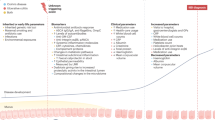
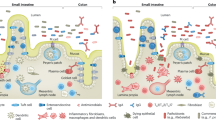
Abstract
Immune-mediated inflammatory diseases (IMID) are chronic conditions with a well-established multifaceted association with the gastrointestinal diseases Crohn’s disease (CD) and ulcerative colitis (UC), commonly known as inflammatory bowel disease (IBD). In this study, we leverage Danish nationwide pedigree and health data, genome-wide association studies, and fecal microbiota data to characterize the association between IBD and other IMIDs and disentangle genetic and environmental contributions. We show that CD and UC have distinct patterns of correlations with other IMIDs within families. By evaluating genetic and gut microbial correlations, we highlight a UC microbiota-linked correlation with multiple sclerosis and systemic lupus erythematosus despite a negative genetic correlation, suggesting a key role for environmental factors. In contrast, we find consistent genetic and microbial convergence between both IBD subtypes and rheumatoid arthritis, whereas genetic factors mainly drive the correlation with psoriasis. Thus, our findings uncover heterogeneity in shared etiological pathways across immune diseases, underscoring the need for stratified approaches to diagnosing and treating IBD.
Similar content being viewed by others
Data availability
This work uses data from the Danish National Health registries (https://sundhedsdatastyrelsen.dk), which is protected by the Danish Act on Processing of Personal Data to protect the personal sensitive data and adhere to the GDPR rules. An application to the Danish Health Data Agency is required to access data, which necessitates approval from your data responsible institution, which must be Danish. The application should only request data necessary to answer the research question, which must be for the benefit of society. You can expect a reply 5–8 weeks after submitting your application. Data should be delivered within 10 weeks. All other data sources are publicly available at GWAS Catalog, GWAS Atlas and NCBI’s BioProject database, and data access information is included in Supplementary Tables 4–6 or available from the authors. The raw numbers for charts and graphs are available in the Source Data files.
Code availability
All analyses were conducted in R (v4.4.0) and Python (v3.9.7). All generated code is available at GitHub: https://github.com/marievibeke/IBD_IMIDs_Correlation34.
References
Bezzio, C. et al. Inflammatory bowel disease and immune-mediated inflammatory diseases: looking at the less frequent associations. Ther. Adv. Gastroenterol. 15, 17562848221115312 (2022).
García, M. J. et al. Impact of immune-mediated diseases in inflammatory bowel disease and implications for therapeutic approach. Sci. Rep. 10, 10731 (2020).
El-Gabalawy, H., Guenther, L. C. & Bernstein, C. N. Epidemiology of immune-mediated inflammatory diseases: incidence, prevalence, natural history, and comorbidities. J. Rheumatol. Suppl. 85, 2–10 (2010).
Garcia Garcia, M. J. et al. P292 Immunomodulator and biological therapy are increased in inflammatory bowel disease patients with associated immune-mediated inflammatory diseases. J. Crohns Colitis 13, S245–S246 (2019).
Attauabi, M., Wewer, M. D., Bendtsen, F., Seidelin, J. B. & Burisch, J. Inflammatory bowel diseases affect the phenotype and disease course of coexisting immune-mediated inflammatory diseases: a systematic review with meta-analysis. Inflamm. Bowel Dis. 28, 1756–1765 (2022).
Park, S. W. et al. Comorbid immune-mediated diseases in inflammatory bowel disease: a nation-wide population-based study. Aliment. Pharmacol. Ther. 49, 165–172 (2019).
Jostins, L. et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Buckley, C. D. et al. Immune-mediated inflammation across disease boundaries: breaking down research silos. Nat. Immunol. 22, 1344–1348 (2021).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Farh, K. K. H. et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–343 (2015).
Metwaly, A., Reitmeier, S. & Haller, D. Microbiome risk profiles as biomarkers for inflammatory and metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 19, 383–397 (2022).
Johnson, J. S. et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 10, 1–11 (2019).
Duvallet, C., Gibbons, S. M., Gurry, T., Irizarry, R. A. & Alm, E. J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 8, 1–10 (2017).
Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930 (2003).
Cerezo, M. et al. The NHGRI-EBI GWAS catalog: standards for reusability, sustainability and diversity. Nucleic Acids Res. 53, D998–D1005 (2025).
Schmidt, M. et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin. Epidemiol. 7, 449 (2015).
Schmidt, M., Pedersen, L. & Sørensen, H. T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 29, 541–549 (2014).
Athanasiadis, G. et al. A comprehensive map of genetic relationships among diagnostic categories based on 48.6 million relative pairs from the Danish genealogy. Proc. Natl. Acad. Sci. Usa. 119, e2118688119 (2022).
Hagberg, A. A., Schult, D. A. & Swart, P. J. Exploring Network Structure, Dynamics, and Function using NetworkX. Proceedings of 7th Python in Science Conference (SciPy2008), 11-15 (2008).
Zhao, W. et al. PyAGH: a Python package to fast construct kinship matrices based on different levels of omic data. BMC Bioinforma. 24, 1–12 (2023).
Agrawal, M. et al. The rising burden of inflammatory bowel disease in denmark over two decades: a Nationwide Cohort Study. Gastroenterology 163, 1547–1554.e5 (2022).
Falconer, D. S. & Mackay, T. F. C. Introduction to quantitative genetics. (Longman Scientific & Technical, 1989).
Olsson, U. Maximum likelihood estimation of the polychoric correlation coefficient. Psychometrika 44, 443–460 (1979).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc.: Ser. B 57, 289–300 (1995).
Watanabe, K. et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat. Genet. 51, 1339–1348 (2019).
Zhang, Y. et al. SUPERGNOVA: local genetic correlation analysis reveals heterogeneous etiologic sharing of complex traits. Genome Biol. 2021 22, 1–30 (2021).
Sayers, E. W. et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 50, D20–D26 (2022).
Vestergaard, M. V. et al. Gut microbiota signatures in inflammatory bowel disease. U. Eur. Gastroenterol. J. 12, 22–33 (2024).
Graspeuntner, S., Loeper, N., Künzel, S., Baines, J. F. & Rupp, J. Selection of validated hypervariable regions is crucial in 16S-based microbiota studies of the female genital tract. Sci. Rep. 8, 1–7 (2018).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792 (2004).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41, D590–D596 (2013).
Vestergaard M. V., Alfaro-Núñez A., Sazonovs A., Athanasiadis G., Jess T. Multimodal analysis disentangles the genetic and environmental association between inflammatory bowel disease and other immune-mediated diseases across a harmonized population framework. IBD_IMIDs_Correlation, https://doi.org/10.5281/zenodo.17943775 (2025).
Acknowledgments
Danish National Research Foundation, DNRF148, TJ, and the Novo Nordisk Foundation, NNF21OC0068631, TJ. Figure 1 was generated by graphic designer Sabrina Hjort Andersen.
Author information
Authors and Affiliations
Contributions
M.V.V. conceptualized the study and was mainly responsible for all data handling and analyses. M.V.V. and A.A.N. wrote the first draft of the manuscript. G.A. supervised and assisted with the data handling and analysis of genealogy data. A.S. supervised the genetics analysis. T.J. was responsible for acquiring ethical permission to work with Danish healthcare data and provided access to data storage and computing resources. T.J. was further responsible for obtaining funding and supervised the entire work on the study. All authors were responsible for reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
TJ reports consultancy for Ferring and Pfizer. The remaining authors have no disclosures.
Peer review
Peer review information
Nature Communications thanks Simone Saibeni (eRef), who co-reviewed with Alice De Bernardi (ECR); Dalin Li and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vestergaard, M.V., Alfaro-Núñez, A., Sazonovs, A. et al. Multimodal analysis disentangles the genetic and microbial associations between inflammatory bowel disease and other immune-mediated diseases across a harmonized population framework. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68564-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68564-4