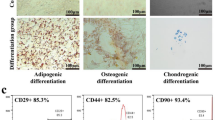
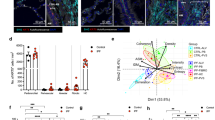
Abstract
The activation and accumulation of lung fibroblasts, leading to excessive ECM deposition, is a pathogenic hallmark of Idiopathic Pulmonary Fibrosis, a lethal and currently untreatable disease. In this report, increased expression of Versican, a multifunctional ECM proteoglycan, is detected in both human and mouse pulmonary fibrosis, mainly in monocytic cells and fibroblasts. Ubiquitous genetic reduction of Versican expression in mice promotes collagen expression and polymerisation, alters pulmonary ECM composition and structure, and exacerbates pulmonary fibrosis, delaying its resolution. Moreover, the decrease in Versican in the ECM and the ensuing reorganisation stimulate Tenascin-C expression from fibroblasts, which is further shown to be a potent Toll-like receptor 4-dependent podosome inducer, promoting ECM invasion. Thus, fibroblast-expressed Versican regulates the underlying ECM composition and structure and suppresses autologous podosome formation, limiting ECM invasion and pulmonary fibrosis.
Similar content being viewed by others
Data availability
The scRNA-seq data utilised in this study are listed in Supplementary Table 2, including accession numbers and hyperlinks. The raw MS proteomics data generated in this study have been deposited in the ProteomeXchange Consortium via the PRIDE92 partner repository under the dataset identifiers PXD060583 and PXD063546. Source data are provided with this paper.
References
Raghu, G. et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 205, e18–e47 (2022).
Martinez, F. J. et al. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 3, 17074 (2017).
Upagupta, C., Shimbori, C., Alsilmi, R. & Kolb, M. Matrix abnormalities in pulmonary fibrosis. Eur Respir Rev. 27, 180033 (2018).
Barbayianni, I. et al. SRC and TKS5 mediated podosome formation in fibroblasts promotes extracellular matrix invasion and pulmonary fibrosis. Nat. Commun. 14, 5882 (2023).
Tomos, I., Kanellopoulou, P., Nastos, D. & Aidinis, V. Pharmacological targeting of ECM homeostasis, fibroblast activation, and invasion for the treatment of pulmonary fibrosis. Expert Opin Ther Targets. 29, 43–57 (2025).
Mouw, J. K., Ou, G. & Weaver, V. M. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 15, 771–785 (2014).
Karamanos, N. K. et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 288, 6850–6912 (2021).
Tomos, I. P. et al. Extracellular matrix remodeling in idiopathic pulmonary fibrosis. It is the ‘bed’ that counts and not ‘the sleepers’. Expert Rev. Respir. Med. 11, 299–309 (2017).
Kanchanawong, P. & Calderwood, D. A. Organization, dynamics and mechanoregulation of integrin-mediated cell–ECM adhesions. Nat. Rev. Mol. Cell Biol. 24, 142–161 (2023).
Theocharis, A. D., Skandalis, S. S., Gialeli, C. & Karamanos, N. K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 97, 4–27 (2016).
Wight, T. N. et al. Versican—a critical extracellular matrix regulator of immunity and inflammation. Front. Immunol. 11, 512 (2020).
Karamanos, N. K. et al. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem. Rev. 118, 9152–9232 (2018).
Tang, F. et al. Defining the versican interactome in lung health and disease. Am. J. Physiol. Cell Physiol. 323, C249–c276 (2022).
Andersson-Sjoland, A. et al. Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology 25, 243–251 (2015).
Chang, W. et al. Plasma versican and plasma exosomal versican as potential diagnostic markers for non-small cell lung cancer. Respir. Res. 24, 140 (2023).
Magkrioti, C. et al. The autotaxin—lysophosphatidic acid axis promotes lung carcinogenesis. Cancer Res. 78, 3634–3644 (2018).
Bensadoun, E. S., Burke, A. K., Hogg, J. C. & Roberts, C. R. Proteoglycan deposition in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 154, 1819–1828 (1996).
Roberts, C. R. & Burke, A. K. Synthesis of the proteoglycan versican in pulmonary fibrosis. Int. J. Exp. Pathol. 81, A21–A22 (2000).
Tashiro, J. et al. Exploring animal models that resemble idiopathic pulmonary fibrosis. Front. Med. 4, 118 (2017).
Mouratis, M. A. & Aidinis, V. Modeling pulmonary fibrosis with bleomycin. Curr. Opin. Pulm. Med. 17, 355–361 (2011).
Barbayianni, I., Ninou, I., Tzouvelekis, A. & Aidinis, V. Bleomycin revisited: a direct comparison of the intratracheal micro-spraying and the oropharyngeal aspiration routes of bleomycin administration in mice. Front. Med. 5, 269 (2018).
Fanidis, D., Moulos, P. & Aidinis, V. Fibromine is a multi-omics database and mining tool for target discovery in pulmonary fibrosis. Sci. Rep. 11, 21712 (2021).
Herrera, J. et al. Registration of the extracellular matrix components constituting the fibroblastic focus in idiopathic pulmonary fibrosis. JCI Insight. 4, e125185 (2019).
Zannikou, M. et al. MAP3K8 regulates Cox-2-mediated prostaglandin E(2) production in the lung and suppresses pulmonary inflammation and fibrosis. J. Immunol. 206, 607–620 (2021).
Galaris, A. et al. Increased lipocalin-2 expression in pulmonary inflammation and fibrosis. Front. Med. 10, 1195501 (2023).
DeQuach, J. A. et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One 5, e13039 (2010).
Kawai, N. et al. Induction of lung-like cells from mouse embryonic stem cells by decellularized lung matrix. Biochem Biophys. Rep. 15, 33–38 (2018).
Choocheep, K. et al. Versican facilitates chondrocyte differentiation and regulates joint morphogenesis. J. Biol. Chem. 285, 21114–21125 (2010).
Segnani, C. et al. Histochemical detection of collagen fibers by sirius red/fast green is more sensitive than van gieson or sirius red alone in normal and inflamed rat colon. PLoS One 10, e0144630 (2015).
Júnior, C. et al. Multi-step extracellular matrix remodelling and stiffening in the development of idiopathic pulmonary fibrosis. Int. J. Mol. Sci. 24, 1708 (2023).
Batasheva, S., Kotova, S., Frolova, A. & Fakhrullin, R. Atomic force microscopy for characterization of decellularized extracellular matrix (dECM) based materials. Sci. Technol. Adv. Mater. 25, 2421739 (2024).
Mjaatvedt, C. H., Yamamura, H., Capehart, A. A., Turner, D. & Markwald, R. R. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev. Biol. 202, 56–66 (1998).
Landolt, R. M., Vaughan, L., Winterhalter, K. H. & Zimmermann, D. R. Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development 121, 2303–2312 (1995).
Hatano, S. et al. Versican/PG-M is essential for ventricular septal formation subsequent to cardiac atrioventricular cushion development. Glycobiology 22, 1268–1277 (2012).
Midwood, K. S., Chiquet, M., Tucker, R. P. & Orend, G. Tenascin-C at a glance. J. Cell Sci. 129, 4321–4327 (2016).
Estany, S. et al. Lung fibrotic tenascin-C upregulation is associated with other extracellular matrix proteins and induced by TGFβ1. BMC Pulm. Med. 14, 120 (2014).
Du, P. et al. Human lung fibroblast-derived matrix facilitates vascular morphogenesis in 3D environment and enhances skin wound healing. Acta Biomater. 54, 333–344 (2017).
Zhang, X., Flores, L. R., Keeling, M. C., Sliogeryte, K. & Gavara, N. Ezrin Phosphorylation at T567 modulates cell migration, mechanical properties, and cytoskeletal organization. Int. J. Mol. Sci. 21, 435 (2020).
Sansilvestri Morel, P. et al. Procollagen C-proteinase enhancer-1 (PCPE-1) deficiency in mice reduces liver fibrosis but not NASH progression. PLoS One 17, e0263828 (2022).
Chen, D. et al. Versican binds collagen via its G3 domain and regulates the organization and mechanics of collagenous matrices. J. Biol. Chem. 300, 107968 (2024).
Perissinotto, D. et al. Avian neural crest cell migration is diversely regulated by the two major hyaluronan-binding proteoglycans PG-M/versican and aggrecan. Development 127, 2823–2842 (2000).
Sand, J. M. B. et al. A serological biomarker of versican degradation is associated with mortality following acute exacerbations of idiopathic interstitial pneumonia. Respir. Res. 19, 82 (2018).
Watanabe, H. Versican and versikine: the dynamism of the extracellular matrix. Proteoglycan Res. 1, e13 (2023).
Gaggar, A. & Weathington, N. Bioactive extracellular matrix fragments in lung health and disease. J. Clin. Invest. 126, 3176–3184 (2016).
Maher, T. M. et al. Biomarkers of extracellular matrix turnover in patients with idiopathic pulmonary fibrosis given nintedanib (INMARK study): a randomised, placebo-controlled study. Lancet Respir. Med. 7, 771–779 (2019).
Li, Y. et al. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J. Exp. Med. 208, 1459–1471 (2011).
Ahangari, F. et al. Saracatinib, a selective Src kinase inhibitor, blocks fibrotic responses in preclinical models of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 206, 1463–1479 (2022).
Jaeger, B. et al. Airway basal cells show a dedifferentiated KRT17(high) phenotype and promote fibrosis in idiopathic pulmonary fibrosis. Nat. Commun. 13, 5637 (2022).
Adams, T. S. et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 6, eaba1983 (2020).
Kellar, G. G. et al. Loss of versican and production of hyaluronan in lung epithelial cells are associated with airway inflammation during RSV infection. J. Biol. Chem. 296, 100076 (2021).
Wynn, T. A. & Vannella, K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462 (2016).
Chang, M. Y. et al. Monocyte-to-macrophage differentiation: synthesis and secretion of a complex extracellular matrix. J. Biol. Chem. 287, 14122–14135 (2012).
Chang, M. Y. et al. Versican is produced by Trif- and type I interferon-dependent signaling in macrophages and contributes to fine control of innate immunity in lungs. Am. J. Physiol. Lung Cell Mol. Physiol. 313, L1069–l1086 (2017).
Chang, M. Y. et al. A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol. 34, 1–12 (2014).
Uhlin-Hansen, L. et al. Proteoglycan metabolism in normal and inflammatory human macrophages. Blood 82, 2880–2889 (1993).
Pappas, A. G. et al. Versican modulates tumor-associated macrophage properties to stimulate mesothelioma growth. Oncoimmunology 8, e1537427 (2019).
Papadas, A. et al. Emerging roles for tumor stroma in antigen presentation and anti-cancer immunity. Biochem. Soc. Trans. 51, 2017–2028 (2023).
Hirani, P. et al. Versican associates with tumor immune phenotype and limits T-cell trafficking via chondroitin sulfate. Cancer Res. Commun. 4, 970–985 (2024).
Emmerich, P. B. et al. Stromal versican accumulation and proteolysis regulate the infiltration of CD8(+) T cells in breast cancer. Cancers 17, 1435 (2025).
Younesi, F. S., Miller, A. E., Barker, T. H., Rossi, F. M. V. & Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 25, 617–638 (2024).
Herrera, J., Henke, C. A. & Bitterman, P. B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Invest. 128, 45–53 (2018).
Nho, R. S., Ballinger, M. N., Rojas, M. M., Ghadiali, S. N. & Horowitz, J. C. Biomechanical force and cellular stiffness in lung fibrosis. Am. J. Pathol. 192, 750–761 (2022).
Artym, V. V. et al. Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. J. Cell Biol. 208, 331–350 (2015).
Juin, A. et al. Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J. Cell Biol. 207, 517–533 (2014).
Zhao, P. et al. The CD44s splice isoform is a central mediator for invadopodia activity. J. Cell Sci. 129, 1355–1365 (2016).
Tucić, M., Stamenković, V. & Andjus, P. The extracellular matrix glycoprotein tenascin C and adult neurogenesis. Front. Cell Dev. Biol. 9, 674199 (2021).
Lowy, C. M. & Oskarsson, T. Tenascin C in metastasis: a view from the invasive front. Cell Adhes. Migr. 9, 112–124 (2015).
Tucker, R. P. & Degen, M. Revisiting the tenascins: Exploitable as cancer targets? Front. Oncol. 12, 908247 (2022).
Selman, M., Pardo, A. & Kaminski, N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs. PLoS Med. 5, e62 (2008).
Carey, W. A., Taylor, G. D., Dean, W. B. & Bristow, J. D. Tenascin-C deficiency attenuates TGF-ß-mediated fibrosis following murine lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 299, L785–L793 (2010).
Bhattacharyya, S. et al. Tenascin-C drives persistence of organ fibrosis. Nat. Commun. 7, 11703 (2016).
Hawkins, A. G. et al. Microenvironmental factors drive tenascin C and Src cooperation to promote invadopodia formation in ewing sarcoma. Neoplasia 21, 1063–1072 (2019).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 (2009).
Bhattacharyya, S. et al. TLR4-dependent fibroblast activation drives persistent organ fibrosis in skin and lung. JCI Insight. 3, e98850 (2018).
Penke, L. R. & Peters-Golden, M. Molecular determinants of mesenchymal cell activation in fibroproliferative diseases. Cell Mol. Life Sci. 76, 4179–4201 (2019).
Atabai, K. et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J. Clin. Invest. 119, 3713–3722 (2009).
Ghanem, M. et al. FGF21 signaling exerts anti-fibrotic properties during pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 211, 486–498 (2024).
Barnes, J. W. et al. Role of fibroblast growth factor 23 and klotho cross talk in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 317, L141–L154 (2019).
Carthy, J. M. et al. Versican V1 overexpression induces a myofibroblast-like phenotype in cultured fibroblasts. PLoS One 10, e0133056 (2015).
Spella, M. et al. Club cells form lung adenocarcinomas and maintain the alveoli of adult mice. Elife. 8, e45571 (2019).
Flores, L. R., Keeling, M. C., Zhang, X., Sliogeryte, K. & Gavara, N. Lifeact-TagGFP2 alters F-actin organization, cellular morphology and biophysical behaviour. Sci. Rep. 9, 3241 (2019).
Narciso, M. et al. Lung micrometastases display ECM depletion and softening while macrometastases are 30-fold stiffer and enriched in fibronectin. Cancers 15, 2404 (2023).
Hughes, C. S. et al. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 14, 68–85 (2019).
Papadopoulos, G. et al. Integrated omics analysis for characterization of the contribution of high fructose corn syrup to non-alcoholic fatty liver disease in obesity. Metabolism 144, 155552 (2023).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Keene, D. R. & Tufa, S. F. Ultrastructural analysis of the extracellular matrix. Methods Cell Biol. 143, 1–39 (2018).
Havaki, S. et al. Altered expression pattern of integrin alphavbeta3 correlates with actin cytoskeleton in primary cultures of human breast cancer. Cancer Cell Int. 7, 16 (2007).
Logotheti, S. et al. in Oncogene-Induced Senescence: Methods and Protocols (eds Vassilis G. Gorgoulis, Maria Cavinato, & Konstantinos Evangelou) 83–112 (Springer, 2025).
Tremi, I. et al. A guide for using transmission electron microscopy for studying the radiosensitizing effects of gold nanoparticles in vitro. Nanomaterials 11, 859 (2021).
Hao, Y. et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 42, 293–304 (2024).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. Omics 16, 284–287 (2012).
Perez-Riverol, Y. et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 53, D543–d553 (2025).
Acknowledgements
This research was funded by the Hellenic Foundation for Research and Innovation (HFRI) under the “2nd Call for HFRI Research Projects to support Faculty Members & Researchers” (#3565 to V.A.). S.H., I.T., and V.G. were supported by an HFRI grant (#2906 to V.G.) under the “1st Call for HFRI Research Projects to support Faculty members and Researchers and the procurement of high-cost research equipment”. The funders had no role in the study’s design, data collection, analysis, or interpretation, nor in the writing of the manuscript or the decision to publish the results.
Author information
Authors and Affiliations
Contributions
V.A. conceptualised, supervised and funded the study. P.K. performed most of the experiments presented, assisted by I.B., E.K., D.N., S.S., M.S., and C.M. A.G. and S.G. performed FACS analyses. V.R. performed and analysed μCT. D.F. analysed transcriptomics data. M.Sa. performed and analysed MS proteomics. A.M.B. and N.G. performed and analysed AFM and F-actin image analysis. I.T., S.H., and V.G. performed and analysed TEM. I.T. and I.V. provided human lung samples and analysed related IHC. H.W. provided resources and co-evaluated results. P.K. and V.A. wrote the manuscript, which was then critically read and edited by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Fuquan Yang, Libang Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kanellopoulou, P., Barbayianni, I., Fanidis, D. et al. Versican expression from lung fibroblasts suppresses pulmonary fibrosis. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68377-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68377-5