Abstract
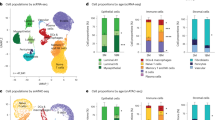
Aging increases breast cancer risk while an early first pregnancy reduces a woman’s life-long risk. Several studies have explored the effect of either aging or pregnancy on mammary stem/progenitor cells, however, the combined effect of both remains unclear. Here, we interrogate the functional and transcriptomic changes at single-cell resolution in the mammary gland of aged nulliparous and parous mice to discover that pregnancy normalizes age-related imbalances in lineage composition, while also inducing a differentiated cell state. Importantly, we uncover a minority population of Il33-expressing epithelial cells that express both luminal and basal markers (i.e. hybrid), which accumulate in aged nulliparous mice but are significantly reduced in aged parous mice. Functionally, IL33 treatment of mammary epithelial cells from young mice phenocopies aged nulliparous epithelial cells, induces proliferation and promotes formation of organoids with Trp53 knockdown. Collectively, our study demonstrates that pregnancy blocks the age-associated imbalances in lineage integrity in the basal layer, including a decrease in Il33+ hybrid cells, that could potentially contribute to pregnancy-induced breast cancer protection.
Similar content being viewed by others

Data availability
Data generated or analyzed during this study are included in this published article (and its supplemental information files). Data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplemental Materials. Single-cell RNA-sequencing data generated in this study has been deposited in the Gene Expression Omnibus (GEO) with the primary accession code GSE272932 and can be explored at https://aging-mouse-pregnancy.cells.ucsc.edu. The published single-cell RNA-sequencing data used in this study are available in the GEO database under accession codes GSE216542, GSE205573, GSM2967054, and GSE195665, and in the ArrayExpress database under the accession code E-MTAB-13664. Source data are provided with this paper.
References
SEER*Explorer Application [Internet]. [cited 2023 Apr 15]. Available from: https://seer.cancer.gov/statistics-network/explorer/application.html?site=1&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&hdn_stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion0.
MacMahon, B. et al. Age at first birth and breast cancer risk. Bull. World Health Organ. 43, 209–221 (1970).
Albrektsen, G., Heuch, I., Tretli, S. & Kvåle, G. Breast cancer incidence before age 55 in relation to parity and age at first and last births: a prospective study of one million Norwegian women. Epidemiology 5, 604–611 (1994).
Albrektsen, G., Heuch, I., Hansen, S. & Kvåle, G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br. J. Cancer Nat. Publ. Group 92, 167–175 (2005).
Russo, J., Tay, L. K., Ciocca, D. R. & Russo, I. H. Molecular and cellular basis of the mammary gland susceptibility to carcinogenesis. Environ. Health Perspect. 49, 185–199 (1983).
Russo, J., Moral, R., Balogh, G. A., Mailo, D. & Russo, I. H. The protective role of pregnancy in breast cancer. Breast Cancer Res. 7, 131–142 (2005).
Medina, D. Breast cancer: the protective effect of pregnancy. Clin. Cancer Res. 10, 380s–384s (2004).
Russo, J. et al. Molecular basis of pregnancy-induced breast cancer protection. Eur. J. Cancer Prev. 15, 306 (2006).
Siwko, S. K. et al. Evidence that an early pregnancy causes a persistent decrease in the number of functional mammary epithelial stem cells—implications for pregnancy-induced protection against breast cancer. Stem Cells 26, 3205–3209 (2008).
Choudhury, S. et al. Molecular profiling of human mammary gland links breast cancer risk to a p27+ cell population with progenitor characteristics. Cell Stem Cell 13, 117–130 (2013).
Santucci-Pereira, J. et al. Genomic signature of parity in the breast of premenopausal women. Breast Cancer Res. 21, 46 (2019).
Meier-Abt, F. et al. Parity induces differentiation and reduces Wnt/Notch signaling ratio and proliferation potential of basal stem/progenitor cells isolated from mouse mammary epithelium. Breast Cancer Res. 15, R36 (2013).
Shalabi, S. F. et al. Evidence for accelerated aging in mammary epithelia of women carrying germline BRCA1 or BRCA2 mutations. Nat. Aging 1, 838–849 (2021).
Pelissier Vatter, F. A. et al. High-dimensional phenotyping identifies age-emergent cells in human mammary epithelia. Cell Rep. 23, 1205–1219 (2018).
Garbe, J. C. et al. Accumulation of multipotent progenitors with a basal differentiation bias during aging of human mammary epithelia. Cancer Res. 72, 3687–3701 (2012).
Pelissier, F. A. et al. Age-related dysfunction in mechanotransduction impairs differentiation of human mammary epithelial progenitors. Cell Rep. Elsevier 7, 1926–1939 (2014).
Lambe, M. et al. Transient increase in the risk of breast cancer after giving birth. N. Engl. J. Med. Mass. Med. Soc. 331, 5–9 (1994).
Husby, A., Wohlfahrt, J., Øyen, N. & Melbye, M. Pregnancy duration and breast cancer risk. Nat. Commun. Nat. Publ. Group 9, 4255 (2018).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 72, 7–33 (2022).
Osterman, M. J. K., Hamilton, B. E., Martin, J. A., Driscoll, A. K. & Valenzuela, C. P. Births: final data for 2021. Natl. Vital. Stat. Rep. 72, 1–53 (2023).
Mathews, T. J. & Hamilton, B. E. Mean Age of Mothers is on the Rise: United States, 2000-2014. NCHS Data Brief 1–8 (2016).
Reed, A. D. et al. A single-cell atlas enables mapping of homeostatic cellular shifts in the adult human breast. Nat. Genet. Nat. Publ. Group 56, 652–662 (2024).
Fu, N. Y., Nolan, E., Lindeman, G. J. & Visvader, J. E. Stem cells and the differentiation hierarchy in mammary gland development. Physiol. Rev. Am. Physiol. Soc. 100, 489–523 (2020).
Li, C. M. C. et al. Aging-associated alterations in mammary epithelia and stroma revealed by single-cell RNA sequencing. Cell Rep. 33, 108566 (2020).
Dong, Q. et al. Aging is associated with an expansion of CD49fhi mammary stem cells that show a decline in function and increased transformation potential. Aging 8, 2754–2776 (2016).
Fu, N. Y. et al. Identification of quiescent and spatially restricted mammary stem cells that are hormone responsive. Nat. Cell Biol. Nat. Publ. Group 19, 164–176 (2017).
Stein, T. et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 6, R75 (2003).
Bai, H. et al. Progressive senescence programs induce intrinsic vulnerability to aging-related female breast cancer. Nat. Commun. Nat. Publ. Group 15, 5154 (2024).
Almanzar, N. et al. Tabula muris consortium. a single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nat. Nat. Publ. Group 583, 590–595 (2020).
Kendrick, H. et al. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genom. 9, 591 (2008).
Wolf, F. A. et al. PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol. 20, 59 (2019).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genom. 19, 477 (2018).
Kang, M. et al. Improved reconstruction of single-cell developmental potential with CytoTRACE 2. Nat. Methods 22, 2258–2263 (2025).
Bu, W. et al. Keratin 6a marks mammary bipotential progenitor cells that can give rise to a unique tumor model resembling human normal-like breast cancer. Oncogene. Nat. Publ. Group 30, 4399–4409 (2011).
Smith, G. H., Mehrel, T. & Roop, D. R. Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth Differ. 1, 161–170 (1990).
Kim, J. Y., Kim, G., Lim, S. C. & Choi, H. S. IL-33-induced transcriptional activation of LPIN1 accelerates breast tumorigenesis. Cancers Multidiscip. Digit. Publ. Inst. 13, 2174 (2021).
Kim, J. Y. et al. Interleukin-33/ST2 axis promotes epithelial cell transformation and breast tumorigenesis via upregulation of COT activity. Oncogene. Nat. Publ. Group 34, 4928–4938 (2015).
Liu J., Shen, J. X., Hu, J. L., Huang, W. H. & Zhang, G. J. Significance of interleukin-33 and its related cytokines in patients with breast cancers. Front. Immunol. 5, 1–7 (2014).
Hu, H. et al. IL-33 facilitates endocrine resistance of breast cancer by inducing cancer stem cell properties. Biochem. Biophys. Res. Commun. 485, 643–650 (2017).
Wasmer, M. H. & Krebs, P. The role of IL-33-dependent inflammation in the tumor microenvironment. Front. Immunol. 7, 682 (2017).
Angarola, B. L. et al. Comprehensive single-cell aging atlas of healthy mammary tissues reveals shared epigenomic and transcriptomic signatures of aging and cancer. Nat. Aging. 5, 1–22 (2024).
Pal, B. et al. Single cell transcriptome atlas of mouse mammary epithelial cells across development. Breast Cancer Res. 23, 69 (2021).
Ursin, G. et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br. J. Cancer Nat. Publ. Group 93, 364–371 (2005).
Liang, J. et al. ERα dysfunction caused by ESR1 mutations and therapeutic pressure promotes lineage plasticity in ER+ breast cancer. Nat. Cancer Nat. Publ. Group 6, 357–371 (2025).
Ventura, A. et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc. Natl. Acad. Sci. USA 101, 10380–10385 (2004).
Alonso-Curbelo, D. et al. A gene–environment-induced epigenetic program initiates tumorigenesis. Nat. Publ. Group 590, 642–648 (2021).
Zhang, Z. et al. Mammary-stem-cell-based somatic mouse models reveal breast cancer drivers causing cell fate dysregulation. Cell Rep. 16, 3146–3156 (2016).
Ngan, E. S. W., Ma, Z. Q., Chua, S. S., DeMayo, F. J. & Tsai, S. Y. Inducible expression of FGF-3 in mouse mammary gland. Proc. Natl. Acad. Sci. USA 99, 11187–11192 (2002).
Burdziak, C. et al. Epigenetic plasticity cooperates with cell-cell interactions to direct pancreatic tumorigenesis. Sci. Am. Assoc. Adv. Sci. 380, eadd5327 (2023).
Topczewska, P. M. et al. ILC2 require cell-intrinsic ST2 signals to promote type 2 immune responses. Front. Immunol. 14, 1130933 (2023).
Kumar, T. et al. A spatially resolved single-cell genomic atlas of the adult human breast. Nat. Publ. Group 620, 181–191 (2023).
Kolodziejczyk, A. A., Kim, J. K., Svensson, V., Marioni, J. C. & Teichmann, S. A. The technology and biology of single-cell RNA sequencing. Mol. Cell 58, 610–620 (2015).
Islam, S. et al. Quantitative single-cell RNA-seq with unique molecular identifiers. Nat. Methods Nat. Publ. Group 11, 163–166 (2014).
Twigger, A. J. et al. Transcriptional changes in the mammary gland during lactation revealed by single cell sequencing of cells from human milk. Nat. Commun. Nat. Publ. Group 13, 562 (2022).
Jena, M. K., Jaswal, S., Kumar, S. & Mohanty, A. K. Molecular mechanism of mammary gland involution: an update. Dev. Biol. 445, 145–155 (2019).
Watson, C. J. & Kreuzaler, P. A. Remodeling mechanisms of the mammary gland during involution. Int. J. Dev. Biol. UPV/EHU press 55, 757–762 (2011).
Stein, T., Salomonis, N. & Gusterson, B. A. Mammary gland involution as a multi-step process. J. Mammary Gland Biol. Neoplasia 12, 25–35 (2007).
Langille, E. et al. Loss of epigenetic regulation disrupts lineage integrity, induces aberrant alveogenesis, and promotes breast cancer. Cancer Discov. 12, 2930–2953 (2022).
Christin, J. R. et al. Stem cell determinant SOX9 Promotes lineage plasticity and progression in basal-like breast cancer. Cell Rep. 31, 107742 (2020).
Van Keymeulen, A. et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature 525, 119–123 (2015).
Koren, S. et al. PIK3CAH1047R induces multipotency and multi-lineage mammary tumours. Nat. Publ. Group 525, 114–118 (2015).
Molyneux, G. et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 7, 403–417 (2010).
Lloyd-Lewis, B. et al. In vivo imaging of mammary epithelial cell dynamics in response to lineage-biased Wnt/β-catenin activation. Cell Rep. 38, 110461 (2022).
Oprean, C. M. et al. Postmenopausal breast cancer in women, clinical and epidemiological factors related to the molecular subtype: a retrospective cohort study in a single institution for 13 years. follow-up data. Int J. Environ. Res. Public Health 17, 8722 (2020).
Davis, F. M. et al. Single-cell lineage tracing in the mammary gland reveals stochastic clonal dispersion of stem/progenitor cell progeny. Nat. Commun. Nat. Publ. Group 7, 13053 (2016).
Van Keymeulen, A. et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature 479, 189–193 (2011).
Rios, A. C., Fu, N. Y., Lindeman, G. J. & Visvader, J. E. In situ identification of bipotent stem cells in the mammary gland. Nat. Nat. Publ. Group 506, 322–327 (2014).
van Amerongen, R., Bowman, A. N. & Nusse, R. Developmental stage and time dictate the fate of wnt/β-catenin-responsive stem cells in the mammary gland. Cell. Stem Cell. Elsevier 11, 387–400 (2012).
Cayrol, C. & Girard, J. P. Interleukin-33 (IL-33): a critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine 156, 155891 (2022).
Centonze, A. et al. Heterotypic cell–cell communication regulates glandular stem cell multipotency. Nat. Nat. Publ. Group 584, 608–613 (2020).
Stier, M. T. et al. IL-33 is a cell-intrinsic regulator of fitness during early B cell development. J. Immunol. 203, 1457–1467 (2019).
Yin, H. et al. IL-33 accelerates cutaneous wound healing involved in upregulation of alternatively activated macrophages. Mol. Immunol. 56, 347–353 (2013).
Wu, K. et al. Basal epithelial stem cells cross an alarmin checkpoint for postviral lung disease. J. Clin. Investig. 131, e149336 (2021).
Kurimoto, M., Watanabe, T., Kamata, K., Minaga, K. & Kudo, M. IL-33 as a Critical Cytokine for Inflammation and Fibrosis in Inflammatory Bowel Diseases and Pancreatitis. Front. Physiol. 12, 1–7 (2021).
Sikandar, S. S. et al. Role of epithelial to mesenchymal transition associated genes in mammary gland regeneration and breast tumorigenesis. Nat. Commun. Nat. Publ. Group 8, 1669 (2017).
Zabala, M. et al. LEFTY1 Is a Dual-SMAD Inhibitor that Promotes Mammary Progenitor Growth and Tumorigenesis. Cell Stem Cell 27, 284–299.e8 (2020).
Sikandar, S. S. et al. Identification of a minority population of LMO2+ breast cancer cells that integrate into the vasculature and initiate metastasis. Sci. Adv. Am. Assoc. Adv. Sci. 8, eabm3548 (2022).
Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cells. Elsevier 8, 281–291.e9 (2019).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods Nat. Publ. Group 16, 1289–1296 (2019).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. Nat. Publ. Group 9, 5233 (2019).
McInnes, L., Healy, J., Saul, N. & Großberger, L. UMAP: Uniform Manifold Approximation and Projection. J. Open Source Softw. 3, 861 (2018).
scanpy_usage/180209_cell_cycle/data/regev_lab_cell_cycle_genes.txt at master · scverse/scanpy_usage [Internet]. GitHub. [cited 2024 July]. Available from: https://github.com/scverse/scanpy_usage/blob/master/180209_cell_cycle/data/regev_lab_cell_cycle_genes.txt.
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Acknowledgements
We thank Bari Nazario and Patricia Lovelace for their help with flow cytometry. The FACS Aria instrument was funded by NIH grant 1S10RR02933801 and Cytoflex RRID SCR_021149 and grant NIH S10OD030423. We thank Benjamin Abrams, UCSC Life Sciences Microscopy Center, RRID: SCR_021135 for technical support during image acquisition and processing. We thank Gunsagar Singh Gulati for advice on data integration and Bryce Manso for flow cytometry analysis of immune cells. We also thank the animal facility core members for animal maintenance. We thank Camilla Forsberg, Lindsay Hinck, Aaron Newman, and members of the Sikandar lab for critical feedback on the manuscript. The authors declare no competing interests. This work was supported by the Hellman Fellows Award and startup funds (to S.S.S), and NIH T-32 (5T32GM133391-04) and NIH/NCI F31 (1F31CA294932-01A1) (to A.O). S.S.S is also supported by the NIH/NCI (R37CA269754).
Author information
Authors and Affiliations
Contributions
S.S.S. and A.O. conceived and designed the study. A.O. performed most of the experiments and analyzed the data with assistance from V.H.A, M.D., and S.K., and under the supervision of S.S.S. V.H.A. collected samples for single-cell RNA sequencing and performed experiments with human cells. P.M. performed all bioinformatic analysis under supervision of S.S.S. A.O., and S.S.S. wrote the manuscript with contributions from P.M. and V.H.A. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olander, A., Medina, P., Haro Acosta, V. et al. Divergent aging of nulliparous and parous mammary glands reveals IL33+ hybrid epithelial cells. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68611-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68611-0