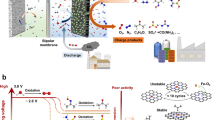
Abstract
Conventional closed batteries are constrained by the electrical energy efficiency of 100%, inevitably leading to the reduction of electricity storage. In contrast, open decoupled batteries offer the possibility to break this limitation, but remain unexplored. Here, we develop a highly efficient and sustainable open decoupled battery through a three-electrodynamic-potential (3E) design, simultaneously realizing waste-to-energy conversion, power generation and energy storage. For decoupled electrodes, we engineer high discharge voltage (ED) incorporating zinc oxidation and oxygen reduction reactions, and low charge voltage (EC) involving zinc-ion reduction and hydrazine (waste) oxidation reactions. Furthermore, we introduce reverse electrodialysis potential (ERED) by decoupling electrolytes. Consequently, the assembled battery demonstrates stability for 1000 cycles at the fast-charging current density of 300 mA cm−2. Moreover, a scaled 20-Ah-capacity battery was performed achieving a high electrical energy efficiency of 375% at 10 mA cm−2. Techno-economic analyses reveal that storing one megawatt-hour of electricity using the open decoupled battery can reduce the cost and carbon emissions of power generation by over 80% compared to conventional batteries. This work establishes a foundation for designing electricity-amplified batteries with economic and environmental benefits.
Similar content being viewed by others
Data availability
The data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided with this paper.
References
Yao, Y., Lei, J., Shi, Y., Ai, F. & Lu, Y.-C. Assessment methods and performance metrics for redox flow batteries. Nat. Energy 6, 582–588 (2021).
Zhou, G., Chen, H. & Cui, Y. Formulating energy density for designing practical lithium–sulfur batteries. Nat. Energy 7, 312–319 (2022).
Wang, J. et al. Toward direct regeneration of spent lithium-ion batteries: a next-generation recycling method. Chem. Rev. 124, 2839–2887 (2024).
Jia, Y. et al. Variable and intelligent catalyst design based on local chemical environments in sulfur redox reactions. Joule 9, 101878 (2025).
Zhang, Q. et al. Constructing bipolar dual-active sites through high-entropy-induced electric dipole transition for decoupling oxygen redox. Adv. Mater. 36, 2401018 (2024).
Li, Y. et al. In situ formation of liquid crystal interphase in electrolytes with soft templating effects for aqueous dual-electrode-free batteries. Nat. Energy 9, 1350–1359 (2024).
Wang, B. et al. Sulfion oxidation assisting self-powered hydrogen production system based on efficient catalysts from spent lithium-ion batteries. Proc. Natl. Acad. Sci. USA 120, e2317174120 (2023).
Liu, J.-N. et al. A data-driven bifunctional oxygen electrocatalyst with a record-breaking ΔE = 0.57 V for ampere-hour-scale zinc-air batteries. Joule 8, 1804–1819 (2024).
Choi, G. et al. Soft-hard zwitterionic additives for aqueous halide flow batteries. Nature 635, 89–95 (2024).
Yang, C. et al. All-temperature zinc batteries with high-entropy aqueous electrolyte. Nat. Sustain. 6, 325–335 (2023).
Song, Y.-W. et al. Phase equilibrium thermodynamics of lithium–sulfur batteries. Nat. Chem. Eng. 1, 588–596 (2024).
Zhong, C. et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc–manganese dioxide batteries. Nat. Energy 5, 440–449 (2020).
Zhong, X. et al. Rechargeable zinc-air batteries with an ultra-large discharge capacity per cycle and an ultra-long cycle life. Adv. Mater. 35, e2301952 (2023).
Xi, D. et al. Mild pH-decoupling aqueous flow battery with practical pH recovery. Nat. Energy 9, 479–490 (2024).
Gong, A. et al. Energy recovery and saving in municipal wastewater treatment engineering practices. Nat. Sustain. 8, 112–119 (2024).
Liu, Z. et al. Unraveling paradoxical effects of large current density on Zn deposition. Adv. Mater. 36 (2024).
Xiao, X. et al. Rational design of flexible Zn-based batteries for wearable electronic devices. ACS Nano 17, 1764–1802 (2023).
Zheng, Z. et al. An extended substrate screening strategy enabling a low lattice mismatch for highly reversible zinc anodes. Nat. Commun. 15, 753 (2024).
Parker, J. F., Ko, J. S., Rolison, D. R. & Long, J. W. Translating materials-level performance into device-relevant metrics for zinc-based batteries. Joule 2, 2519–2527 (2018).
Huang, S. et al. A universal coulombic efficiency compensation strategy for zinc-based flow batteries. Adv. Mater. 36, e2406366 (2024).
Wang, Q. et al. Rescue of dead MnO2 for stable electrolytic Zn-Mn redox-flow battery: a metric of mediated and catalytic kinetics. Natl. Sci. Rev. 11, nwae230 (2024).
Hammer, B. & Norskov, J. K. Why gold is the noblest of all the metals. Nature 376, 238–240 (1995).
Taylor, C. D. The transition from metal–metal bonding to metal–solvent interactions during a dissolution event as assessed from electronic structure. Chem. Phys. Lett. 469, 99–103 (2009).
Sharma, S. et al. Metal dissolution from first principles: potential-dependent kinetics and charge transfer. Electrochim. Acta 437, 141443 (2023).
Cao, L. et al. Solvation structure design for aqueous Zn metal batteries. J. Am. Chem. Soc. 142, 21404–21409 (2020).
Roy, K. et al. How solvation energetics dampen the hydrogen evolution reaction to maximize zinc anode stability. Adv. Energy Mater. 14, 2303998 (2024).
Toney, M. F. et al. Voltage-dependent ordering of water molecules at an electrode–electrolyte interface. Nature 368, 444–446 (1994).
Wang, Y. H. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021).
Li, Y. et al. Main-group element-boosted oxygen electrocatalysis of Cu-N-C sites for zinc-air battery with cycling over 5000 h. Nat. Commun. 15, 8365 (2024).
Wei, Z. et al. Starch-mediated colloidal chemistry for highly reversible zinc-based polyiodide redox flow batteries. Nat. Commun. 15, 3841 (2024).
Wang, C. et al. High-voltage and dendrite-free zinc-iodine flow battery. Nat. Commun. 15, 6234 (2024).
Xie, C. et al. Reversible multielectron transfer I−/IO3− cathode enabled by a hetero-halogen electrolyte for high-energy-density aqueous batteries. Nat. Energy 9, 714–724 (2024).
Lv, X.-L. et al. Modular dimerization of organic radicals for stable and dense flow battery catholyte. Nat. Energy 8, 1109–1118 (2023).
Zhao, Z. et al. Air-stable naphthalene derivative-based electrolytes for sustainable aqueous flow batteries. Nat. Sustain. 7, 1273–1282 (2024).
Lei, J. et al. An active and durable molecular catalyst for aqueous polysulfide-based redox flow batteries. Nat. Energy 8, 1355–1364 (2023).
Schmidt, O., Hawkes, A., Gambhir, A. & Staffell, I. The future cost of electrical energy storage based on experience rates. Nat. Energy 2, 1–8 (2017).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Yokoi, R., Watari, T. & Motoshita, M. Future greenhouse gas emissions from metal production: gaps and opportunities towards climate goals. Energy Environ. Sci. 15, 146–157 (2022).
Turconi, R., Boldrin, A. & Astrup, T. Life cycle assessment (LCA) of electricity generation technologies: overview, comparability and limitations. Renew. Sustain. Energy Rev. 28, 555–565 (2013).
Wang, L. et al. A comparative life-cycle assessment of hydro-, nuclear and wind power: a China study. Appl. Energy 249, 37–45 (2019).
Zhu, L. et al. Active site recovery and N-N bond breakage during hydrazine oxidation boosting the electrochemical hydrogen production. Nat. Commun. 14, 1997 (2023).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152 (2005).
Acknowledgements
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2023B1515120099, G.Z.), Guangdong Innovative and Entrepreneurial Research Team Program (Grant No. 2021ZT09L197, G.Z.), Shenzhen Science and Technology Program (Grant No. KQTD20210811090112002, G.Z.), Guangxi Young Elite Scientist Sponsorship Program (Grant No. GXYESS2025063, B.W.), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2025A1515011765, X.Z.), Shenzhen Science and Technology Program (Grant No. JCYJ20240813094606009, X.Z.), and the National Natural Science Foundation of China (Grant No. 22309077 and No. 22579077, X.Z.). The first author would like to thank Jianyu Xie from Southern University of Science and Technology, Zhiyuan Zhang, Zhiyuan Han and Yeyang Jia from Tsinghua University for their useful discussion.
Author information
Authors and Affiliations
Contributions
G.Z., Z.Z., B.W. and X.Z. conceived the project. Z.Z. and B.W. synthesized the materials. Z.Z. and F.Z. carried out the materials characterization and analyzed the data. B.W. conducted the TEAs. B.H. conducted theoretical simulations. J.X., Z.X., Z.L. and J.L. provided experimental insights. All authors participated in manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Michael Purdy, and the other, anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, Z., Zheng, FY., Huang, B. et al. An open decoupled cell design achieving electricity generation and amplification through waste-to-energy conversion. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68550-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68550-w