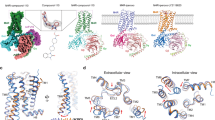
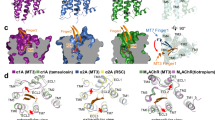
Abstract
Hard ticks depend upon an ability to precisely and dynamically regulate their saliva to successfully evade host haemostatic and immune defences during extended blood feeding. Although pilocarpine, an exogenous muscarinic acetylcholine receptor (mAChR) agonist, can stimulate salivation experimentally, the endogenous control of saliva secretion by acetylcholine remains poorly understood. Here, we identify and characterise two pharmacologically distinct mAChRs (type A and B) in the genome of the medically important tick Ixodes ricinus. Molecular dynamics simulations and targeted mutagenesis reveal that type B mAChRs exhibit an atypical muscarinic profile, suggesting unconventional receptor signalling. Combining immunolabelling, in vivo pharmacology, and proteomics, we show that specific central neurons interact with distinct salivary gland regions via mAChR type-specific axons, coordinating fluid and protein secretion through separate acini and likely acting upstream of a neuropeptide-dependent cascade. This previously unrecognised mechanism of neural control offers new insights into how ticks modulate their saliva advancing our understanding of vector-host interactions, with potential implications for disrupting pathogen transmission.
Similar content being viewed by others
Data availability
All data generated or analysed in this study are included in the article and the supplementary information files. Mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium via the PRIDE87 repository under dataset identifier PXD055362 (https://proteomecentral.proteomexchange.org). Source data are provided with this paper.
References
Kaufman, W. R. Gluttony and sex in female ixodid ticks: how do they compare to other blood-sucking arthropods? J. Insect Physiol. 53, 264–273 (2007).
Šimo, L., Kazimirova, M., Richardson, J. & Bonnet, S. I. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 7, 281 (2017).
Mans, B. J. Chemical equilibrium at the tick–host feeding interface: a critical examination of biological relevance in hematophagous behavior. Front. Physiol. 10, 530 (2019).
Šimo, L. 50 Years since Kaufman and Phillips’ groundbreaking trilogy elucidating ion and water homeostasis in ixodid ticks. Pathogens 12, 385 (2023).
Nuttall, P. A. Tick saliva and its role in pathogen transmission. Wien. Klin. Wochenschr. 135, 165–176 (2023).
Binnington, K. C. Sequential changes in salivary gland structure during attachment and feeding of the cattle tick, Boophilus microplus. Int. J. Parasitol. 8, 97–115 (1978).
Kim, D., Šimo, L., Vancová, M., Urban, J. & Park, Y. Neural and endocrine regulation of osmoregulatory organs in tick: Recent discoveries and implications. Gen. Comp. Endocrinol. 278, 42–49 (2019).
Mateos-Hernández, L. et al. Cholinergic axons regulate type I acini in salivary glands of Ixodes ricinus and Ixodes scapularis ticks. Sci. Rep. 10, 16054 (2020).
Šimo, L., Žitňan, D. & Park, Y. Neural control of salivary glands in ixodid ticks. J. Insect Physiol. 58, 459–466 (2012).
Vancová, M. et al. Ultrastructural mapping of salivary gland innervation in the tick Ixodes ricinus. Sci. Rep. 9, 6860 (2019).
Kaufman, W. R. Actions of some transmitters and their antagonists on salivary secretion in a tick. Am. J. Physiol. 235, R76–R81 (1978).
Kaufman, W. R. & Phillips, J. E. Ion and water balance in the ixodid tick Dermacentor andersoni: II. Mechanism and control of salivary secretion. J. Exp. Biol. 58, 537–547 (1973).
Kaufman, W. R. The influence of adrenergic agonists and their antagonists on isolated salivary glands of ixodid ticks. Eur. J. Pharmacol. 45, 61–68 (1977).
Tatchell, R. J. A modified method for obtaining tick oral secretion. J. Parasitol. 53, 1106–1107 (1967).
Kim, D., Šimo, L. & Park, Y. Orchestration of salivary secretion mediated by two different dopamine receptors in the blacklegged tick Ixodes scapularis. J. Exp. Biol. 217, 3656–3663 (2014).
Šimo, L., Koči, J., Žitňan, D. & Park, Y. Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PLoS ONE 6, e16158 (2011).
Šimo, L., Koči, J., Kim, D. & Park, Y. Invertebrate specific D1-like dopamine receptor in control of salivary glands in the black-legged tick Ixodes scapularis. J. Comp. Neurol. 522, 2038–2052 (2014).
Binnington, K. C. & Rice, M. J. A technique for recording efferent neurone activity from normal and poisoned cattle ticks [boophilus Microplus (canestrini)]. Aust. J. Entomol. 21, 161–166 (1982).
Kaufman, W. R. & Harris, R. A. Neural pathways mediating salivary fluid secretion in the ixodid tick Amblyomma hebraeum. Can. J. Zool. 61, 1976–1980 (1983).
Kaufman, W. R., Aeschlimann, A. A. & Diehl, P. A. Regulation of body volume by salivation in a tick challenged with fluid loads. Am. J. Physiol. 238, R102–112 (1980).
Roller, L. et al. Orcokinin-like immunoreactivity in central neurons innervating the salivary glands and hindgut of ixodid ticks. Cell Tissue Res. 360, 209–222 (2015).
Šimo, L., Slovák, M., Park, Y. & Zitnan, D. Identification of a complex peptidergic neuroendocrine network in the hard tick, Rhipicephalus appendiculatus. Cell Tissue Res. 335, 639–655 (2009).
Šimo, L., Žitňan, D. & Park, Y. Two novel neuropeptides in innervation of the salivary glands of the black-legged tick, ixodes scapularis: myoinhibitory peptide and SIFamide. J. Comp. Neurol. 517, 551–563 (2009).
Ren, G. R., Folke, J., Hauser, F., Li, S. & Grimmelikhuijzen, C. J. P. The A- and B-type muscarinic acetylcholine receptors from Drosophila melanogaster couple to different second messenger pathways. Biochem. Biophys. Res. Commun. 462, 358–364 (2015).
Xia, R.-Y. et al. A new family of insect muscarinic acetylcholine receptors. Insect Mol. Biol. 25, 362–369 (2016).
Birdsall, N. J., Burgen, A. S. & Hulme, E. C. The binding of agonists to brain muscarinic receptors. Mol. Pharm. 14, 723–736 (1978).
Meyer, E. M. & Otero, D. H. Pharmacological and ionic characterizations of the muscarinic receptors modulating [3H]acetylcholine release from rat cortical synaptosomes. J. Neurosci. 5, 1202–1207 (1985).
Collin, C. et al. Two types of muscarinic acetylcholine receptors in Drosophila and other arthropods. Cell. Mol. Life Sci. 70, 3231–3242 (2013).
Nìng, C., Heckmann, A., Mateos-Hernández, L., Karadjian, G. & Šimo, L. Functional characterization of three G protein-coupled acetylcholine receptors in parasitic nematode Trichinella spiralis. Int. J. Parasitol. Drugs Drug Resist. 23, 130–139 (2023).
Rang, H. P., Dale, M. M., Ritter, J. M. & Moore, P. K. Pharmacology 5th edn 797 pp. (Churchill Livingstone, 2003).
Lee, Y. S. et al. Characterization of GAR-2, a novel G protein-linked acetylcholine receptor from Caenorhabditis elegans. J. Neurochem. 75, 1800–1809 (2000).
Kimber, M. J. et al. Identification of an Ascaris G protein-coupled acetylcholine receptor with atypical muscarinic pharmacology. Int. J. Parasitol. 39, 1215–1222 (2009).
Maeda, S. et al. Structure and selectivity engineering of the M1 muscarinic receptor toxin complex. Science 369, 161–167 (2020).
Dong, C. & Wu, G. Regulation of anterograde transport of alpha2-adrenergic receptors by the N termini at multiple intracellular compartments. J. Biol. Chem. 281, 38543–38554 (2006).
Yamanaka, N. et al. Regulation of insect steroid hormone biosynthesis by innervating peptidergic neurons. Proc. Natl. Acad. Sci. USA 103, 8622–8627 (2006).
Grimmelikhuijzen, C. J. & Spencer, A. N. FMRFamide immunoreactivity in the nervous system of the medusa Polyorchis penicillatus. J. Comp. Neurol. 230, 361–371 (1984).
Nichols, R. Signaling pathways and physiological functions of Drosophila melanogaster FMRFamide-related peptides. Annu. Rev. Entomol. 48, 485–503 (2003).
Leander, M. et al. Cardiac contractility structure-activity relationship and ligand-receptor interactions; the discovery of unique and novel molecular switches in myosuppressin signaling. PLoS ONE 10, e0120492 (2015).
Waldman, J. et al. Neuropeptides in Rhipicephalus microplus and other hard ticks. Ticks Tick. Borne Dis. 13, 101910 (2022).
Chen, Y., Veenstra, J. A., Davis, N. T. & Hagedorn, H. H. A comparative study of leucokinin-immunoreactive neurons in insects. Cell Tissue Res. 276, 69–83 (1994).
Medla, M. et al. Identification and expression of short neuropeptide F and its receptors in the tick Ixodes ricinus. J. Insect Physiol. 147, 104524 (2023).
Yamanaka, N. et al. Bombyx orcokinins are brain-gut peptides involved in the neuronal regulation of ecdysteroidogenesis. J. Comp. Neurol. 519, 238–246 (2011).
Terhzaz, S., Rosay, P., Goodwin, S. F. & Veenstra, J. A. The neuropeptide SIFamide modulates sexual behavior in Drosophila. Biochem. Biophys. Res. Commun. 352, 305–310 (2007).
Li, Z., Macaluso, K. R., Foil, L. D. & Swale, D. R. Inward rectifier potassium (Kir) channels mediate salivary gland function and blood feeding in the lone star tick, Amblyomma americanum. PLoS Negl. Trop. Dis. 13, e0007153 (2019).
Li, Z. et al. ATP-sensitive inward rectifier potassium channels regulate secretion of pro-feeding salivary proteins in the lone star tick (Amblyomma americanum). Int. J. Biol. Macromol. 253, 126545 (2023).
Nathanson, N. M. A multiplicity of muscarinic mechanisms: enough signaling pathways to take your breath away. Proc. Natl. Acad. Sci. USA 97, 6245–6247 (2000).
Daaka, Y., Luttrell, L. M. & Lefkowitz, R. J. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390, 88–91 (1997).
Binnington, K. C. Ultrastructural identification of neurohaemal sites in a tick: evidence that the dorsal complex may be a true endocrine gland. Tissue Cell 15, 317–327 (1983).
Lapied, B., Le Corronc, H. & Hue, B. Sensitive nicotinic and mixed nicotinic-muscarinic receptors in insect neurosecretory cells. Brain Res 533, 132–136 (1990).
Lees, K. et al. Functional characterisation of a nicotinic acetylcholine receptor α subunit from the brown dog tick, Rhipicephalus sanguineus. Int. J. Parasitol. 44, 75–81 (2014).
Le Mauff, A. et al. Nicotinic acetylcholine receptors in the synganglion of the tick Ixodes ricinus: functional characterization using membrane microtransplantation. Int. J. Parasitol. Drugs Drug Resist. 14, 144–151 (2020).
Ito, K. et al. A systematic nomenclature for the insect brain. Neuron 81, 755–765 (2014).
Boussaine, K. et al. Isolation and electrophysiological recording of Ixodes ricinus synganglion neurons. J. Pharmacol. Toxicol. Methods 124, 107473 (2023).
Trendelenburg, A.-U., Gomeza, J., Klebroff, W., Zhou, H. & Wess, J. Heterogeneity of presynaptic muscarinic receptors mediating inhibition of sympathetic transmitter release: a study with M2- and M4-receptor-deficient mice. Br. J. Pharmacol. 138, 469–480 (2003).
Salvaterra, P. M. & McCaman, R. E. Choline acetyltransferase and acetylcholine levels in Drosophila melanogaster: a study using two temperature-sensitive mutants. J. Neurosci. 5, 903–910 (1985).
Kim, D., Urban, J., Boyle, D. L. & Park, Y. Multiple functions of Na/K-ATPase in dopamine-induced salivation of the Blacklegged tick, Ixodes scapularis. Sci. Rep. 6, 21047 (2016).
Mateos-Hernández, L., Rakotobe, S., Defaye, B., Cabezas-Cruz, A. & Šimo, L. A capsule-based model for immature hard tick stages infestation on laboratory mice. J. Vis. Exp. https://doi.org/10.3791/61430 (2020).
Almazán, C. et al. A versatile model of hard tick infestation on laboratory rabbits. J. Vis. Exp. https://doi.org/10.3791/57994 (2018).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Cieslikiewicz-Bouet, M. et al. Functional characterization of multifunctional ligands targeting acetylcholinesterase and alpha 7 nicotinic acetylcholine receptor. Biochem Pharma 177, 114010 (2020).
Cartereau, A., Martin, C. & Thany, S. H. Neonicotinoid insecticides differently modulate acetycholine-induced currents on mammalian α7 nicotinic acetylcholine receptors. Br. J. Pharmacol. 175, 1987–1998 (2018).
Papke, R. L. et al. Persistent activation of α7 nicotinic ACh receptors associated with stable induction of different desensitized states. Br. J. Pharmacol. 175, 1838–1854 (2018).
Papke, R. L. & Porter Papke, J. K. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br. J. Pharmacol. 137, 49–61 (2002).
Gulsevin, A. et al. Allosteric agonism of α7 nicotinic acetylcholine receptors: receptor modulation outside the orthosteric site. Mol. Pharm. 95, 606–614 (2019).
Vernon, W. I. & Printen, J. A. Assay for intracellular calcium using a codon-optimized aequorin. BioTechniques 33, 730–734 (2002).
Offermanns, S. & Simon, M. I. Gα15 and Gα16 couple a wide variety of receptors to phospholipase C (∗). J. Biol. Chem. 270, 15175–15180 (1995).
Hallgren, J. et al. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural network. Preprint at https://doi.org/10.1101/2022.04.08.487609 (2022).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Schrödinger Release 2022-1: Maestro (Schrödinger. LLC, 2021).
Madhavi Sastry, G., Adzhigirey, M., Day, T., Annabhimoju, R. & Sherman, W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 27, 221–234 (2013).
Mahoney, M. W. & Jorgensen, W. L. A five-site model for liquid water and the reproduction of the density anomaly by rigid, nonpolarizable potential functions. J. Chem. Phys. 112, 8910–8922 (2000).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Huang, J. & MacKerell, A. D. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Zoete, V., Cuendet, M. A., Grosdidier, A. & Michielin, O. SwissParam: a fast force field generation tool for small organic molecules. J. Comput. Chem. 32, 2359–2368 (2011).
Bowers, K. J. et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proc. 2006 ACM/IEEE Conference on Supercomputing 84–es (Association for Computing Machinery, New York, NY, USA, 2006).
Evans, D. J. & Holian, B. L. The nose–Hoover thermostat. J. Chem. Phys. 83, 4069–4074 (1985).
Martyna, G. J., Tobias, D. J. & Klein, M. L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 101, 4177–4189 (1994).
Tuckerman, M., Berne, B. J. & Martyna, G. J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 97, 1990–2001 (1992).
Humphrey, W., Dalke, A. & Schulten, K. V. M. D. Visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Šimo, L., Koči, J. & Park, Y. Receptors for the neuropeptides, myoinhibitory peptide and SIFamide, in control of the salivary glands of the blacklegged tick Ixodes scapularis. Insect Biochem. Mol. Biol. 43, 376–387 (2013).
Šimo, Daniel, S., E., Park, Y. & Žitňan, D. The Nervous and Sensory Systems: Structure, Function, Proteomics and Genomics. In Biology of Ticks (eds. Sonenshine, D. E. & Roe, R. M.) vol. 1 309–367 (Oxford Univ. Press, New York, 2014).
Cardona, A. et al. TrakEM2 software for neural circuit reconstruction. PLoS ONE 7, e38011 (2012).
Belevich, I., Joensuu, M., Kumar, D., Vihinen, H. & Jokitalo, E. Microscopy image browser: a platform for segmentation and analysis of multidimensional datasets. PLoS Biol. 14, e1002340 (2016).
Koči, J., Šimo, L. & Park, Y. Validation of internal reference genes for real-time quantitative polymerase chain reaction studies in the tick, Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 50, 79–84 (2013).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25, 402–408 (2001).
Kozelková, T. et al. Insight into the dynamics of the ixodes ricinus nymphal midgut proteome. Mol. Cell. Proteom. 22, 100663 (2023).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Kim, D., Šimo, L. & Park, Y. Molecular characterization of neuropeptide elevenin and two elevenin receptors, IsElevR1 and IsElevR2, from the blacklegged tick, Ixodes scapularis. Insect Biochem. Mol. Biol. 101, 66–75 (2018).
Acknowledgements
This study was supported by the French National Research Agency (ANR-21-CE14-0012, project AxoTick; L.Š). UMR BIPAR was supported by the French Government’s Investissement d’Avenir programme, Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (ANR-10-LABX-62-IBEID; institutional support). Additional support was provided by the Czech Science Foundation (22-18424M; J.P.), (22-30920S; R.Š.), (21-08826S; P.K.), and the Ministry of Health of the Czech Republic (NU20-05-00396 R.Š.). The study was also supported by a grant from the Centre-Val de Loire Region, under the Electro-CELL, Appel à projet d’intérêt regional (APR IR 21060LBL; S.H.T.). We acknowledge the BC CAS core facility LEM supported by MEYS CR (LM2023050 Czech-BioImaging and OP VVV CZ.02.1.01/0.0/0.0/18_046/0016045; M.V.). We are grateful to Jan Veenstra from the University of Bordeaux, France, for providing the anti-SIFamide and anti-leucokinin antibodies.
Author information
Authors and Affiliations
Contributions
L. Šimo designed the study and supervised all work. C.N., L.M.-H., S.R., L.A.-D., L. Šofranková, K.B., A.C., E.T., H.F., V.U., T.K., F.D., O.H., R.Š., J.T., T.B., M.T., M.V., J.P., S.H.T., and L. Šimo performed the experiments and analysed and interpreted the data. J.J.V. performed the molecular dynamics analyses and wrote this part of the manuscript. N.H., M.S., H.A., P.K., and J.P. provided resources. L. Šimo prepared the final figures and wrote the manuscript. All authors revised and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Philip Ahring and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nìng, C., Valdés, J.J., Mateos-Hernández, L. et al. Two types of axonal muscarinic acetylcholine receptors mediate formation of saliva cocktail in the tick Ixodes ricinus. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68654-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68654-3