Abstract
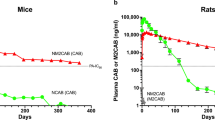
Ultra-long-acting (ULA) antiretroviral parenteral formulations, with low injection volumes, high resistance barriers, and short pharmacokinetic (PK) tails, can transform HIV-1 therapeutics. Here, we converted bictegravir (BIC), a potent daily oral antiretroviral drug, into monomeric and homodimeric ester prodrugs. The homodimeric prodrug nanosuspension, NMXBIC, shows sustained plasma BIC levels > 16 times the protein-adjusted 95% inhibitory concentration (PA-IC95) for six months after a single injection in Sprague Dawley rats. The results paralleled a short PK tail with the potential for late dose forgiveness. The monomeric prodrug nanosuspension, NM2BIC, shows lower year-long plasma BIC concentrations above PA-IC95 after a single injection. After repeated injections, NMXBIC and NM2BIC are well tolerated in New Zealand White rabbits. NMXBIC’s physicochemical properties and high BIC loading/unit mass of the prodrug contribute to its unique ULA PK profile. These results support its development as a ULA formulation for HIV-1 treatment and prevention.
Similar content being viewed by others
Data availability
Source data are provided in the Figshare database under Digital Object Identifier. https://doi.org/10.6084/m9.figshare.30879314 (Fig. 1); https://doi.org/10.6084/m9.figshare.30879389 (Fig. 2); https://doi.org/10.6084/m9.figshare.30879395 (Fig. 3); https://doi.org/10.6084/m9.figshare.30879398 (Fig. 4); https://doi.org/10.6084/m9.figshare.30879437 (Fig. 5); https://doi.org/10.6084/m9.figshare.30881102 (Supplementary Materials). Source data are provided with this paper.
References
Iacob, S. A., Iacob, D. G. & Jugulete, G. Improving the adherence to antiretroviral therapy, a difficult but essential task for a successful HIV treatment-clinical points of view and practical considerations. Front. Pharmacol. 8, 831 (2017).
Foka, F. E. T. & Mufhandu, H. T. Current ARTs, virologic failure, and implications for AIDS management: a systematic review. Viruses 15, 1732 (2023).
Cobb, D. A. et al. Long-acting approaches for delivery of antiretroviral drugs for prevention and treatment of HIV: a review of recent research. Expert Opin. Drug Deliv. 17, 1227–1238 (2020).
Swindells, S. et al. Week 96 extension results of a phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment. AIDS 36, 185 (2022).
Orkin, C. et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N. Engl. J. Med. 382, 1124–1135 (2020).
Smith, G. H. et al. Efficacy, safety, and durability of long-acting cabotegravir and rilpivirine in adults with human immunodeficiency virus type 1 infection: 5-year results from the LATTE-2 study. Open Forum Infect. Dis. 8, ofab439 (2021).
Margolis, D. A. et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 390, 1499–1510 (2017).
Cooper, S. E., Rosenblatt, J. & Gulick, R. M. Barriers to uptake of long-acting antiretroviral products for treatment and prevention of human immunodeficiency virus (HIV) in high-income countries. Clin. Infect. Dis. 75, S541–S548 (2022).
Landovitz, R. J. et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV 7, e472–e481 (2020).
Hodge, D. et al. Pharmacokinetics and drug-drug interactions of long-acting intramuscular cabotegravir and rilpivirine. Clin. Pharmacokinet. 60, 835–853 (2021).
Parikh, U. M., Koss, C. A. & Mellors, J. W. Long-acting injectable cabotegravir for HIV prevention: What do we know and need to know about the risks and consequences of cabotegravir resistance?. Curr. HIV/AIDS Rep. 19, 384–393 (2022).
Oliveira, M. et al. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology 15, 1–14 (2018).
Segal-Maurer, S. et al. Capsid inhibition with lenacapavir in multidrug-resistant HIV-1 infection. N. Engl. J. Med. 386, 1793–1803 (2022).
Gupta, S. K. et al. Lenacapavir administered every 26 weeks or daily in combination with oral daily antiretroviral therapy for initial treatment of HIV: a randomised, open-label, active-controlled, phase 2 trial. Lancet HIV 10, e15–e23 (2023).
Tsiang, M. et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob. Agents Chemother. 60, 7086–7097 (2016).
Young, I. C. et al. Ultra-long-acting in-situ forming implants with cabotegravir protect female macaques against rectal SHIV infection. Nat. Commun. 14, 708 (2023).
Matthews, R. P. et al. Safety and pharmacokinetics of islatravir subdermal implant for HIV-1 pre-exposure prophylaxis: a randomized, placebo-controlled phase 1 trial. Nat. Med. 27, 1712–1717 (2021).
Gunawardana, M. et al. Multispecies Evaluation of a Long-Acting Tenofovir Alafenamide Subdermal Implant for HIV Prophylaxis. Front. Pharmacol. 11, 569373 (2020).
Massud, I. et al. Safety and efficacy of a biodegradable implant releasing tenofovir alafenamide for vaginal protection in a macaque model. J. Antimicrob. Chemother. 77, 2964–2971 (2022).
Karunakaran, D. et al. Design and Testing of a Cabotegravir Implant for HIV Prevention. J. Control. Release 330, 658–668 (2021).
Johnson, L. M. et al. Characterization of a reservoir-style implant for sustained release of tenofovir alafenamide (TAF) for HIV pre-exposure prophylaxis (PrEP). Pharmaceutics 11, 315 (2019).
Su, J. T. et al. A subcutaneous implant of tenofovir alafenamide fumarate causes local inflammation and tissue necrosis in rabbits and macaques. Antimicrob. Agents Chemother. 64, https://doi.org/10.1128/aac.01893-19 (2020).
Thakur, R. R. S., McMillan, H. L. & Jones, D. S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 176, 8–23 (2014).
Romano J. W. et al. Tenofovir Alafenamide for HIV Prevention: Review of the Proceedings from the Gates Foundation Long-Acting TAF Product Development Meeting. AIDS Res Hum Retroviruses. 37, 409–420 (2021).
Zhou, T. et al. Optimizing the preparation and stability of decorated antiretroviral drug nanocrystals. Nanomedicine 13, 871–885 (2018).
Deodhar, S. et al. Transformation of dolutegravir into an ultra-long-acting parenteral prodrug formulation. Nat. Commun. 13, 3226 (2022).
Kulkarni, T. A. et al. A year-long extended release nanoformulated cabotegravir prodrug. Nat. Mater. 19, 910–920 (2020).
Turner, P. V. et al. Administration of substances to laboratory animals: routes of administration and factors to consider. J. Am. Assoc. Lab Anim. Sci. 50, 600–613 (2011).
Darville, N. et al. Intramuscular administration of paliperidone palmitate extended-release injectable microsuspension induces a subclinical inflammatory reaction modulating the pharmacokinetics in rats. J. Pharm. Sci. 103, 2072–2087 (2014).
Darville, N. et al. The effect of macrophage and angiogenesis inhibition on the drug release and absorption from an intramuscular sustained-release paliperidone palmitate suspension. J. Control. Release 230, 95–108 (2016).
Gautam, N. et al. Lipophilic nanocrystal prodrug-release defines the extended pharmacokinetic profiles of a year-long cabotegravir. Nat. Commun. 12, 3453 (2021).
Darville, N. et al. Modeling the Time Course of the Tissue Responses to Intramuscular Long-acting Paliperidone Palmitate Nano-/Microcrystals and Polystyrene Microspheres in the Rat. Toxicol Pathol. 44, 189–210 (2016).
Gallant, J. E. et al. Antiviral activity, safety, and pharmacokinetics of bictegravir as 10-day monotherapy in HIV-1-infected adults. J. Acquir. Immune Defic. Syndr. 75, 61–66 (2017).
Yamaguchi, K. & Anderson, J. M. Biocompatibility studies of naltrexone sustained release formulations. J. Control. release 19, 299–314 (1992).
Anderson, J. M. Biological responses to materials. Annu. Rev. Mater. Res. 31, 81–110 (2001).
Anderson, J. M., Rodriguez, A. & Chang, D. T. Foreign body reaction to biomaterials. in Semin. Immunol. 20, 86–100 (2008).
Grundy, S. M. Cholesterol: factors determining blood levels. in Encyclopedia of Human Nutrition 3rd edn (ed. Caballero, B.) 335–340 (Academic Press, 2013).
Senyilmaz-Tiebe, D. et al. Dietary stearic acid regulates mitochondria in vivo in humans. Nat. Commun. 9, 3129 (2018).
Trezza, C. et al. Formulation and pharmacology of long-acting cabotegravir. Curr. Opin. HIV AIDS 10, 239–245 (2015).
Scarsi, K. K. & Swindells, S. The promise of improved adherence with long-acting antiretroviral therapy: What are the data?. J. Int Assoc. Provid AIDS Care 20, 23259582211009011 (2021).
Smith, S. J. et al. Efficacies of Cabotegravir and Bictegravir against drug-resistant HIV-1 integrase mutants. Retrovirology 15, 37 (2018).
Doan, J. et al. Expanding therapeutic options: lenacapavir + bictegravir as a potential treatment for HIV. Expert Opin. Pharmacother. 24, 1949–1956 (2023).
Study to compare bictegravir/lenacapavir versus current therapy in people with HIV-1 who are successfully treated with a complicated Regimen Clinicaltrials.gov: NIH National Library of Medicine. [cited 2023 Oct 9] (2022).
Zhou, T. et al. Creation of a nanoformulated cabotegravir prodrug with improved antiretroviral profiles. Biomaterials 151, 53–65 (2018).
Sillman, B. et al. Creation of a long-acting nanoformulated dolutegravir. Nat. Commun. 9, 443 (2018).
Cobb, D. A. et al. Transformation of tenofovir into stable ProTide nanocrystals with long-acting pharmacokinetic profiles. Nat. Commun. 12, 5458 (2021).
Acknowledgements
The authors thank the University of Nebraska Medical Center (UNMC) cores for NMR (Ed Ezell), Elutriation and Cell Separation (Myhanh Che, Na Ly, and Li Wu), Electron Microscopy, Histology (Tissue Sciences Facility), MALDI/TOF Mass Spectrometry, as well as Comparative Medicine for technical assistance and animal care. The authors also wish to thank Gilead Sciences Inc. for designing and funding the injection site tolerability study in NZW rabbits performed at Labcorp Early Development Laboratories Inc. This research is supported by the University of Nebraska Foundation, which includes donations from the Carol Swarts, M.D. Emerging Neuroscience Research Laboratory, the Margaret R. Larson Professorship, the Frances and Louie Blumkin Endowment, the Community Pride of Nebraska Professorship, the Sylvia L. Havlik Centennial Professorship of Oncology, and the Harriet Singer Endowment; the Vice Chancellor’s Office of the University of Nebraska Medical Center for Core Facilities; Nickolus Badami Fellowship, The Nebraska Neuroscience Alliance Award, and the National Institutes of Health grants R01AI145542, R01AI158160, and R01AI163042.
Author information
Authors and Affiliations
Contributions
M.N.—study design, synthesized the prodrugs and formulations, design and execution of experiments, data acquisition, data analysis and interpretation, co-wrote manuscript; S.A. and A.K.D.—assisted with solid state data acquisition; C.Z., P.K.D., B.G., B.S., S. Das, and N.T.H.L.—assisted with animal PK and efficacy study data acquisition; B.H., A.S., and S. Deodhar—assisted with LC-MS/MS sample analyses; S.M.C.—acquisition and interpretation of toxicology data sets; H.E.G.—design of experiments; data interpretation, supervision of experiments, co-wrote the manuscript, and funding acquisition; B.E.—conceived project, study design, supervision of experiments, data analysis and interpretation, co-wrote the manuscript and funding acquisition. All authors critically evaluated the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
B.E. and H.E.G. are cofounders of Exavir Therapeutics, Inc. and are inventors on patents that cover ULA BIC formulations. The authors declare that this work was produced solely by the authors and that no other individuals or entities influenced any aspects of the work, including, but not limited to, the study conception and design, data acquisition, analyses, and interpretation, and writing of the manuscript. The authors further declare that they have received no financial compensation from any other third parties for any aspects of the published work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Daniel Reker and Vicente Soriano for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nayan, M.U., Sillman, B., Das, S. et al. An ultra-long-acting dimeric bictegravir prodrug defined by a short pharmacokinetic tail. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68501-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68501-5