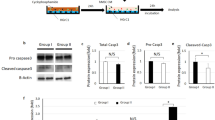
Abstract
Cyclophosphamide (CTX) is a primary medicine for curing breast cancer which often causes premature ovarian insufficiency (POI). Our recent publication reveals that CTX induces POI by promoting the expression of SLC1A4, a transporter of serine efflux, in ovarian granulosa cells (GCs). Here, we report that there is a closed connection between the reduction of serum serine and ovarian hypofunction in the breast cancer patients treated with CTX or women of childbearing age who are suffered from the staying-up-late. Additionally, we observe that dietary serine supplementation protects mice from CTX-induced POI without altering its anti-breast cancer. Furthermore, we demonstrate that the elevated serine promotes S1P synthesis, and in turn, inhibits the nuclear translocation of Nrf2 and consequent HO-1 expression, to suppress ferroptosis in GCs. Our study reveals that the chemotherapy-induced or idiopathic POI share the same mechanisms, indicating that serine is a critical factor for maintaining ovarian function.
Similar content being viewed by others
Data availability
The RNA-seq data in this study have been deposited in the GEO database under accession number GSE314714. The amino acid-targeted metabolomics data in this study have been deposited in the MetaboLights database under accession number MTBLS13561. All other relevant source data supporting the key findings of this study are provided in this paper. Source data are provided with this paper.
References
Zhang, S. et al. Advances in biomaterials and regenerative medicine for primary ovarian insufficiency therapy. Bioact. Mater. 6, 1957–1972 (2021).
Anderson, R. A. et al. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diab. Endocrinol. 3, 556–567 (2015).
Guo, Y. et al. Ovarian microenvironment: challenges and opportunities in protecting against chemotherapy-associated ovarian damage. Hum. Reprod. Update 30, 614–647 (2024).
Condorelli, M. & Demeestere, I. Challenges of fertility preservation in non-oncological diseases. Acta Obstet. Gynecol. Scand. 98, 638–646 (2019).
European Society for Human R et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 31, 926–937 (2016).
Touraine, P. et al. Premature ovarian insufficiency. Nat. Rev. Dis. Prim. 10, 63 (2024).
Huhtaniemi, I. et al. Advances in the molecular pathophysiology, genetics, and treatment of primary ovarian insufficiency. Trends Endocrinol. Metab. 29, 400–419 (2018).
Gargus, E., Deans, R., Anazodo, A. & Woodruff, T. K. Management of primary ovarian insufficiency symptoms in survivors of childhood and adolescent cancer. J. Natl. Compr. Canc Netw. 16, 1137–1149 (2018).
Maltaris, T. et al. Cancer and fertility preservation: fertility preservation in breast cancer patients. Breast Cancer Res. 10, 206 (2008).
Emadi, A., Jones, R. J. & Brodsky, R. A. Cyclophosphamide and cancer: golden anniversary. Nat. Rev. Clin. Oncol. 6, 638–647 (2009).
Abogresha, N. M. et al. Diosmin mitigates cyclophosphamide induced premature ovarian insufficiency in rat model. Int. J. Mol. Sci. 22, 3044 (2021).
Ibarra, I., Erlich, Y., Muthuswamy, S. K., Sachidanandam, R. & Hannon, G. J. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 21, 3238–3243 (2007).
Lavafian, A., Pezeshki, P. S. & Rezaei, N. Investigation of the female infertility risk associated with anti-cancer therapy. Clin. Transl. Oncol. 25, 1893–1905 (2023).
Ostensen, M. Sexual and reproductive health in rheumatic disease. Nat. Rev. Rheumatol. 13, 485–493 (2017).
Gershenson, D. M. Menstrual and reproductive function after treatment with combination chemotherapy for malignant ovarian germ cell tumors. J. Clin. Oncol. 6, 270–275 (1988).
Lu, Z. et al. The landscape of cancer research and cancer care in China. Nat. Med. 29, 3022–3032 (2023).
Lei, S. et al. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 41, 1183–1194 (2021).
Teng, Y. et al. Cancer statistics for young adults aged 20 to 49 years in China from 2000 to 2017: a population-based registry study. Sci. China Life Sci. 67, 711–719 (2024).
Ruddy, K. J. et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J. Clin. Oncol. 32, 1151–1156 (2014).
Letourneau, J. M. et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 118, 1710–1717 (2012).
Oktay, K. et al. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 36, 1994–2001 (2018).
Liu, T. et al. miR-15b induces premature ovarian failure in mice via inhibition of alpha-Klotho expression in ovarian granulosa cells. Free Radic. Biol. Med. 141, 383–392 (2019).
Gu, H. C. et al. Human urine stem cells protect against cyclophosphamide-induced premature ovarian failure by inhibiting SLC1A4-mediated outflux of intracellular serine in ovarian granulosa cells. Cell Mol. Biol. Lett. 30, 21 (2025).
Handzlik, M. K. & Metallo, C. M. Sources and sinks of serine in nutrition, health, and disease. Annu Rev. Nutr. 43, 123–151 (2023).
Jiang, J., Li, B., He, W. & Huang, C. Dietary serine supplementation: friend or foe? Curr. Opin. Pharm. 61, 12–20 (2021).
El-Hattab, A. W. Serine biosynthesis and transport defects. Mol. Genet. Metab. 118, 153–159 (2016).
Holecek, M. Serine metabolism in health and disease and as a conditionally essential amino acid. Nutrients 14, 1987 (2022).
Kurniawan, H. et al. Glutathione restricts serine metabolism to preserve regulatory T cell function. Cell Metab. 31, 920–936 e927 (2020).
Gantner, M. L. et al. Serine and lipid metabolism in macular disease and peripheral neuropathy. N. Engl. J. Med. 381, 1422–1433 (2019).
Handzlik, M. K. et al. Insulin-regulated serine and lipid metabolism drive peripheral neuropathy. Nature 614, 118–124 (2023).
Perea-Gil, I. et al. Serine biosynthesis as a novel therapeutic target for dilated cardiomyopathy. Eur. Heart J. 43, 3477–3489 (2022).
Rachedi, N. S. et al. Dietary intake and glutamine-serine metabolism control pathologic vascular stiffness. Cell Metab. 36, 1335–1350 e1338 (2024).
Wu, D. et al. A comprehensive review on signaling attributes of serine and serine metabolism in health and disease. Int. J. Biol. Macromol. 260, 129607 (2024).
Maffioli, E. et al. Insulin and serine metabolism as sex-specific hallmarks of Alzheimer’s disease in the human hippocampus. Cell Rep. 40, 111271 (2022).
Lu, X. et al. Increased serine synthesis in cumulus cells of young infertile women with diminished ovarian reserve. Hum. Reprod. 38, 1723–1732 (2023).
Duffin, K., Howie, R., Kelsey, T. W., Wallace, H. B. & Anderson, R. A. Long-term follow-up to assess criteria for ovarian tissue cryopreservation for fertility preservation in young women and girls with cancer. Hum. Reprod. 38, 1076–1085 (2023).
Ruan, X. et al. Practice guideline on ovarian tissue cryopreservation and transplantation in the prevention and treatment of iatrogenic premature ovarian insufficiency. Maturitas 182, 107922 (2024).
Chen, Y., Wang, S. & Zhang, C. The differentiation fate of granulosa cells and the regulatory mechanism in ovary. Reprod. Sci. 32, 1414–1426 (2025).
Wang, H. The role of granulosa cells in oocyte development and aging: mechanisms and therapeutic opportunities. Semin Cell Dev. Biol. 171, 103614 (2025).
Schramm, R. D. & Bavister, B. D. Granulosa cells from follicle stimulating hormone-primed monkeys enhance the development competence of in-vitro-matured oocytes from non-stimulated rhesus monkeys. Hum. Reprod. 11, 1698–1702 (1996).
Gilardi, M., Ramos, M. & Hollern, D. B cells secrete GABA, which provokes a pro-tumor immune microenvironment. Cancer Cell 40, 17–19 (2022).
Jiang, X. et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 18, 10 (2019).
Reynolds, A. C. & McKenzie, L. J. Cancer treatment-related ovarian dysfunction in women of childbearing potential: management and fertility preservation options. J. Clin. Oncol. 41, 2281–2292 (2023).
Spears, N. et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 25, 673–693 (2019).
Dolmans, M. M., Donnez, J. & Cacciottola, L. Fertility preservation: the challenge of freezing and transplanting ovarian tissue. Trends Mol. Med. 27, 777–791 (2021).
Zhang, S. et al. Chemotherapy impairs ovarian function through excessive ROS-induced ferroptosis. Cell Death Dis. 14, 340 (2023).
Yang, Y. et al. Metformin protects ovarian granulosa cells in chemotherapy-induced premature ovarian failure mice through AMPK/PPAR-gamma/SIRT1 pathway. Sci. Rep. 14, 1447 (2024).
Ai, G. et al. Adipose-derived stem cells promote the repair of chemotherapy-induced premature ovarian failure by inhibiting granulosa cells apoptosis and senescence. Stem Cell Res. Ther. 14, 75 (2023).
Liu, M. et al. MicroRNA-144-3p protects against chemotherapy-induced apoptosis of ovarian granulosa cells and activation of primordial follicles by targeting MAP3K9. Eur. J. Med. Res. 28, 264 (2023).
Chen, X. et al. Heat shock pretreatment of mesenchymal stem cells for inhibiting the apoptosis of ovarian granulosa cells enhanced the repair effect on chemotherapy-induced premature ovarian failure. Stem Cell Res. Ther. 9, 240 (2018).
Liang, F. G. et al. OPA1 promotes ferroptosis by augmenting mitochondrial ROS and suppressing an integrated stress response. Mol. Cell 84, 3098–3114.e3096 (2024).
Dixon, S. J. & Olzmann, J. A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 25, 424–442 (2024).
Gao, M. et al. Role of mitochondria in ferroptosis. Mol. Cell 73, 354–363.e353 (2019).
Zhong, S. et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis. Redox Biol. 63, 102760 (2023).
Zhao, Y. et al. Human umbilical cord mesenchymal stem cells restore the ovarian metabolome and rescue premature ovarian insufficiency in mice. Stem Cell Res. Ther. 11, 466 (2020).
Guo, L., Ou, X., Li, H. & Han, Z. Roles of sphingosine-1-phosphate in reproduction. Reprod. Sci. 21, 550–554 (2014).
Pascuali, N. et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum. Reprod. 33, 844–859 (2018).
Ishizuka, B. Current understanding of the etiology, symptomatology, and treatment options in premature ovarian insufficiency (POI). Front. Endocrinol. 12, 626924 (2021).
Blumenfeld, Z. Preservation of fertility and ovarian function and minimalization of chemotherapy associated gonadotoxicity and premature ovarian failure: the role of inhibin-A and -B as markers. Mol. Cell Endocrinol. 187, 93–105 (2002).
Federici, S. et al. Primary ovarian insufficiency: update on clinical and genetic findings. Front. Endocrinol. 15, 1464803 (2024).
Weng, L. et al. Sleep deprivation triggers the excessive activation of ovarian primordial follicles via beta2 adrenergic receptor signaling. Adv. Sci. 11, e2402393 (2024).
Yan, J. et al. Sleep deprivation causes gut dysbiosis impacting on systemic metabolomics leading to premature ovarian insufficiency in adolescent mice. Theranostics 14, 3760–3776 (2024).
Sang, D. et al. Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals. Cell 186, 5500–5516.e5521 (2023).
Wang, F. et al. BNC1 deficiency-triggered ferroptosis through the NF2-YAP pathway induces primary ovarian insufficiency. Nat. Commun. 13, 5871 (2022).
Kaplan, E. et al. ASCT1 (Slc1a4) transporter is a physiologic regulator of brain D-serine and neurodevelopment. Proc. Natl. Acad. Sci. USA 115, 9628–9633 (2018).
Tapanes, S. A. et al. Inhibition of glial D-serine release rescues synaptic damage after brain injury. Glia 70, 1133–1152 (2022).
Lin, L., Yee, S. W., Kim, R. B. & Giacomini, K. M. SLC transporters as therapeutic targets: emerging opportunities. Nat. Rev. Drug Discov. 14, 543–560 (2015).
Maugard, M., Vigneron, P. A., Bolanos, J. P. & Bonvento, G. l-Serine links metabolism with neurotransmission. Prog. Neurobiol. 197, 101896 (2021).
Mardinoglu, A. et al. Genome-scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non-alcoholic fatty liver disease. Nat. Commun. 5, 3083 (2014).
Padron-Barthe, L. et al. Activation of serine one-carbon metabolism by calcineurin Abeta1 reduces myocardial hypertrophy and improves ventricular function. J. Am. Coll. Cardiol. 71, 654–667 (2018).
Ma, H. et al. Astaxanthin from Haematococcus pluvialis ameliorates the chemotherapeutic drug (doxorubicin) induced liver injury through the Keap1/Nrf2/HO-1 pathway in mice. Food Funct. 11, 4659–4671 (2020).
Chen, H. et al. Renal UTX-PHGDH-serine axis regulates metabolic disorders in the kidney and liver. Nat. Commun. 13, 3835 (2022).
Le Douce, J. et al. Impairment of glycolysis-derived l-serine production in astrocytes contributes to cognitive deficits in Alzheimer’s disease. Cell Metab. 31, 503–517.e508 (2020).
Zheng, J. & Conrad, M. Ferroptosis: when metabolism meets cell death. Physiol. Rev. 105, 651–706 (2025).
Tong, H. et al. Dual impacts of serine/glycine-free diet in enhancing antitumor immunity and promoting evasion via PD-L1 lactylation. Cell Metab. 36, 2493–2510.e2499 (2024).
Geeraerts, S. L., Heylen, E., De Keersmaecker, K. & Kampen, K. R. The ins and outs of serine and glycine metabolism in cancer. Nat. Metab. 3, 131–141 (2021).
Yang, M. & Vousden, K. H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 (2016).
Lau, A., Blenis, J. & Burgos-Barragan, G. Decoding serine metabolism: unveiling novel pathways for evolving cancer therapies. Cancer Res. 84, 1191–1194 (2024).
Beroukhim, G., Esencan, E. & Seifer, D. B. Impact of sleep patterns upon female neuroendocrinology and reproductive outcomes: a comprehensive review. Reprod. Biol. Endocrinol. 20, 16 (2022).
Freidman, N. et al. Amino acid transporters and exchangers from the SLC1A family: structure, mechanism and roles in physiology and cancer. Neurochem Res. 45, 1268–1286 (2020).
Acknowledgements
We extend a special thanks to the Laboratory Animal Center of the Institute of Translational Medicine, Nanchang University. We especially thank Suzhou PANOMIX Biomedical Tech. Co. Ltd. for its contribution to amino acid-targeted metabolomic sequencing. We especially thank Novogene for its contribution to transcriptomic sequencing. This work was supported by the National Key Research and Development Program of China (2022YFA1104300 to Hongbo Xin and Keyu Deng), the National Natural Science Foundation of China (82470454 and 82270302 to Hongbo Xin, 81970256 to Keyu Deng and 82560089 to Lingfang Wang), the Natural Science Foundation of Jiangxi Province, China (2025BAC240701 to Lingfang Wang), and the Jiangxi Province Key Laboratory of Bioengineering Drugs (No. 2024SSY07061).
Author information
Authors and Affiliations
Contributions
H.B.X. and K.Y.D. led the project and contributed to the conception and design of the study, data analysis and interpretation, and manuscript revising. H.C.G. performed the most experiments and data analysis. Y.Q.Z., Y.W.Z., Y.K.W., H.T.L., J.Q.W., S.Q.T., and X.Y.W. performed animal models and cell experiments. D.S.L., L.Q.C., Z.H.L., and Y.Y.W. were responsible for clinical sample collection and patient testing result collection. Y.Q.Z. was responsible for clinical sample metabolome sequencing and result analysis. H.C.G. and L.F.W. wrote the manuscript draft. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, HC., Zhuo, YQ., Wang, LF. et al. Serine inhibits granulosa cell ferroptosis to maintain ovarian function. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68440-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68440-1