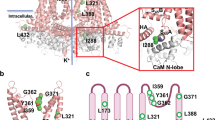
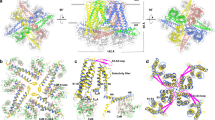
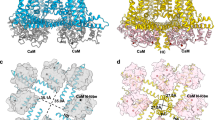
Abstract
The small-conductance calcium-activated potassium (SK1-3 or KCa2) channels regulate the intrinsic excitability and firing frequency of excitable cells. SK channels are modulated by a variety of distinct modulators; however, the underlying mechanisms remain elusive. Here, we present four cryoelectron microscopy structures of the human SK2-calmodulin complex bound with apamin, UCL1684, AP30663, and CAD-1883, elucidating their distinct binding sites and regulatory mechanisms. Apamin and UCL1684 compete for a similar binding site above the selectivity filter, which is formed by the distinct S3-S4 linker of SK2. CAD-1883 glues the N-lobe of calmodulin and the S4-S5 linker of SK2, reinforcing the open state. In contrast, AP30663 resides in the central cavity of SK2, blocking ion conductance. This study reveals multiple modulation sites in SK2 and the molecular mechanisms for the inhibition and potentiation of SK channels, which could advance rational drug design targeting SK2 channel for the treatment of cardiovascular and neurological disorders.
Similar content being viewed by others
Data availability
The cryo-EM density maps of human SK2-apamin, SK2-UCL1684, SK2-CAD-1883, and SK2-AP30663 have been deposited into the Electron Microscopy Data Bank (EMDB) under accession codes EMD-65357, EMD-65359, EMD-65358, and EMD-65356, respectively. The coordinates of human SK2-apamin, SK2-UCL1684, SK2-CAD, and SK2-AP have been deposited into the Protein Data Bank (PDB) under accession codes 9VUA, 9VUC, 9VUB, and 9VU9, respectively. Previously published data for the structures used in this study are available with PDB accession codes: SK4, 6CNO, 6CNM, and 6CNN; apamin, 7OXF; Shaker Kv channel, 7SJ1; rat SK2, 8V2H and 8V3G; human SK2-4 chimera, 9O52. The source data underlying Figs. 2–4 and Supplementary Figs. 1, 7, 9, and 10 are provided as a Source Data file. Source data are provided with this paper.
References
Adelman, J. P., Maylie, J. & Sah, P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev. Physiol. 74, 245–269 (2012).
Bond, C. T. et al. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J. Neurosci. 24, 5301–5306 (2004).
Stocker, M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat. Rev. Neurosci. 5, 758–770 (2004).
Zhang, W. H. et al. Chronic stress causes projection-specific adaptation of amygdala neurons via small-conductance calcium-activated potassium channel downregulation. Biol. Psychiatry 85, 812–828 (2019).
Wolfart, J., Neuhoff, H., Franz, O. & Roeper, J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J. Neurosci. 21, 3443–3456 (2001).
Stackman, R. W. et al. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J. Neurosci. 22, 10163–10171 (2002).
Hammond, R. S. et al. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J. Neurosci. 26, 1844–1853 (2006).
Kohler, M. et al. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273, 1709–1714 (1996).
Stocker, M. & Pedarzani, P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol. Cell Neurosci. 15, 476–493 (2000).
Ishii, T. M. et al. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. USA 94, 11651–11656 (1997).
Joiner, W. J., Wang, L. Y., Tang, M. D. & Kaczmarek, L. K. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc. Natl. Acad. Sci. USA 94, 11013–11018 (1997).
Sailer, C. A. et al. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J. Neurosci. 22, 9698–9707 (2002).
Xia, X. M. et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395, 503–507 (1998).
Fanger, C. M. et al. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J. Biol. Chem. 274, 5746–5754 (1999).
Lee, C. H. & MacKinnon, R. Activation mechanism of a human SK-calmodulin channel complex elucidated by cryo-EM structures. Science 360, 508–513 (2018).
Nam, Y. W. et al. Cryo-EM structures of the small-conductance Ca(2+)-activated K(Ca)2.2 channel. Nat. Commun. 16, 3690 (2025).
Cassell Samantha, J., Li, W. eiyan, Krautwald, S. imon, Khoshouei, M. aryam, Lee Yan, T. ony, Hou, J. oyce, Guan, W. endy, Peukert, S. tefan, Weihofen Wilhelm, A. & Whicher Jonathan, R. Mechanism of SK2 channel gating and its modulation by the bee toxin apamin and small molecules. eLife 14, RP107733 (2025).
Hugues, M., Romey, G., Duval, D., Vincent, J. P. & Lazdunski, M. Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: voltage-clamp and biochemical characterization of the toxin receptor. Proc. Natl. Acad. Sci. USA 79, 1308–1312 (1982).
Messier, C. et al. Effect of apamin, a toxin that inhibits Ca(2+)-dependent K+ channels, on learning and memory processes. Brain Res. 551, 322–326 (1991).
Burgess, G. M., Claret, M. & Jenkinson, D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J. Physiol. 317, 67–90 (1981).
Grunnet, M., Jensen, B. S., Olesen, S. P. & Klaerke, D. A. Apamin interacts with all subtypes of cloned small-conductance Ca2+-activated K+ channels. Pflug. Arch. 441, 544–550 (2001).
Strobaek, D., Jorgensen, T. D., Christophersen, P., Ahring, P. K. & Olesen, S. P. Pharmacological characterization of small-conductance Ca(2+)-activated K(+) channels stably expressed in HEK 293 cells. Br. J. Pharm. 129, 991–999 (2000).
Ishii, T. M., Maylie, J. & Adelman, J. P. Determinants of apamin and d-tubocurarine block in SK potassium channels. J. Biol. Chem. 272, 23195–23200 (1997).
Nolting, A., Ferraro, T., D’Hoedt, D. & Stocker, M. An amino acid outside the pore region influences apamin sensitivity in small conductance Ca2+-activated K+ channels. J. Biol. Chem. 282, 3478–3486 (2007).
Lamy, C. et al. Allosteric block of KCa2 channels by apamin. J. Biol. Chem. 285, 27067–27077 (2010).
Weatherall, K. L., Seutin, V., Liégeois, J. F. & Marrion, N. V. Crucial role of a shared extracellular loop in apamin sensitivity and maintenance of pore shape of small-conductance calcium-activated potassium (SK) channels. Proc. Natl. Acad. Sci. USA 108, 18494–18499 (2011).
Finlayson, K. et al. Characterisation of [(125)I]-apamin binding sites in rat brain membranes with HE293 cells transfected with SK channel subtypes. Neuropharmacology 41, 341–350 (2001).
Castle, N. A., Haylett, D. G., Morgan, J. M. & Jenkinson, D. H. Dequalinium: a potent inhibitor of apamin-sensitive K+ channels in hepatocytes and of nicotinic responses in skeletal muscle. Eur. J. Pharm. 236, 201–207 (1993).
Campos Rosa, J. et al. Synthesis, molecular modeling, and pharmacological testing of bis-quinolinium cyclophanes: potent, non-peptidic blockers of the apamin-sensitive Ca(2+)-activated K(+) channel. J. Med. Chem. 43, 420–431 (2000).
Chen, J. Q., Galanakis, D., Ganellin, C. R., Dunn, P. M. & Jenkinson, D. H. bis-Quinolinium cyclophanes: 8,14-diaza-1,7(1, 4)-diquinolinacyclotetradecaphane (UCL 1848), a highly potent and selective, nonpeptidic blocker of the apamin-sensitive Ca(2+)-activated K(+) channel. J. Med. Chem. 43, 3478–3481 (2000).
Wulff, H., Kolski-Andreaco, A., Sankaranarayanan, A., Sabatier, J. M. & Shakkottai, V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr. Med. Chem. 14, 1437–1457 (2007).
Brown, B. M., Shim, H., Christophersen, P. & Wulff, H. Pharmacology of small- and intermediate-conductance calcium-activated potassium channels. Annu Rev. Pharm. Toxicol. 60, 219–240 (2020).
Bentzen, B. H. et al. Mechanisms of action of the KCa2-negative modulator AP30663, a novel compound in development for treatment of atrial fibrillation in man. Front. Pharm. 11, 610 (2020).
Gal, P. et al. First clinical study with AP30663 - a K(Ca) 2 channel inhibitor in development for conversion of atrial fibrillation. Clin. Transl. Sci. 13, 1336–1344 (2020).
Holst, A. G. et al. Inhibition of the K(Ca)2 potassium channel in atrial fibrillation: a randomized phase 2 trial. Nat. Med. 30, 106–111 (2024).
Yfanti, C. et al. A phase 1 trial of AP30663, a K(Ca)2 channel inhibitor in development for conversion of atrial fibrillation. Br. J. Clin. Pharm. 90, 1027–1035 (2024).
Cho, L. T. et al. An intracellular allosteric modulator binding pocket in sk2 ion channels is shared by multiple chemotypes. Structure 26, 533–544.e533 (2018).
Zhang, M., Pascal, J. M., Schumann, M., Armen, R. S. & Zhang, J. F. Identification of the functional binding pocket for compounds targeting small-conductance Ca(2)(+)-activated potassium channels. Nat. Commun. 3, 1021 (2012).
Zhang, M., Pascal, J. M. & Zhang, J. F. Unstructured to structured transition of an intrinsically disordered protein peptide in coupling Ca(2)(+)-sensing and SK channel activation. Proc. Natl. Acad. Sci. USA 110, 4828–4833 (2013).
Wemmer, D. & Kallenbach, N. R. Structure of apamin in solution: a two-dimensional nuclear magnetic resonance study. Biochemistry 22, 1901–1906 (1983).
Le-Nguyen, D. et al. Role of Asn(2) and Glu(7) residues in the oxidative folding and on the conformation of the N-terminal loop of apamin. Biopolymers 86, 447–462 (2007).
Kuzmenkov, A. I. et al. Apamin structure and pharmacology revisited. Front Pharm. 13, 977440 (2022).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Labbe-Jullie, C. et al. Binding and toxicity of apamin. Characterization of the active site. Eur. J. Biochem 196, 639–645 (1991).
Bagneris, C. et al. Prokaryotic NavMs channel as a structural and functional model for eukaryotic sodium channel antagonism. Proc. Natl. Acad. Sci. USA 111, 8428–8433 (2014).
Simó-Vicens, R. et al. A new negative allosteric modulator, AP14145, for the study of small conductance calcium-activated potassium (K(Ca) 2) channels. Br. J. Pharm. 174, 4396–4408 (2017).
Tan, X. F. et al. Structure of the Shaker Kv channel and mechanism of slow C-type inactivation. Sci. Adv. 8, eabm7814 (2022).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Acknowledgments
We thank Dr. B. Xu at the Cryo-EM Center of the School of Advanced Agricultural Sciences of Peking University and D. Sun at the Cryo-EM Facility at the Institute of Physics, Chinese Academy of Science & Beijing Branch of Songshan Lake Materials Laboratory for support in cryo-EM data collection. We thank Dr. Lu Ma and Wei Fan for their research assistance service. This work is funded by the National Natural Science Foundation of China (32222034 and U25A2080 to W.Z.; 32271272 and T2221001 to D.J.; 82430048 and 82125010 to B.P.), the Natural Science Foundation of Jiangxi Province (20252BAC230007 to W.Z.; 20242BAB24003 to B.P.), and the Taishan Scholars Program (tsqnz20231243 to C.C.). This work is also supported by the Medical-Engineering Interdisciplinary Talent Development Program and the Fund of the School of Basic Medical Sciences, Jiangxi Medical College, Nanchang University.
Author information
Authors and Affiliations
Contributions
D.J., W.Z., and B.P. conceived and designed the experiments. B.M. prepared samples for the cryo-EM study and made all the constructs. D.W., B.M., Z.X., and C.C. collected the cryo-EM data. D.W. and D.J. processed the data and built and refined the models. E.C. and Z.W. performed the electrophysiology experiments. B.M. and D.W. prepared the figures. B.M., D.W., E.C., C.C., L.S., B.P., W.Z., and D.J. analysed and interpreted the results. D.J. and D.W. wrote the paper, and all the authors reviewed and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Wei Lü, who co-reviewed with Sushant Kumar, Heike Wulff and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, B., Wu, D., Cao, E. et al. Structural mechanisms for inhibition and activation of human small-conductance Ca2+-activated potassium channel SK2. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68475-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68475-4