Abstract
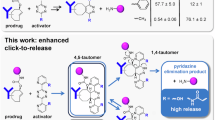
The advancement of bioorthogonal bond-breaking chemistry requires precise spatiotemporal control over chemical reactions. In this study, we introduce a click-release strategy based on the reaction between mono-alkyl-hydroxylamine and cyclooctyne (COT), enabling rapid and nearly complete payload release. We demonstrate that mono-alkyl-hydroxylamine and hydroxylamine react with COT to form nitrone and oxime, respectively, facilitating a versatile and efficient release mechanism. By conjugating mono-alkyl-hydroxylamine with responsive cleavable groups, we convert its inherent reactivity into an on-demand activation system. In vivo, this strategy is applied in a 4T1 mouse breast tumor model, showing significant tumor inhibition compared to the parent anticancer drug. Additionally, in local anesthesia, the anesthetic effect could be modulated by light exposure, allowing for repeated activation. This click-release system combines fast kinetics, high release efficiency, and precise spatiotemporal control, offering promising applications in chemical biology and drug delivery.
Similar content being viewed by others
Data availability
All data supporting the findings of this manuscript, including full characterization data for new compounds and detailed experimental procedures, are provided within the main text and Supplementary Information, and are available from the corresponding authors upon request. Source data are provided with this paper.
References
Prescher, J. A., Dube, D. H. & Bertozzi, C. R. Chemical remodelling of cell surfaces in living animals. Nature 430, 873–877 (2004).
Sletten, E. M. & Bertozzi, C. R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 48, 6974–6998 (2009).
Sletten, E. M. & Bertozzi, C. R. From mechanism to mouse: a tale of two bioorthogonal reactions. Acc. Chem. Res. 44, 666–676 (2011).
Takayama, Y., Kusamori, K. & Nishikawa, M. Click chemistry as a tool for cell engineering and drug delivery. Molecules 24, 172 (2019).
Parker, C. G. & Pratt, M. R. Click chemistry in proteomic investigations. Cell 180, 605–632 (2020).
Scinto, S. L. et al. Bioorthogonal chemistry. Nat. Rev. Methods Primers 1, 30 (2021).
Flores, J., White, B. M., Brea, R. J., Baskin, J. M. & Devaraj, N. K. Lipids: chemical tools for their synthesis, modification, and analysis. Chem. Soc. Rev. 49, 4602–4614 (2020).
George, J. T. & Srivatsan, S. G. Bioorthogonal chemistry-based RNA labeling technologies: evolution and current state. Chem. Commun. 56, 12307–12318 (2020).
Porte, K., Riomet, M., Figliola, C., Audisio, D. & Taran, F. Click and bio-orthogonal reactions with mesoionic compounds. Chem. Rev. 121, 6718–6743 (2020).
Li, J. & Chen, P. R. Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat. Chem. Biol. 12, 129–137 (2016).
Ji, X. et al. Click and release: bioorthogonal approaches to “on-demand” activation of prodrugs. Chem. Soc. Rev. 48, 1077–1094 (2019).
Deb, T., Tu, J. & Franzini, R. M. Mechanisms and substituent effects of metal-free bioorthogonal reactions. Chem. Rev. 121, 6850–6914 (2021).
Wang, J., Wang, X., Fan, X. & Chen, P. R. Unleashing the power of bond cleavage chemistry in living systems. ACS Cent. Sci. 7, 929–943 (2021).
Xue, Z. et al. Organelle-directed staudinger reaction enabling fluorescence-on resolution of mitochondrial electropotentials via a self-immolative charge reversal probe. Anal. Chem. 90, 2954–2962 (2018).
Khan, I., Seebald, L. M., Robertson, N. M., Yigit, M. V. & Royzen, M. Controlled in-cell activation of RNA therapeutics using bond-cleaving bio-orthogonal chemistry. Chem. Sci. 8, 5705–5712 (2017).
Zeng, T. et al. Unraveling the cleavage reaction of hydroxylamines with cyclopropenones considering biocompatibility. J. Am. Chem. Soc. 146, 35077–35089 (2024).
Li, J., Jia, S. & Chen, P. R. Diels-Alder reaction–triggered bioorthogonal protein decaging in living cells. Nat. Chem. Biol. 10, 1003–1005 (2014).
Luo, J., Liu, Q., Morihiro, K. & Deiters, A. Small-molecule control of protein function through Staudinger reduction. Nat. Chem. 8, 1027–1034 (2016).
Zhang, G. et al. Bioorthogonal chemical activation of kinases in living systems. ACS Cent. Sci. 2, 325–331 (2016).
Weiss, J. T. et al. Extracellular palladium-catalysed dealkylation of 5-fluoro-1-propargyl-uracil as a bioorthogonally activated prodrug approach. Nat. Commun. 5, 3277 (2014).
Tu, J., Xu, M., Parvez, S., Peterson, R. T. & Franzini, R. M. Bioorthogonal removal of 3-isocyanopropyl groups enables the controlled release of fluorophores and drugs in vivo. J. Am. Chem. Soc. 140, 8410–8414 (2018).
Oliveira, B. L. et al. Platinum-triggered bond-cleavage of pentynoyl amide and N-propargyl handles for drug-activation. J. Am. Chem. Soc. 142, 10869–10880 (2020).
Wang, C. et al. A Cationic micelle as in vivo catalyst for tumor-localized cleavage chemistry. Angew. Chem. Int. Ed. 60, 19750–19758 (2021).
Zhou, Z., Feng, S., Zhou, J., Ji, X. & Long, Y.-Q. On-demand activation of a bioorthogonal prodrug of SN-38 with fast reaction kinetics and high releasing efficiency in vivo. J. Med. Chem. 65, 333–342 (2021).
Xu, X. et al. Development of cyclooctyne-nitrone based click release chemistry for bioorthogonal prodrug activation both in vitro and in vivo. J. Am. Chem. Soc. 147, 34425–34437 (2025).
Wang, Q. et al. Prodrug activation by 4,4’-bipyridine-mediated aromatic nitro reduction. Nat. Commun. 15, 8643 (2024).
Wang, D. et al. A click-and-release approach to CO prodrugs. Chem. Commun. 50, 15890–15893 (2014).
Wang, Q. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579, 421–426 (2020).
Chen, Y. et al. Design and development of a bioorthogonal, visualizable and mitochondria-targeted hydrogen sulfide (H2S) delivery system. Angew. Chem. Int. Ed 61, e202112734 (2021).
Deng, Y. et al. A membrane-embedded macromolecular catalyst with substrate selectivity in live cells. J. Am. Chem. Soc. 145, 1262–1272 (2022).
Versteegen, R. M., Rossin, R., ten Hoeve, W., Janssen, H. M. & Robillard, M. S. Click to release: instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem. Int. Ed. 52, 14112–14116 (2013).
Versteegen, R. M. et al. Click-to-release from trans-cyclooctenes: mechanistic insights and expansion of scope from established carbamate to remarkable ether cleavage. Angew. Chem. Int. Ed. 57, 10494–10499 (2018).
Li, H., Conde, J., Guerreiro, A. & Bernardes, G. J. L. Tetrazine carbon nanotubes for pretargeted in vivo “click-to-release” bioorthogonal tumour imaging. Angew. Chem. Int. Ed. 59, 16023–16032 (2020).
Wang, X. et al. Dual-locked enzyme-activatable bioorthogonal fluorescence turn-on imaging of senescent cancer cells. J. Am. Chem. Soc. 146, 22689–22698 (2024).
Ko, J. et al. Spatiotemporal multiplexed immunofluorescence imaging of living cells and tissues with bioorthogonal cycling of fluorescent probes. Nat Biotechnol 40, 1654–1662 (2022).
Ligthart, N. A. M. et al. A lysosome-targeted tetrazine for organelle-specific click-to-release chemistry in antigen presenting cells. J. Am. Chem. Soc. 145, 12630–12640 (2023).
Spitzberg, J. D. et al. Multiplexed analysis of EV reveals specific biomarker composition with diagnostic impact. Nat. Commun. 14, 1239 (2023).
Mejia Oneto, J. M., Khan, I., Seebald, L. & Royzen, M. In vivo bioorthogonal chemistry enables local hydrogel and systemic pro-drug to treat soft tissue sarcoma. ACS Cent. Sci. 2, 476–482 (2016).
Rossin, R. et al. Triggered drug release from an antibody–drug conjugate using fast “click-to-release” chemistry in mice. Bioconjugate Chem 27, 1697–1706 (2016).
Czuban, M. et al. Bio-orthogonal chemistry and reloadable biomaterial enable local activation of antibiotic prodrugs and enhance treatments against staphylococcus aureus infections. ACS Cent. Sci. 4, 1624–1632 (2018).
Rossin, R. et al. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice. Nat. Commun. 9, 1484 (2018).
McFarland, J. M. et al. Click chemistry selectively activates an auristatin protodrug with either intratumoral or systemic tumor-targeting agents. ACS Cent. Sci. 9, 1400–1408 (2023).
He, X. et al. An all-in-one tetrazine reagent for cysteine-selective labeling and bioorthogonal activable prodrug construction. Nat. Commun. 15, 2831 (2024).
Srinivasan, S. et al. SQ3370, the first clinical click chemistry-activated cancer therapeutic, shows safety in humans and translatability across species. BioRxiv (Accessed 7-10-2025) (2023).
Zheng, Y. et al. Enrichment-triggered prodrug activation demonstrated through mitochondria-targeted delivery of doxorubicin and carbon monoxide. Nat. Chem. 10, 787–794 (2018).
Liu, L., Zhang, D., Johnson, M. & Devaraj, N. K. Light-activated tetrazines enable precision live-cell bioorthogonal chemistry. Nat. Chem. 14, 1078–1085 (2022).
Knittel, C., Chadwick, S., Kuehling, C. & Devaraj, N. Enzymatic activation of caged tetrazines for cell-specific bioconjugation. Preprint at https://doi.org/10.26434/chemrxiv-2024-78kxz (2024).
Cheng, P. et al. Urinary bioorthogonal reporters for the monitoring of the efficacy of chemotherapy for lung cancer and of associated kidney injury. Nat. Biomed. Eng. 9, 686–699 (2025).
Kang, D. & Kim, J. Bioorthogonal retro-cope elimination reaction of N,N-dialkylhydroxylamines and strained alkynes. J. Am. Chem. Soc. 143, 5616–5621 (2021).
Kang, D., Lee, S. & Kim, J. Bioorthogonal click and release: a general, rapid, chemically revertible bioconjugation strategy employing enamine N-oxides. Chem 8, 2260–2277 (2022).
Yan, Z. et al. A Bioorthogonal decaging chemistry of N-oxide and silylborane for prodrug activation both in vitro and in vivo. J. Am. Chem. Soc. 145, 24698–24706 (2023).
Lee, M. H. et al. Disulfide-cleavage-triggered chemosensors and their biological applications. Chem. Rev. 113, 5071–5109 (2013).
Benoit, P. W., Yagiela, J. A. & Fort, N. F. Pharmacologic correlation between local anesthetic-induced myotoxicity and disturbances of intracellular calcium distribution. Toxicol. Appl. Pharmacol. 52, 187–198 (1980).
Li, J. et al. A tetrazine amplification system for visual detection of trace analytes via click-release reactions. Angew. Chem. Int. Ed. 64, e202414246 (2025).
Zeng, T. et al. Unraveling the cleavage reaction of hydroxylamines with cyclopropenones for biocompatible applications. J. Am. Chem. Soc. 146, 35077–35089 (2024).
Tu, J. et al. Stable, reactive, and orthogonal tetrazines: dispersion forces promote the cycloaddition with isonitriles. Angew. Chem. Int. Ed. 58, 9043–9048 (2019).
Eddins, A. J. et al. Quantitative protein labeling in live cells by controlling the redox state of encoded tetrazines. J. Am. Chem. Soc. 147, 23625–23634 (2025).
Acknowledgements
The authors are gratefully thankful for the support from the National Natural Science Foundation of China, China (22377147 and 22577156), and the Specialized Research Funds from the State Key Laboratory of Natural Medicines, China Pharmaceutical University (SKLNMZZ2024JS38).
Author information
Authors and Affiliations
Contributions
X.X., X.T. and Y.S. contributed equally. Y.Z. and W.G. conceived the initial idea and supervised the study and the overall manuscript preparation and revision process. X.X. designed and performed the experiments and drafted the manuscript. X.X. and X.T. synthesized and characterized the related compounds. X.T. also revised the manuscript. Y.S., X.W., and M.C. conducted studies of the reaction kinetics and some of the synthesis. Y.C. and X.X. conducted the in vitro biology studies. X.X., Y.S., X.W., S.C., and Y.W. evaluated the antitumor and local anesthetic activities. Y.L. designed and supervised the pharmacokinetic studies. Y.S. and H.H. performed the pharmacokinetic studies. All authors reviewed, edited, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks anonymous reviewers for their contribution to the peer review of this work. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, X., Tong, X., Shi, Y. et al. Spatiotemporally controlled drug release via a click-release system utilizing mono-alkyl-hydroxylamine and cyclooctyne chemistry. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68502-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68502-4