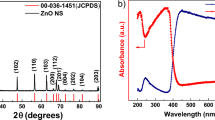
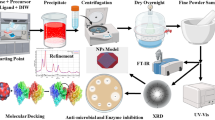
Abstract
Acne, caused by Cutibacterium acnes, triggers inflammatory lesions. The hypoxic biofilm microenvironment exacerbates acne progression, while inadequate hydrogen peroxide and dense biofilm barriers hinder oxygen generation and nanomedicine penetration. Here, we develop microneedles patch loaded with near infrared-driven self-oxygenating Z@P-M nanomotors for photothermal therapy of acne. Zinc peroxide nanoparticles are asymmetrically modified with polydopamine, followed by in-situ manganese dioxide growth on polydopamine to form Z@P-M nanomotors. Z@P-M nanomotors loaded microneedles penetrate the stratum corneum to deliver antibacterial nanoparticles into the dermis. In female murine acne, Zinc peroxide slowly releases hydrogen peroxide in acidic biofilm, catalyzed by manganese dioxide to generate oxygen, thus alleviating hypoxia and skin inflammation. After near infrared laser irradiation, the thermal gradient generated by the asymmetrically modified polydopamine coating endows the nanomotors with enhanced diffusion to promote biofilm penetration, further improving photothermal therapy efficacy and showing a potential for active acne treatment.
Similar content being viewed by others
Data availability
Source data are provided with this paper. The source data produced by this study can be found in the Supplementary Information/Source Data file. The source data are available in Figshare dataset: https://doi.org/10.6084/m9.figshare.30226972. All data supporting the findings of this study are available from corresponding authors upon request. Source data are provided with this paper.
References
Williams, H. C., Dellavalle, R. P. & Garner, S. Acne vulgaris. Lancet 379, 361–372 (2012).
Layton, A. M. & Ravenscroft, J. Adolescent acne vulgaris: current and emerging treatments. Lancet Child Adolesc. Health 7, 136–144 (2023).
Brüggemann, H. et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science 305, 671–673 (2004).
Xiang, Y. et al. Ultrasound-triggered interfacial engineering-based microneedle for bacterial infection acne treatment. Sci. Adv. 9, eadf0854 (2023).
Zhang, T. et al. Active pharmaceutical ingredient poly(ionic liquid)-based microneedles for the treatment of skin acne infection. Acta Biomater. 115, 136–147 (2020).
Kobayashi, T. et al. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell 176, 982–997.e916 (2019).
Gan, Y. et al. Commensal microbe regulation of skin cells in disease. Cell Host Microbe 32, 1264–1279 (2024).
Xu, Y. et al. Innate lymphoid cell-based immunomodulatory hydrogel microspheres containing Cutibacterium acnes extracellular vesicles for the treatment of psoriasis. Acta Biomater. 184, 296–312 (2024).
Reynolds, R.V. et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 90, 1006 (2024).
Wang, B. et al. Hyaluronic acid-based CuS nanoenzyme biodegradable microneedles for treating deep cutaneous fungal Infection without drug resistance. Nano Lett. 23, 1327–1336 (2023).
Wu, C. et al. Microneedles as transdermal drug delivery system for enhancing skin disease treatment. Acta Pharm. Sin. B 14, 5161–5180 (2024).
Zheng, B. et al. Microorganism microneedle micro-engine depth drug delivery. Nat. Commun. 15, 8947 (2024).
Liu, Z. et al. Janus nanoparticles targeting extracellular polymeric substance achieve flexible elimination of drug-resistant biofilms. Nat. Commun. 14, 5132 (2023).
Gao, J. et al. Hyperthermia-triggered biomimetic bubble nanomachines. Nat. Commun. 14, 4867 (2023).
Yan, D. et al. Adding flying wings: butterfly-shaped NIR-II AIEgens with multiple molecular rotors for photothermal combating of bacterial biofilms. J. Am. Chem. Soc. 145, 25705–25715 (2023).
Liu, T. et al. Controlled propulsion of micro/nanomotors: operational mechanisms, motion manipulation and potential biomedical applications. Chem. Soc. Rev. 51, 10083–10119 (2022).
Xu, W. et al. Self-propelled ferroptosis nanoinducer for enhanced cancer therapy. Int. J. Extrem. Manuf. 7, 035501 (2025).
Zhang, H. et al. A bioinspired virus-like mechano–bactericidal nanomotor for ocular multidrug-resistant bacterial infection treatment. Adv. Mater. 37, 2408221 (2025).
Zhang, X. et al. Alginate lyase immobilized Chlamydomonas algae microrobots: minimally invasive therapy for biofilm penetration and eradication. Acta Pharm. Sin. B 15, 3259–3272 (2025).
Ji, X. et al. multifunctional parachute-like nanomotors for enhanced skin penetration and synergistic antifungal therapy. ACS Nano 15, 14218–14228 (2021).
Hu, Z. et al. NIR-actuated ferroptosis nanomotor for enhanced tumor penetration and therapy. Adv. Mater. 36, 2412227 (2024).
Jia, D. et al. A self-supplied hydrogen peroxide and nitric oxide-generating nanoplatform enhances the efficacy of chemodynamic therapy for biofilm eradication. J. Colloid Interface Sci. 678, 20–29 (2025).
Wang, J. et al. Carrier-free nanoprodrug for p53-mutated tumor therapy via concurrent delivery of zinc-manganese dual ions and ROS. Bioact. Mater. 20, 404–417 (2023).
Li, L. et al. A MnO2 nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano 13, 14283–14293 (2019).
Zhang, L. et al. Tailored synthesis of octopus-type janus nanoparticles for synergistic actively-targeted and chemo-photothermal therapy. Angew. Chem. 55, 2118–2121 (2016).
Tiwari, N. et al. Nanocarriers for skin applications: where do we stand? Angew. Chem. 61, e202107960 (2022).
Sabri, A. H. et al. Intradermal and transdermal drug delivery using microneedles – fabrication, performance evaluation and application to lymphatic delivery. Adv. Drug Deliv. Rev. 153, 195–215 (2020).
Yu, W. et al. Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Mater. Sci. Eng. C. Mater. Biol. Appl 80, 187–196 (2017).
Wang, J. et al. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces 4, 4024–4030 (2012).
Herring, N. P., Panchakarla, L. S. & El-Shall, M. S. P-type nitrogen-doped ZnO nanostructures with controlled shape and doping level by facile microwave synthesis. Langmuir 30, 2230–2240 (2014).
Cheng, S. et al. Soft-template synthesis and characterization of ZnO2 and ZnO hollow spheres. J. Phys. Chem. C. 113, 13630–13635 (2009).
Escobedo-Morales, A. et al. Structural and vibrational properties of hydrothermally grown ZnO2 nanoparticles. J. Cryst. Growth 316, 37–41 (2011).
Razzaq, H. A. A. et al. Bioactive films based on barley β-glucans and ZnO for wound healing applications. Carbohydr. Polym. 272, 118442 (2021).
Salehi, N. et al. Synergistic photothermal and photodynamic therapy to promote bacteria-infected wound healing using ZnO@PDA/Ag-integrated waterborne polyurethane films. J. Mater. Chem. B 13, 6177–6198 (2025).
Wang, Z. et al. A thrombin-activated peptide-templated nanozyme for remedying ischemic stroke via thrombolytic and neuroprotective actions. Adv. Mater. 36, 2210144 (2024).
Ma, H. et al. Mesoporous Silica-based nanomotors loaded with rapamycin for synergistic treatment of rheumatoid arthritis. ACS Nano 19, 22914–22930 (2025).
Du, Y. et al. Synergistic provoking of pyroptosis and STING pathway by multifunctional manganese-polydopamine nano-Immunomodulator for enhanced renal cell carcinoma immunotherapy. Adv. Healthc. Mater. 14, 2500141 (2025).
Zeng, W. et al. Dual-response oxygen-generating MnO2 nanoparticles with polydopamine modification for combined photothermal-photodynamic therapy. Chem. Eng. J. 389, 124494 (2020).
Wang, C. et al. A self-regulated phototheranostic nanosystem with single wavelength-triggered energy switching and oxygen supply for multimodal synergistic therapy of bacterial biofilm infections. Aggregate 5, e587 (2024).
Lai, S. et al. Fluorescent microneedle-based theranostic patch for naked-eye monitoring and on-demand photo-therapy of bacterial biofilm infections. Adv. Funct. Mater. 35, 2415559 (2025).
Gao, S. et al. Chlorella-loaded antibacterial microneedles for microacupuncture oxygen therapy of diabetic bacterial infected wounds. Adv. Mater. 36, 2307585 (2024).
Zhao, T. et al. Mesoporous nano-badminton with asymmetric mass distribution: how nanoscale architecture affects the blood flow dynamics. J. Am. Chem. Soc. 145, 21454–21464 (2023).
Yang, Z.-R. et al. Endogenous stimuli-responsive separating microneedles to inhibit hypertrophic scar through remodeling the pathological microenvironment. Nat. Commun. 15, 2038 (2024).
Achermann, Y., Goldstein Ellie, J. C., Coenye, T. & Shirtliff Mark, E. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin. Microbiol. Rev. 27, 419–440 (2014).
Chen, Z., Wang, Z., Ren, J. & Qu, X. Enzyme mimicry for combating bacteria and biofilms. Acc. Chem. Res 51, 789–799 (2018).
Xie, S. et al. Self-propelling nanomotors integrated with biofilm microenvironment-activated NO release to accelerate healing of bacteria-infected diabetic wounds. Adv. Healthc. Mater. 11, 2201323 (2022).
Tyner, H. & Patel, R. Propionibacterium acnes biofilm – a sanctuary for Staphylococcus aureus? Anaerobe 40, 63–67 (2016).
Wang, S. et al. Nanoenzyme-reinforced injectable hydrogel for healing diabetic wounds infected with multidrug resistant bacteria. Nano Lett. 20, 5149–5158 (2020).
Wei, Y. et al. Kill two birds with one stone: dual-metal MOF-nanozyme-decorated hydrogels with ROS-scavenging, oxygen-generating, and antibacterial abilities for accelerating Infected diabetic wound healing. Small 20, 2403679 (2024).
Zhang, X. et al. Wireless power supply near-infrared light drug-loaded microneedle system for the treatment of linear skin wounds. Chem. Eng. J. 500, 157110 (2024).
Zhao, Y. et al. All-in-one bioactive properties of photothermal nanofibers for accelerating diabetic wound healing. Biomaterials 295, 122029 (2023).
Ye, J. et al. Biomimetic dual-driven heterojunction nanomotors for targeted catalytic immunotherapy of glioblastoma. Adv. Funct. Mater. 35, 2416265 (2024).
Yuan, Z. et al. A photo-therapeutic nanocomposite with bio-responsive oxygen self-supplying combats biofilm infections and inflammation from drug-resistant bacteria. Adv. Funct. Mater. 33, 2302908 (2023).
Li, J. et al. Targeting endogenous hydrogen peroxide at bone defects promotes bone repair. Adv. Funct. Mater. 32, 2111208 (2022).
Zhu, J. et al. Biomimetic hyaluronic acid-stabilized zinc oxide nanoparticles in acne treatment: A preclinical and clinical approach. J. Control Release 382, 113754 (2025).
Jahns, A. C. & Alexeyev, O. A. Three dimensional distribution of Propionibacterium acnes biofilms in human skin. Exp. Dermatol 23, 687–689 (2014).
Kim, Y., Lee, J. & Lee, J. Antibiofilm activities of fatty acids including myristoleic acid against Cutibacterium acnes via reduced cell hydrophobicity. Phytomedicine 91, 153710 (2021).
Sharma, M., Schoop, R., Suter, A. & Hudson, J. B. The potential use of Echinacea in acne: control of Propionibacterium acnes growth and inflammation. Phytother. Res 25, 517–521 (2011).
Lou, S. et al. Efficient organic nanoparticles for photothermal antibacterial activity and immune regulation to promote maxillofacial wound healing and scarless repair. Biomaterials 324, 123534 (2026).
Wang, P. et al. Microecology in vitro model replicates the human skin microbiome interactions. Nat. Commun. 16, 3085 (2025).
Dréno, B., Dagnelie, M. A., Khammari, A. & Corvec, S. The skin microbiome: a new actor in inflammatory acne. Am. J. Clin. Dermatol 21, 18–24 (2020).
Lekbua, A. et al. SkinCom, a synthetic skin microbial community, enables reproducible investigations of the human skin microbiome. Cell Rep Methods. 4, 100832 (2024).
Fyhrquist, N. et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat. Commun. 10, 4703 (2019).
Yang, Z. et al. Nano-oxygenated hydrogels for locally and permeably hypoxia relieving to heal chronic wounds. Biomaterials 282, 121401 (2022).
Greywal, T. et al. Evidence-based recommendations for the management of acne fulminans and its variants. J. Am. Acad. Dermatol 77, 109–117 (2017).
Liu, X. et al. A tough, antibacterial and antioxidant hydrogel dressing accelerates wound healing and suppresses hypertrophic scar formation in infected wounds. Bioact. Mater. 34, 269–281 (2024).
Peng, V. et al. Ornithine decarboxylase supports ILC3 responses in infectious and autoimmune colitis through positive regulation of IL-22 transcription. Proc. Natl. Acad. Sci. USA 119, e2214900119 (2022).
Sajiir, H. et al. Harnessing IL-22 for metabolic health: promise and pitfalls. Trends Mol. Med 31, 574–584 (2024).
Xu, Z. et al. Nanoparticles-incorporated hydrogel microneedle for biomedical applications: Fabrication strategies, emerging trends and future prospects. Asian J. Pharm. Sci. 20, 101069 (2025).
González-Vázquez, P. et al. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J. Control Release 265, 30–40 (2017).
Ye, Y. et al. Apoptotic tumor DNA activated nanomotor chemotaxis. Nano Lett. 21, 8086–8094 (2021).
Acknowledgements
This work was supported by National Key Research and Development Program of China (2022YFA1206900, Y.T.), and National Natural Science Foundation of China (22175083, Y.T. and 22375224, F.P.).
Author information
Authors and Affiliations
Contributions
Z.H. and Y.G. contributed equally to this work. Z.H. and Y.T. participated in the conceptualization of the project. Z.H. and Y.S. were involved in the synthesis and characterization of nanomotors. Z.H., H.Q., and L.L. (Lu Liu) perform experiments in vitro. Z.H., Y.G., L.L. (Limeng Liu), and Z.F. contributed to the animal experiments. Z.H., Y.G. and Y.T. wrote the manuscript. Y.T., Z.F. and F.P. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Peer review
Peer review information
Nature Communications thanks Tijana Miseljic, who co-reviewed with Ziqiao Li, Tzanko Tzanov, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, Z., Gan, Y., Song, Y. et al. Near-infrared light-driven nanomotors-based microneedles for the active therapy of bacterial infected acne. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68376-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68376-6