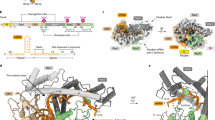
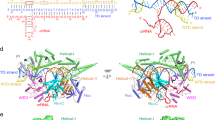
Abstract
CRISPR-Cas12a, an RNA-based DNA targeting system, is widely used for genome editing and biomarker detection. To mitigate the off-target DNA cleavage of Cas12a, we previously developed a Francisella novicida Cas12a variant (FnoCas12aKD2P) by introducing double proline substitutions (K969P/D970P) in a conserved arginine-rich helix called the bridge helix (BH). In this work, we use a combinatorial approach to understand the molecular mechanisms of BH-mediated activation of Cas12a for DNA cleavage. We report five structures of FnoCas12aKD2P that are at different states of conformational activation. Comparison of the variant and wild-type (FnoCas12aWT) structures, along with activity assays and computational simulations, establishes the loop-to-helical transition and bending of the BH as an allosteric trigger for RNA-DNA hybrid propagation. These changes track with the previously reported coupled remodeling of BH and helix 1 of RuvC motif-II as well as the REC lobe movements needed to accommodate the growing hybrid. The transition of the BH is essential for the loop-to-helical transition of the “lid”, which in turn opens the RuvC active site pocket for DNA entry and cleavage. Pairwise 3D structural comparison of the BH and RuvC of Cas12 and Cas9 families provides insight into the diversity of BH’s structural organization in these mechanistically similar enzymes.
Similar content being viewed by others
Data availability
All data are included in the manuscript, Supplementary Information, and public data repositories as mentioned below. The atomic coordinates and cryo-EM density maps are available in the Protein Data Bank and Electron Microscopy Data Bank under accession codes 9MKT/ EMD-48337 for FnoCas12aKD2P-state 1, 9MKU/ EMD-48338 for FnoCas12aKD2P-state 2, 9MKV/ EMD-48339 for FnoCas12aKD2P-state 3), 9MKW/ EMD-48340 for FnoCas12aKD2P-state 4a, and 9MKX/ EMD-48341 for FnoCas12aKD2P-state 4b. The molecular dynamics data generated in this study have been deposited in the GitHub database74. DNA sequencing files for the engineered Cas12a variants, the full raw dataset used to generate Fig. 3e, f, Supplementary Fig. 18a, b, Supplementary Table 11, and the sequences used to generate Fig. 5 and Supplementary Fig. 23 are provided in the Source Data file. Uncropped gel images for all the replications of the DNA cleavage assay are included in Supplementary Information (Appendix, pages 96 to 104). Source data are provided with this paper.
References
Mojica, F. J., Diez-Villasenor, C., Garcia-Martinez, J. & Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 60, 174–182 (2005).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Ganguly, C., Rostami, S., Long, K., Aribam, S. D. & Rajan, R. Unity among the diverse RNA-guided CRISPR-Cas interference mechanisms. J. Biol. Chem. 300, 107295 (2024).
Stella, S., Alcon, P. & Montoya, G. Class 2 CRISPR-Cas RNA-guided endonucleases: Swiss Army knives of genome editing. Nat. Struct. Mol. Biol. 24, 882–892 (2017).
Adashi, E. Y., Gruppuso, P. A. & Cohen, I. G. CRISPR Therapy of Sickle Cell Disease: The Dawning of the Gene Editing Era. Am. J. Med 137, 390–392 (2024).
Jinek, M. et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 343, 1247997 (2014).
Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759–771 (2015).
Murugan, K., Babu, K., Sundaresan, R., Rajan, R. & Sashital, D. G. The Revolution Continues: Newly Discovered Systems Expand the CRISPR-Cas Toolkit. Mol. Cell 68, 15–25 (2017).
Sundaresan, R., Parameshwaran, H. P., Yogesha, S. D., Keilbarth, M. W. & Rajan, R. RNA-Independent DNA Cleavage Activities of Cas9 and Cas12a. Cell Rep. 21, 3728–3739 (2017).
Makarova, K. S. et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol 18, 67–83 (2020).
Nishimasu, H. et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156, 935–949 (2014).
Yamano, T. et al. Crystal Structure of Cpf1 in Complex with Guide RNA and Target DNA. Cell 165, 949–962 (2016).
Babu, K. et al. Bridge Helix of Cas9 Modulates Target DNA Cleavage and Mismatch Tolerance. Biochemistry 58, 1905–1917 (2019).
Parameshwaran, H. P. et al. The bridge helix of Cas12a imparts selectivity in cis-DNA cleavage and regulates trans-DNA cleavage. FEBS Lett. 595, 892–912 (2021).
Bratovic, M. et al. Bridge helix arginines play a critical role in Cas9 sensitivity to mismatches. Nat. Chem. Biol. 16, 587–595 (2020).
Worle, E., Jakob, L., Schmidbauer, A., Zinner, G. & Grohmann, D. Decoupling the bridge helix of Cas12a results in a reduced trimming activity, increased mismatch sensitivity and impaired conformational transitions. Nucleic Acids Res. 49, 5278–5293 (2021).
Worle, E., Newman, A., D’Silva, J., Burgio, G. & Grohmann, D. Allosteric activation of CRISPR-Cas12a requires the concerted movement of the bridge helix and helix 1 of the RuvC II domain. Nucleic Acids Res. 50, 10153–10168 (2022).
Ma, E. et al. Improved genome editing by an engineered CRISPR-Cas12a. Nucleic Acids Res. 50, 12689–12701 (2022).
Dagdas, Y. S., Chen, J. S., Sternberg, S. H., Doudna, J. A. & Yildiz, A. A conformational checkpoint between DNA binding and cleavage by CRISPR-Cas9. Sci. Adv. 3, eaao0027 (2017).
Stella, S. et al. Conformational Activation Promotes CRISPR-Cas12a Catalysis and Resetting of the Endonuclease Activity. Cell 175, 1856–1871.e1821 (2018).
Strohkendl, I. et al. Cas12a domain flexibility guides R-loop formation and forces RuvC resetting. Mol. Cell 84, 2717–2731.e2716 (2024).
Stella, S., Alcon, P. & Montoya, G. Structure of the Cpf1 endonuclease R-loop complex after target DNA cleavage. Nature 546, 559–563 (2017).
Swarts, D. C., van der Oost, J. & Jinek, M. Structural Basis for Guide RNA Processing and Seed-Dependent DNA Targeting by CRISPR-Cas12a. Mol. Cell 66, 221–233.e224 (2017).
Swarts, D. C. & Jinek, M. Mechanistic Insights into the cis- and trans-Acting DNase Activities of Cas12a. Mol. Cell 73, 589–600.e584 (2019).
Jianwei, L. et al. Structures of apo Cas12a and its complex with crRNA and DNA reveal the dynamics of ternary complex formation and target DNA cleavage. PLoS Biol. 21, e3002023 (2023).
Dong, D. et al. The crystal structure of Cpf1 in complex with CRISPR RNA. Nature 532, 522–526 (2016).
Saha, A. et al. An alpha-helical lid guides the target DNA toward catalysis in CRISPR-Cas12a. Nat. Commun. 15, 1473 (2024).
Baranova, S. V. et al. Thermodynamic parameters obtained for the formation of the Cas12a-RNA/DNA complex. Biochem Biophys. Res Commun. 743, 151176 (2025).
Jeon, Y. et al. Direct observation of DNA target searching and cleavage by CRISPR-Cas12a. Nat. Commun. 9, 2777 (2018).
Naqvi, M. M., Lee, L., Montaguth, O. E. T., Diffin, F. M. & Szczelkun, M. D. CRISPR-Cas12a-mediated DNA clamping triggers target-strand cleavage. Nat. Chem. Biol. 18, 1014–1022 (2022).
Zhang, H. et al. Structural Basis for the Inhibition of CRISPR-Cas12a by Anti-CRISPR Proteins. Cell Host Microbe 25, 815–826.e814 (2019).
Babu, K. et al. Coordinated Actions of Cas9 HNH and RuvC Nuclease Domains Are Regulated by the Bridge Helix and the Target DNA Sequence. Biochemistry 60, 3783–3800 (2021).
Wu, D. et al. Structural integrity and side-chain interaction at the loop region of the bridge helix modulate Cas9 substrate discrimination. Nucleic Acids Res. 53, https://doi.org/10.1093/nar/gkaf557 (2025).
Jiang, F. et al. Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science 351, 867–871 (2016).
Jiang, F., Zhou, K., Ma, L., Gressel, S. & Doudna, J. A. STRUCTURAL BIOLOGY. A Cas9-guide RNA complex preorganized for target DNA recognition. Science 348, 1477–1481 (2015).
Yamano, T. et al. Structural Basis for the Canonical and Non-canonical PAM Recognition by CRISPR-Cpf1. Mol. Cell 67, 633–645.e633 (2017).
Patel, A., Toso, D., Litvak, A. & Nogales, E. Efficient graphene oxide coating improves cryo-EM sample preparation and data collection from tilted grids. bioRxiv, 2021.2003.2008.434344 https://doi.org/10.1101/2021.03.08.434344 (2021).
Kumar, A., Sengupta, N. & Dutta, S. Simplified Approach for Preparing Graphene Oxide TEM Grids for Stained and Vitrified Biomolecules. Nanomaterials (Basel) 11, https://doi.org/10.3390/nano11030643 (2021).
Gao, P., Yang, H., Rajashankar, K. R., Huang, Z. & Patel, D. J. Type V CRISPR-Cas Cpf1 endonuclease employs a unique mechanism for crRNA-mediated target DNA recognition. Cell Res. 26, 901–913 (2016).
Finn, R. D., Clements, J. & Eddy, S. R. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 39, W29–W37 (2011).
Holm, L. Using Dali for Protein Structure Comparison. Methods Mol. Biol. 2112, 29–42 (2020).
Shmakov, S. et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol 15, 169–182 (2017).
Sasnauskas, G. et al. TnpB structure reveals minimal functional core of Cas12 nuclease family. Nature 616, 384–389 (2023).
Tsuchida, C. A. et al. Chimeric CRISPR-CasX enzymes and guide RNAs for improved genome editing activity. Mol. Cell 82, 1199–1209.e1196 (2022).
Hino, T. et al. An AsCas12f-based compact genome-editing tool derived by deep mutational scanning and structural analysis. Cell 186, 4920–4935.e4923 (2023).
Pausch, P. et al. DNA interference states of the hypercompact CRISPR-CasPhi effector. Nat. Struct. Mol. Biol. 28, 652–661 (2021).
Carabias, A. et al. Structure of the mini-RNA-guided endonuclease CRISPR-Cas12j3. Nat. Commun. 12, 4476 (2021).
Xiao, R. et al. Structural basis of target DNA recognition by CRISPR-Cas12k for RNA-guided DNA transposition. Mol. Cell 81, 4457–4466.e4455 (2021).
Li, Z., Zhang, H., Xiao, R., Han, R. & Chang, L. Cryo-EM structure of the RNA-guided ribonuclease Cas12g. Nat. Chem. Biol. 17, 387–393 (2021).
Zhang, B. et al. Structural insights into target DNA recognition and cleavage by the CRISPR-Cas12c1 system. Nucleic Acids Res. 50, 11820–11833 (2022).
Zhang, B. et al. Mechanistic insights into the R-loop formation and cleavage in CRISPR-Cas12i1. Nat. Commun. 12, 3476 (2021).
Huang, X. et al. Structural basis for two metal-ion catalysis of DNA cleavage by Cas12i2. Nat. Commun. 11, 5241 (2020).
Sun, A. et al. The compact Caspi (Cas12l) ‘bracelet’ provides a unique structural platform for DNA manipulation. Cell Res. 33, 229–244 (2023).
Chen, J. S. et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436–439 (2018).
Cofsky, J. C. et al. CRISPR-Cas12a exploits R-loop asymmetry to form double-strand breaks. Elife 9, https://doi.org/10.7554/eLife.55143 (2020).
Martin, L., Rostami, S. & Rajan, R. Optimized protocols for the characterization of Cas12a activities. Methods Enzymol. 679, 97–129 (2023).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. Biol. Crystallogr 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. Biol. Crystallogr 66, 213–221 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D. Biol. Crystallogr 68, 352–367 (2012).
Williams, C. J. et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Webb, B. & Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinforma. 54, 5 6 1–5 6 37 (2016).
Olsson, M. H., Sondergaard, C. R., Rostkowski, M. & Jensen, J. H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput 7, 525–537 (2011).
Sondergaard, C. R., Olsson, M. H., Rostkowski, M. & Jensen, J. H. Improved Treatment of Ligands and Coupling Effects in Empirical Calculation and Rationalization of pKa Values. J. Chem. Theory Comput 7, 2284–2295 (2011).
Roe, D. R. & Cheatham, T. E. 3rd PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput 9, 3084–3095 (2013).
Case, D. A. et al. AmberTools. J. Chem. Inf. Model 63, 6183–6191 (2023).
Zgarbova, M. et al. Refinement of the Cornell et al. Nucleic Acids Force Field Based on Reference Quantum Chemical Calculations of Glycosidic Torsion Profiles. J. Chem. Theory Comput 7, 2886–2902 (2011).
Zgarbova, M. et al. Refinement of the Sugar-Phosphate Backbone Torsion Beta for AMBER Force Fields Improves the Description of Z- and B-DNA. J. Chem. Theory Comput 11, 5723–5736 (2015).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23, 127–128 (2007).
Alberto Monteiro dos Santos, Y. S., and Rakhi Rajan Bridge helix of Cas12a is an allosteric regulator of R-loop formation and RuvC activation. https://doi.org/10.5281/zenodo.17834622 (2025).
Acknowledgements
We thank the OU Protein Production and Characterization Core (PPCC) facility for protein purification services and instrument support and the Biomolecular Structure Core (BSC)-Norman for cryo-EM experimental support. The OU PPCC and BSC-Norman are supported by IDeA grants from NIGMS [grant number P20GM103640 and P30GM145423]. The data for FnoCas12a-crRNA-DNA complex was collected at Stanford-SLAC Cryo-EM Center (S2C2), which is supported by the National Institutes of Health Common Fund Transformative High-Resolution Cryo-Electron Microscopy program (grant number U24 GM129541) and National Institute of General Medical Sciences (1R24GM154186). Some of this work was performed at the National Center for CryoEM Access and Training (NCCAT) and the Simons Electron Microscopy Center located at the New York Structural Biology Center, supported by the NIH Common Fund Transformative High Resolution Cryo-Electron Microscopy program (U24 GM129539 and NIGMS R24 GM154192) and by grants from the Simons Foundation (SF349247) and NY State Assembly. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would also like to thank S2C2 members (Dr. Wah Chiu, Dr. Nathan D. Burrows, and Dr. Xu Yang) and NCCAT members (Dr. Edward Eng, Dr. Christina Zimanyi, and Dr. Aaron Owji) for their invaluable support and guidance for establishing cryo-EM studies in the Rajan laboratory. We also thank Dr. Martin Lawrence and Luke Findlay for valuable advice on cryo-EM troubleshooting. We acknowledge Dr. Fares Najar at the High-Performance Computing Center of the Oklahoma State University for building the HMM profile for the BH and RuvC-H1 region. Work reported here was supported in part by grants from the National Science Foundation [MCB-1716423 and MCB-2424888], grants from the Research Council of the University of Oklahoma Norman Campus to R.R. and through the Dodge Family College of Arts and Sciences (DFCAS) Dissertation Research Fellowship awarded to C.G. and DFCAS Dissertation Completion Fellowship awarded to L.M. The computational work was financed in part by the National Institutes of Health (NIH) grants R35GM153297 and R44GM133270 awarded to Y.S. We also acknowledge the access to the computational resources of the PETE Supercomputer provided by the High-Performance Computing Center of Oklahoma State University (OSU) and the OU Supercomputing Center for Education & Research (OSCER) at the University of Oklahoma. Financial support for publication was partly provided by the University of Oklahoma Libraries’ Open Access Fund.
Author information
Authors and Affiliations
Contributions
R.R. conceived the idea. R.R., C.G., and L.M. designed and performed the cryo-EM experiments. L.T. assisted in cryo-EM experiments and model building. C.G., S.D.A., and R.R. designed and performed the biochemical experiments. S.R.A. performed the bioinformatics and structural comparisons of BH and RuvC-H1. A.M.S. and Y.S. performed computational simulations and data analysis. All authors contributed to data analysis, manuscript writing, and editing.
Corresponding author
Ethics declarations
Competing interests
R.R. is an inventor on US patents related to FnoCas12a bridge helix variants (US11459552B2 and US12163166B2; status = granted) that were filed by the University of Oklahoma. These patents describe how amino acid substitutions in the bridge helix of Cas12a increase DNA cleavage selectivity. This manuscript presents the structure and mechanism of one of the bridge helix variants (FnoCas12a-K969P/D970P, abbreviated as FnoCas12aKD2P) that was described in the patents. All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chunyi Hu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ganguly, C., Aribam, S.D., dos Santos, A.M. et al. Bridge helix of Cas12a is an allosteric regulator of R-loop formation and RuvC activation. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68657-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68657-0