as a degradation mechanism in planar perovskite solar cells. Adv. Energy Mater. 10, 202000310 (2020).
Knight, A. J. et al. Halide segregation in mixed-halide perovskites: influence of a-site cations. Acs. Energy Lett. 6, 799–808 (2021).
Tan, J. et al. Hydrogel protection strategy to stabilize water-splitting photoelectrodes. Nat. Energy 7, 537–547 (2022).
Bashkatov, A. et al. Performance enhancement of electrocatalytic hydrogen evolution through coalescence-induced bubble dynamics. J. Am. Chem. Soc. 146, 10177–10186 (2024).
Iwata, R. C. et al. Bubble growth and departure modes on wettable/non-wettable porous foams in alkaline water splitting. Joule 5, 887–900 (2021).
Qiu, H. H. et al. Quantitative description of bubble formation in response to electrolyte engineering. Langmuir 39, 4993–5001 (2023).
Park, S., Lohse, D., Krug, D. & Koper, M. T. M. Electrolyte design for the manipulation of gas bubble detachment during hydrogen evolution reaction. Electrochim. Acta 485, 144084 (2024).
Cao, Z. S. et al. Mass transfer mechanism during bubble evolution on the surface of photoelectrode. Electrochim. Acta 434, 141293 (2022).
Moon, S. et al. Mechanistic insights into biomassed-derived dual hydrogen generation via vacancy-engineered NiO catalyst. Appl. Catal. B. Environ. 381, 125828 (2026).
Park, Y. S. et al. Efficient solar-driven hydrogen peroxide production enabled by a perovskite electrochemical device integrated with a cobalt-based chiral catalyst. Energy Environ. Sci. https://doi.org/10.1039/D5EE04313A (2026).
Acknowledgements
This research was supported by National R&D Program through the National Research Foundation (NRF) of Korea (No. 2021R1A3B1068920 and 2021M3H4A1A03049662) funded by the Ministry of Science and ICT. This research was also supported by the Yonsei Signature Research Cluster Program of 2021 (2021-22-0002) and the Yonsei Fellowship, funded by Lee Youn Jae.
Author information
Authors and Affiliations
Contributions
C.-S.J. and J.M. conceived the idea and designed the cathode. C.-S.J., W.J., and J.Y. fabricated the perovskite-based electrodes and wrote the initial draft. W.J. and J.Y. conducted in-situ Raman spectroscopy and DLCP measurements, while J.Y. and C.-S.J. performed IMVS/IMPS tests and collected ex-situ data. C.-S.J. and W.J. analyzed the operando electrochemical data, and B.K. and C.-S.J. carried out high-speed profiling of bubble dynamics. D.K. and B.K. developed FEM modeling of Marangoni convection, with Hyungsoo L. and J.H.K. preparing the experimental setup. C.-S.J., W.J., J.W., B.K., D.K., Hyungsoo L., J.H.K., Hyungsuk L., and J.M. contributed to the discussion and revision of the manuscript. J.M. supervised the overall project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jingshan Luo and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jeong, CS., Jeong, W., Yun, J. et al. Operando insights into stability of perovskite-based solar water splitting devices. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68357-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68357-9