Abstract
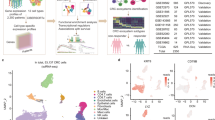
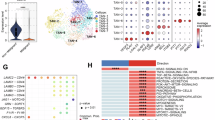
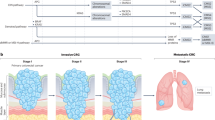
Colorectal cancer (CRC) remains refractory to most immunotherapies, with cancer vaccines failing due to an immunosuppressive tumor microenvironment. Here, we show that β-glucan–induced trained immunity overcomes these barriers by reprogramming macrophages through H3K4me3-dependent epigenetic modifications and metabolic rewiring. In female mice vaccinated with peptide-coated adenovirus-based vaccine PeptiCrad, training enhances glycolysis with creatine metabolism sustaining CXCL9/10 production, enabling macrophages to recruit NK cells via CXCR3. In turn, NK cells produce CCL5, driving cDC1 infiltration and antigen presentation, which together amplify effector memory CD8⁺ T cell responses. Moreover, with human peripheral blood mononuclear cells and CRC patient-derived organoids, trained macrophages boost NK migration, antigen-specific T cell activation, and tumor killing. These findings highlight trained immunity as a powerful adjuvant to reinvigorate colorectal cancer vaccination.
Similar content being viewed by others
Data availability
All scRNA sequencing data generated in this study have been deposited in the Genome Sequence Archive (GSA) under the accession number CRA024974 (https://ngdc.cncb.ac.cn/gsa/browse/CRA024974). The full dataset of the metabolomics data can be found in the Source Data (Supplementary Fig. 7A). The remaining data supporting the findings of this study are available within the Article or its Supplementary Information. Source data are provided with this paper.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021).
Morgan, E. et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72, 338–344 (2023).
Sung, H. et al. Colorectal cancer incidence trends in younger versus older adults: an analysis of population-based cancer registry data. Lancet Oncol. 26, 51–63 (2025).
Mariotto, A. B., Thompson, T. D., Johnson, C., Wu, X. C. & Pollack, L. A. Breast and colorectal cancer recurrence-free survival estimates in the US: modeling versus active data collection. Cancer Epidemiol. 85, 102370 (2023).
Yan, S. et al. Immune checkpoint inhibitors in colorectal cancer: limitation and challenges. Front Immunol. 15, 1403533 (2024).
Moreau, M. et al. A multicenter study evaluating efficacy of immune checkpoint inhibitors in advanced non-colorectal digestive cancers with microsatellite instability. Eur. J. Cancer 202, 959–8049 (2024).
André, T., Cohen, R. & Salem, M. E. Immune checkpoint blockade therapy in patients with colorectal cancer harboring microsatellite instability/mismatch repair deficiency in 2022. American Society of Clinical Oncology Educational Book 233–241 https://doi.org/10.1200/EDBK_349557 (2022).
Yamaguchi, K. et al. Efficacy of pembrolizumab in microsatellite-stable, tumor mutational burden-high metastatic colorectal cancer: genomic signatures and clinical outcomes. ESMO Open 10, 104108 (2025).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Le, D. T. et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409 (2017).
Hazama, S. et al. A phase ΙI study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study). J. Transl. Med. 12, 108 (2014).
Murahashi, M. et al. Phase I clinical trial of a five-peptide cancer vaccine combined with cyclophosphamide in advanced solid tumors. Clin. Immunol. 166–167, 48–58 (2016).
Harris, J. E. et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group study E5283. J. Clin. Oncol. 18, 148–157 (2000).
Toh, H. C. et al. Clinical benefit of allogeneic melanoma cell lysate-pulsed autologous dendritic cell vaccine in mage-positive colorectal cancer patients. Clin. Cancer Res. 15, 7726–7736 (2009).
Saito, T. et al. Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 22, 679–684 (2016).
Loddenkemper, C. et al. In situ analysis of FOXP3+ regulatory T cells in human colorectal cancer. J. Transl. Med. 4, 1–8 (2006).
Bonertz, A. et al. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J. Clin. Invest. 119, 3311 (2009).
Zhang, B. et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One 8, e57114 (2013).
Brandau, S., Moses, K. & Lang, S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: cousins, siblings or twins?. Semin Cancer Biol. 23, 171–182 (2013).
Wang, P. F. et al. Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: a meta-analysis of 40 studies. Oncoimmunology 7, e1494113 (2018).
Li, J. et al. Tumor-associated macrophage infiltration and prognosis in colorectal cancer: systematic review and meta-analysis. Int J. Colorectal Dis. 35, 1203–1210 (2020).
Zhou, Q. et al. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J. Transl. Med. 8, 13 (2010).
Forssell, J. et al. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin. Cancer Res. 13, 1472–1479 (2007).
Koelzer, V. H. et al. Phenotyping of tumor-associated macrophages in colorectal cancer: Impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology 5, e1106677 (2015).
Zhu, Q., Han, X., Peng, J., Qin, H. & Wang, Y. The role of CXC chemokines and their receptors in the progression and treatment of tumors. J. Mol. Histol. 43, 699–713 (2012).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234 (2018).
Zhang, Y., Rajput, A., Jin, N. & Wang, J. Mechanisms of immunosuppression in colorectal cancer. Cancers (Basel) 12, 3850 (2020).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020). 2020 20:6.
Ciarlo, E. et al. Trained immunity confers broad-spectrum protection against bacterial infections. J. Infect. Dis. 222, 1869 (2019).
Moorlag, S. J. C. F. M. et al. β-Glucan induces protective trained immunity against Mycobacterium tuberculosis infection: a key role for IL-1. Cell Rep. 31, 107634 (2020).
Ciarlo, E. et al. Trained immunity confers broad-spectrum protection against bacterial infections. J. Infect. Dis. 222, 1869–1881 (2020).
Khan, N. et al. β-Glucan reprograms neutrophils to promote disease tolerance against influenza A virus. Nat. Immunol. 26, 174–187 (2025).
Starr, S. E., Visintine, A. M., Tomeh, M. O. & Nahmias, A. J. Effects of immunostimulants on resistance of newborn mice to herpes simplex type 2 infection. Proc. Soc. Exp. Biol. Med. 152, 57–60 (1976).
Suenaga, T., Okuyama, T., Yoshida, I. & Azuma, M. Effect of Mycobacterium tuberculosis BCG infection on the resistance of mice to ectromelia virus infection: participation of interferon in enhanced resistance. Infect. Immun. 20, 312–314 (1978).
Spencer, J. C., Ganguly, R. & Waldman, R. H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with bacille calmette-guerin. J. Infect. Dis. 136, 171–175 (1977).
Floc’h, F. & Werner, G. H. Increased resistance to virus infections of mice inoculated with BCG (Bacillus calmette-guérin). Ann. Immunol. (Paris) 127, 173–186 (1976).
Masuda, Y., Yamashita, S., Nakayama, Y., Shimizu, R. & Konishi, M. Maitake beta-glucan enhances the therapeutic effect of trastuzumab via antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Biol. Pharm. Bull. 47, 840–847 (2024).
Kalafati, L. et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 183, 771–785.e12 (2020).
Wattenberg, M. M. et al. Cancer immunotherapy via synergistic coactivation of myeloid receptors CD40 and Dectin-1. Sci. Immunol. 8, eadj5097 (2023).
Geller, A. E. et al. The induction of peripheral trained immunity in the pancreas incites anti-tumor activity to control pancreatic cancer progression. Nat. Commun. 13, 1–20 (2022).
Feola, S. et al. Peptides-Coated Oncolytic Vaccines for Cancer Personalized Medicine. Front Immunol. 13, 826164 (2022).
Capasso, C. et al. Oncolytic adenoviruses coated with MHC-I tumor epitopes increase the antitumor immunity and efficacy against melanoma. Oncoimmunology 5, e1105429 (2015).
Lee, H. G., Cho, M. Z. & Choi, J. M. Bystander CD4+ T cells: crossroads between innate and adaptive immunity. Exp. Mol. Med. 52, 1255–1263 (2020).
Feola, S. et al. A novel immunopeptidomic-based pipeline for the generation of personalized oncolytic cancer vaccines. Elife 11, e71156 (2022).
Dufva, O. et al. Single-cell functional genomics reveals determinants of sensitivity and resistance to natural killer cells in blood cancers. Immunity 56, 2816–2835.e13 (2023).
Barry, K. C. et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med 24, 1178–1191 (2018).
Böttcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14 (2018).
Ray, A. et al. Targeting CD206+ macrophages disrupts the establishment of a key antitumor immune axis. J. Exp. Med. 222, e20240957 (2025).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 938 (2014).
Doedens, A. L. et al. Macrophage expression of hypoxia-inducible factor-1α suppresses T-cell function and promotes tumor progression. Cancer Res. 70, 7465–7475 (2010).
Mitchem, J. B. et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 73, 1128–1141 (2013).
Nixon, B. G. et al. Tumor-associated macrophages expressing the transcription factor IRF8 promote T cell exhaustion in cancer. Immunity 55, 2044–2058.e5 (2022).
Park, M. D. et al. TREM2 macrophages drive NK cell paucity and dysfunction in lung cancer. Nat. Immunol. 24, 792–801 (2023).
Peranzoni, E. et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti–PD-1 treatment. Proc. Natl. Acad. Sci. USA 115, E4041–E4050 (2018).
Ruffell, B. et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26, 623–637 (2014).
Subtil, B., Cambi, A., Tauriello, D. V. F. & de Vries, I. J. M. The therapeutic potential of tackling tumor-induced dendritic cell dysfunction in colorectal cancer. Front Immunol. 12, 724883 (2021).
Zhu, B. et al. Plasticity of Ly-6Chi myeloid cells in T cell regulation. J. Immunol. 187, 2418 (2011).
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl. Acad. Sci. USA 109, E3186–E3195 (2012).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Ferreira, A. V., Domínguez-Andrés, J., Merlo Pich, L. M., Joosten, L. A. B. & Netea, M. G. Metabolic regulation in the induction of trained immunity. Semin Immunopathol. 46, 7 (2024).
Peng, Z. & Saito, S. Creatine supplementation enhances anti-tumor immunity by promoting adenosine triphosphate production in macrophages. Front Immunol. 14, 1176956 (2023).
Bhagat, A., Lyerly, H. K., Morse, M. A. & Hartman, Z. C. CEA vaccines. Hum. Vaccin Immunother. 19, 2291857 (2023).
Evers, M. et al. Novel chimerized IgA CD20 antibodies: Improving neutrophil activation against CD20-positive malignancies. MAbs 12, 1795505 (2020).
Acknowledgements
The flow cytometry analysis was performed at the HiLife Flow Cytometry Unit, University of Helsinki. We acknowledge the Helsinki Metabolomics Center, supported by HiIFE and Biocenter Finland. The single-cell RNA sequencing was performed with FIMM Single-Cell Analytics and Sequencing units supported by HiLIFE and Biocenter Finland. We are also grateful for Dr. Cristian Smerdou (Cima Universidad de Navarra) for kindly gifting us the MC38 cell line. We would also like to acknowledge bachelor student, Karim Hamdan, for helping in some experiments. This work has been supported by European Research Council (ERC), Horizon 2020 (H2020) framework (Agreement No. 681219) (V.C.), Magnus Ehrnrooth Foundation (project No. 4706235) (V.C.), Jane and Aatos Erkko Foundation (Project No. 4705796) (V.C.), Finnish Cancer Foundation (project No. 4706116) (V.C.), Helsinki Institute of Life Science (HiLIFE) (project No. 797011004) (V.C.), Digital Precision Cancer Medicine Flagship iCAN (V.C.), GeneCellNano flagship (V.C.), HiLIFE HiPOC (FH), Research Council of Finland (TTS) and iCAN Digital Precision Cancer Medicine Flagship (TTS), and research grants by Jane and Aatos Erkko Foundation (TTS), Sigrid Juselius Foundation (TTS), Mary and Georg Ehrnrooth Foundation (TTS), Cancer Foundation Finland (TTS), Relander Foundation (TTS), and HUS(TTS) and Pirha state research funding (TTS).
Author information
Authors and Affiliations
Contributions
S.G. and F.D have contributed equally to this work. Specific contributions: Conceptualization: F.H, S.G and V.C. Investigation: F.H, S.G, F.D, Y.G, J.K, M.Fu, E.Z, S.R, O.I, P.B, J.C, G.A, M.Fe, V.S, M.S, J.S, A.N, O.E, KM.A. Data Curation: F.H., S.G., A.I.N., and F.D. Formal analysis. F.H., S.G., A.I.N., F.D and V.C. Visualization: F.H. Project administration: F.H. and V.C. Writing original draft: F.H., S.G., M.G. and V.C. Writing-Review editing: All authors. Funding acquisition: N.Z, O.E, S.M, T.TS, M.G and V.C
Corresponding author
Ethics declarations
Competing interests
V.C. is co-founder and shareholder of Valo Therapeutics LTD. H.C. and L.K. are stakeholders of Valo Therapeutics LTD. Toni T. Seppälä reports consultation fees from Mehiläinen, Nouscom, Orion Pharma, Amgen, and Tillots Pharma, and a position in the Clinical Advisory Board and as a minor shareholder of Lynsight Ltd. S.M. has received honoraria and research funding from Novartis, Pfizer, and Bristol-Myers Squibb and honoraria from Dren-Bio (not related to this study). V.S. is an employee and shareholder of AstraZeneca. All other named authors declare that they have no competing interests, financial or otherwise.
Peer review
Peer review information
Nature Communications thanks Xiaojun Xia, Jun Yan, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hamdan, F., Gandolfi, S., D’Alessio, F. et al. Leveraging glucan-induced trained immunity for the epigenetic and metabolic rewiring of macrophages to enhance colorectal cancer vaccine response. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68466-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68466-5