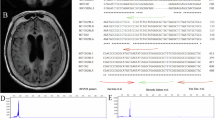
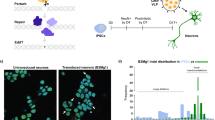
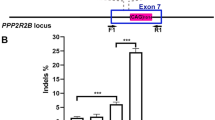
Abstract
Neuronal intranuclear inclusion disease (NIID) is an adult-onset neurodegenerative disease caused by expanded GGC repeats in the 5’ untranslated region of the human-specific NOTCH2NLC gene. The high sequence similarity between NOTCH2NLC and its paralogs poses a significant challenge for precise gene editing. Here, we develop a CRISPR/spCas9-based gene-editing strategy that precisely excises the expanded GGC repeats in NOTCH2NLC without detectable off-target effects on the highly homologous NOTCH2/NOTCH2NL family genes (<2% sequence divergence at this locus). The efficacy, specificity and safety of this approach are rigorously validated across multiple experimental models, including human cell lines, NIID iPSCs, and our previously established transgenic NIID mouse model. Our results demonstrate that precise excision of the expanded GGC repeats effectively alleviates NIID-related neuropathological, molecular and behavioral abnormalities. This study establishes the proof of concept for genome editing as a therapeutic strategy for NIID and other related repeat expansion disorders.
Similar content being viewed by others
Data availability
The raw RNA-seq data from mice have been deposited in the Gene Expression Omnibus under accession code GSE295763. The raw RNA-seq data from human NPCs and the raw WGS data from human iPSCs have been deposited in the Genome Sequence Archive for Human under accession code HRA011636 and HRA014016, respectively. The deposition and sharing of the raw data have been approved by the Human Genetics Resource Office in China (registration number: 2025BAT00839). All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary data files. Source data are provided with this paper.
References
Depienne, C. & Mandel, J. L. 30 years of repeat expansion disorders: what have we learned and what are the remaining challenges? Am. J. Hum. Genet. 108, 764–785 (2021).
Malik, I., Kelley, C. P., Wang, E. T. & Todd, P. K. Molecular mechanisms underlying nucleotide repeat expansion disorders. Nat. Rev. Mol. Cell Biol. 22, 589–607 (2021).
Verkerk, A. J. M. H. et al. Identification of a gene containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914 (1991).
Pieretti, M. et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66, 817–822 (1991).
Duyao, M. et al. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat. Genet. 4, 387–392 (1993).
Banfi, S. et al. Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat. Genet. 7, 513–520 (1994).
DeJesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011).
Renton, A. E. et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 (2011).
Sone, J. et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat. Genet. 51, 1215–1221 (2019).
Cortese, A. et al. Biallelic expansion of an intronic repeat in RFC1 is a common cause of late-onset ataxia. Nat. Genet. 51, 649–658 (2019).
Pellerin, D. et al. Deep intronic FGF14 GAA repeat expansion in late-onset cerebellar ataxia. N. Engl. J. Med. 388, 128–141 (2023).
Figueroa, K. P. et al. A GGC-repeat expansion in ZFHX3 encoding polyglycine causes spinocerebellar ataxia type 4 and impairs autophagy. Nat. Genet. 56, 1080–1089 (2024).
Zhou, Z.-D., Jankovic, J., Ashizawa, T. & Tan, E.-K. Neurodegenerative diseases associated with non-coding CGG tandem repeat expansions. Nat. Rev. Neurol. 18, 145–157 (2022).
Takahashi-Fujigasaki, J. Neuronal intranuclear hyaline inclusion disease. Neuropathology 23, 351–359 (2003).
Sone, J. et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain 139, 3170–3186 (2016).
Tian, Y. et al. Clinical features of NOTCH2NLC-related neuronal intranuclear inclusion disease. J. Neurol. Neurosurg. Psychiatry 93, 1289–1298 (2022).
Liu, Y. H. et al. Neuronal intranuclear inclusion disease in patients with adult-onset non-vascular leukoencephalopathy. Brain 145, 3010–3021 (2022).
Tai, H. et al. Clinical features and classification of neuronal intranuclear inclusion disease. Neurol. Genet 9, e200057 (2023).
Chen, H. et al. Re-defining the clinicopathological spectrum of neuronal intranuclear inclusion disease. Ann. Clin. Transl. Neurol. 7, 1930–1941 (2020).
Huang, X. R., Tang, B. S., Jin, P. & Guo, J. F. The phenotypes and mechanisms of NOTCH2NLC-related GGC repeat expansion disorders: a comprehensive review. Mol. Neurobiol. 59, 523–534 (2022).
Liu, Y. et al. Clinical and mechanism advances of neuronal intranuclear inclusion disease. Front. Aging Neurosci. 14, 934725 (2022).
Tian, Y. et al. Expansion of human-specific GGC repeat in neuronal intranuclear inclusion disease-related disorders. Am. J. Hum. Genet. 105, 166–176 (2019).
Ishiura, H. et al. Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nat. Genet. 51, 1222–1232 (2019).
Deng, J. et al. Long-read sequencing identified repeat expansions in the 5’UTR of the NOTCH2NLC gene from Chinese patients with neuronal intranuclear inclusion disease. J. Med. Genet. 56, 758–764 (2019).
Sun, Q. Y. et al. Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain 143, 222–233 (2019).
Yuan, Y. et al. Identification of GGC repeat expansion in the NOTCH2NLC gene in amyotrophic lateral sclerosis. Neurology 95, e3394–e3405 (2020).
Shi, C. H. et al. NOTCH2NLC intermediate-length repeat expansions are associated with Parkinson disease. Ann. Neurol. 89, 182–187 (2021).
Yan, Y. et al. Assessing the NOTCH2NLC GGC expansion in essential tremor patients from eastern China. Brain 144, e1–e1 (2021).
Okubo, M. et al. GGC repeat expansion of NOTCH2NLC in adult patients with leukoencephalopathy. Ann. Neurol. 86, 962–968 (2019).
Fang, P. et al. Repeat expansion scanning of the NOTCH2NLC gene in patients with multiple system atrophy. Ann. Clin. Transl. Neurol. 7, 517–526 (2020).
Jiao, B. et al. Identification of expanded repeats in NOTCH2NLC in neurodegenerative dementias. Neurobiol. Aging 89, 142.e1–142.e7 (2020).
Yu, J. et al. The GGC repeat expansion in NOTCH2NLC is associated with oculopharyngodistal myopathy type 3. Brain 144, 1819–1832 (2021).
Zeng, T. et al. Neuronal intranuclear inclusion disease with NOTCH2NLC GGC repeat expansion: a systematic review and challenges of phenotypic characterization. Aging Dis. 16, 578–597 (2024).
Ehrlich, M. E. & Ellerby, L. M. Neuronal intranuclear inclusion disease: polyglycine protein is the culprit. Neuron 109, 1757–1760 (2021).
Boivin, M. et al. Translation of GGC repeat expansions into a toxic polyglycine protein in NIID defines a novel class of human genetic disorders: the polyG diseases. Neuron 109, 1825–1835.e5 (2021).
Zhong, S. et al. Upstream open reading frame with NOTCH2NLC GGC expansion generates polyglycine aggregates and disrupts nucleocytoplasmic transport: implications for polyglycine diseases. Acta Neuropathol. 142, 1003–1023 (2021).
Yu, J. et al. CGG repeat expansion in NOTCH2NLC causes mitochondrial dysfunction and progressive neurodegeneration in Drosophila model. Proc. Natl. Acad. Sci. USA 119, e2208649119 (2022).
Fan, Y. et al. GGC repeat expansion in NOTCH2NLC induces dysfunction in ribosome biogenesis and translation. Brain 146, 3373–3391 (2023).
Liu, Q. et al. Expression of expanded GGC repeats within NOTCH2NLC causes behavioral deficits and neurodegeneration in a mouse model of neuronal intranuclear inclusion disease. Sci. Adv. 8, eadd6391 (2022).
Pan, Y. et al. Expression of expanded GGC repeats within NOTCH2NLC causes cardiac dysfunction in mouse models. Cell Biosci. 13, 157 (2023).
Zhong, S. et al. Microglia contribute to polyG-dependent neurodegeneration in neuronal intranuclear inclusion disease. Acta Neuropathol. 148, 21 (2024).
Fiddes, I. T. et al. Human-specific NOTCH2NL genes affect notch signaling and cortical neurogenesis. Cell 173, 1356 (2018).
Suzuki, I. K. et al. Human-specific NOTCH2NL genes expand cortical neurogenesis through Delta/Notch regulation. Cell 173, 1370 (2018).
Florio, M. et al. Evolution and cell-type specificity of human-specific genes preferentially expressed in progenitors of fetal neocortex. Elife 7, e32332 (2018).
Yang, S. et al. CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington’s disease. J. Clin. Investig. 127, 2719–2724 (2017).
Yan, S. et al. Cas9-mediated replacement of expanded CAG repeats in a pig model of Huntington’s disease. Nat. Biomed. Eng. 7, 629–646 (2023).
Chan, K. Y. et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179 (2017).
Sun, Q. Y. et al. Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain 143, 222–233 (2020).
Ng, A. S. L. et al. NOTCH2NLC GGC repeat expansions are associated with sporadic essential tremor: variable disease expressivity on long-term follow-up. Ann. Neurol. 88, 614–618 (2020).
Yan, Y. et al. Assessing the NOTCH2NLC GGC expansion in essential tremor patients from eastern China. Brain 144, e1 (2021).
Zhou, X. et al. Clinical features and reclassification of essential tremor with NOTCH2NLC GGC repeat expansions based on a long-term follow-up. Eur. J. Neurol. 29, 3600–3610 (2022).
Zhang, W. et al. GGC repeat expansions in NOTCH2NLC causing a phenotype of lower motor neuron syndrome. J. Neurol. 269, 4469–4477 (2022).
Wan, M. et al. Intermediate-length GGC repeat expansion in NOTCH2NLC was identified in chinese patients with amyotrophic lateral sclerosis. Brain Sci. 13, 85 (2023).
Wang, Y. C. et al. NOTCH2NLC expanded GGC repeats in patients with cerebral small vessel disease. Stroke Vasc. Neurol. 8, 161–168 (2022).
Liao, Y. C. et al. NOTCH2NLC GGC repeat expansion in patients with vascular leukoencephalopathy. Stroke 54, 1236–1245 (2023).
Wu, C. et al. The genetic and phenotypic spectra of adult genetic leukoencephalopathies in a cohort of 309 patients. Brain 146, 2364–2376 (2022).
Wu, W. et al. Intermediate-length CGG repeat expansion in NOTCH2NLC is associated with pathologically confirmed Alzheimer’s disease. Neurobiol. Aging 120, 189–195 (2022).
Ma, D. et al. Association of NOTCH2NLC repeat expansions with Parkinson disease. JAMA Neurol. 77, 1559–1563 (2020).
Wang, H. et al. GGC repeat expansion in the NOTCH2NLC gene is associated with a phenotype of predominant motor-sensory and autonomic neuropathy. Front. Genet. 12, 694790 (2021).
Liao, Y. C. et al. GGC repeat expansion of NOTCH2NLC in Taiwanese patients with inherited neuropathies. Neurology 98, e199–e206 (2022).
Ogasawara, M. et al. CGG expansion in NOTCH2NLC is associated with oculopharyngodistal myopathy with neurological manifestations. Acta Neuropathol. Commun. 8, 204 (2020).
Cao, L., Yan, Y. & Zhao, G. NOTCH2NLC-related repeat expansion disorders: an expanding group of neurodegenerative disorders. Neurol. Sci. 42, 4055–4062 (2021).
Fan, Y., Xu, Y. & Shi, C. NOTCH2NLC-related disorders: the widening spectrum and genotype-phenotype correlation. J. Med. Genet. 59, 1–9 (2022).
Pan, Y. et al. HnRNP M expression rescues neurodegeneration in neuronal intranuclear inclusion disease mouse model by restoring dysregulated RNA splicing and transcription. Cell Biosci. 15, 134 (2025).
Hagerman, R. J. et al. Intention tremor, Parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 57, 127–130 (2001).
Gu, Y., Shen, Y., Gibbs, R. A. & Nelson, D. L. Identification of FMR2, a novel gene associated with the FRAXE CCG repeat and CpG island. Nat. Genet. 13, 109–113 (1996).
Gecz, J., Gedeon, A. K., Sutherland, G. R. & Mulley, J. C. Identification of the gene FMR2, associated with FRAXE mental retardation. Nat. Genet. 13, 105–108 (1996).
Fan, Y. et al. GIPC1 CGG repeat expansion is associated with movement disorders. Ann. Neurol. 91, 704–715 (2022).
Pan, Y. et al. Assessment of GGC repeat expansion in GIPC1 in patients with Parkinson’s disease. Mov. Disord. 37, 1557–1559 (2022).
Brais, B. et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat. Genet. 18, 164–167 (1998).
Ishiura, H. Recent progress in oculopharyngodistal myopathy research from clinical and genetic viewpoints. J. Neuromuscul. Dis. 12, 303–311 (2025).
Park, C. Y. et al. Reversion of FMR1 methylation and silencing by editing the triplet repeats in fragile X iPSC-derived neurons. Cell Rep. 13, 234–241 (2015).
Xie, N. et al. Reactivation of FMR1 by CRISPR/Cas9-mediated deletion of the expanded CGG-repeat of the fragile X chromosome. PLoS ONE 11, e0165499 (2016).
Wang, X. H. et al. Modeling neuronal intranuclear inclusion disease: a review of animal and human-derived cellular models and mechanistic insights. Zool. Res. 46, 1565–1574 (2025).
Yin, P., Li, S., Li, X. J. & Yang, W. New pathogenic insights from large animal models of neurodegenerative diseases. Protein Cell 13, 707–720 (2022).
Malinin, N. L. et al. Defining genome-wide CRISPR-Cas genome-editing nuclease activity with GUIDE-seq. Nat. Protoc. 16, 5592–5615 (2021).
Liu, Q. et al. Cerebellum-enriched protein INPP5A contributes to selective neuropathology in mouse model of spinocerebellar ataxias type 17. Nat. Commun. 11, 1101 (2020).
Yardeni, T., Eckhaus, M., Morris, H. D., Huizing, M. & Hoogstraten-Miller, S. Retro-orbital injections in mice. Lab Anim. (NY) 40, 155–160 (2011).
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Xu, K. et al. Detecting anomalous anatomic regions in spatial transcriptomics with STANDS. Nat. Commun. 15, 8223 (2024).
Deng, T. et al. LEGEND: Identifying co-expressed genes in multimodal transcriptomic sequencing data. Genom. Proteom. Bioinform. 23, qzaf056 (2025).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Chen, K. et al. BreakDancer: an algorithm for high-resolution mapping of genomic structural variation. Nat. Methods 6, 677–681 (2009).
Abyzov, A., Urban, A. E., Snyder, M. & Gerstein, M. CNVnator: an approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res. 21, 974–984 (2011).
Bae, S., Park, J. & Kim, J. S. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475 (2014).
Acknowledgements
The authors would like to thank Peng Jin and Yujing Li from School of Medicine, Emory University; Hao Wu from School of Computer Science and Control Engineering, Shenzhen University of Advanced Technology; Su Yang from Guangdong-Hongkong-Macau Institute of CNS Regeneration, Jinan University; Ying Cheng from Institute of biomedical research, Yunnan University for providing valuable advice to the study. This study was supported by the National Natural Science Foundation of China (82394421 and 82394420 to B.T., 82394422 and 82371874 to X.-J.L., 82271902 and U24A6013 to S.L., 32071037 to Q.L., 82171843 to Y.P., 82101946 to N.X., 82171256 to Q.S.), the National Key R&D Program of China (2021YFA0805200 to H.J.), and the Natural Science Foundation of Hunan Province (2023JJ10097 to Q.L., 2025JJ20079 to Y.P., 2022JJ40832 to N.X.).
Author information
Authors and Affiliations
Contributions
Q.L., Y.P., S.L., and X.-J.L. designed the study, supervised the study, and revised the manuscript; N.X., Y.P., and Q.L. performed experiments, analyzed data, and wrote the manuscript; H.T., Y.L., Y.J., Z.W., J.W., W.Z., X.W., X.S., S.Y., P.Y., Q.S., C.Q., and Y.T. provided important technical assistance to the study; L.S., H.J., D.L., and B.T. provided insightful advice to the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hye Young Lee, who co-reviewed with Seo-Jun Kang; the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, N., Pan, Y., Tong, H. et al. Precise excision of expanded GGC repeats in NOTCH2NLC via CRISPR/Cas9 for treating neuronal intranuclear inclusion disease. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68385-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68385-5