Abstract
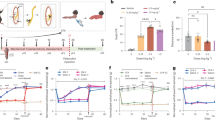
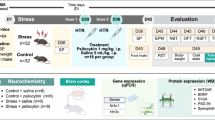
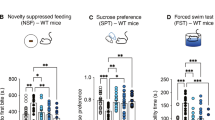
The psychedelic psilocybin may have lasting therapeutic effects for patients with chronic pain syndromes. Some preclinical data suggest these putative benefits derive from direct analgesic effects; however, this possibility has not been comprehensively tested in preclinical models. Here, we evaluated the analgesic properties of a single exposure to psilocybin at acute and chronic time points in Complete Freund’s Adjuvant-induced inflammatory pain, spared nerve injury model of neuropathic pain, and acid-induced muscle pain. Across these models, we tested a range of doses (0.3, 2, and 10 mg/kg i.p.) in male and female mice using multiple behavioral assays evaluating sensory aspects (von Frey, cold plate, Hargreaves, thermal place preference, and muscle withdrawal threshold) and functional aspects of pain (marble burying). We further tested the effects of psilocybin on the affective dimension of pain in a surgical model of acute pain (mouse grimace scale). Except for cold sensitivity, we found no effect of psilocybin across pain models, behavioral assays, drug doses, or sex. The apparent reduction in cold sensitivity may be explained by profound hypothermia induced by psilocybin rather than true analgesia.
Similar content being viewed by others
Data availability
Source data are provided with this paper. The individual sex data generated in this study are provided in Source Data 2. Source data are provided with this paper.
References
Raison, C. L. et al. Single-Dose Psilocybin treatment for major depressive disorder: a randomized clinical trial. JAMA 330, 843–853 (2023).
Goodwin, G. M. et al. Single-Dose Psilocybin for a treatment-resistant episode of major depression. N. Engl. J. Med 387, 1637–1648 (2022).
Bogenschutz, M. P. et al. Percentage of heavy drinking days following Psilocybin-Assisted Psychotherapy vs Placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry 79, 953–962 (2022).
von Rotz, R. et al. Single-dose psilocybin-assisted therapy in major depressive disorder: a placebo-controlled, double-blind, randomised clinical trial. eClinicalMedicine 56, 1–13 (2023).
Dworkin, R. H. et al. If the Doors of Perception Were Cleansed, Would Chronic Pain be Relieved? Evaluating the Benefits and Risks of Psychedelics. J. Pain. 23, 1666–1679 (2022).
Kast, E. C. & Collins, V. J. Study of Lysergic Acid Diethylamide as an Analgesic Agent. Anesth. Analges. 43, 285 (1964).
Grof, S., Goodman, L. E., Richards, W. A. & Kurland, A. A. LSD-Assisted Psychotherapy in Patients with Terminal Cancer. Int. Pharmacopsych. 8, 129–144 (1973).
Griffiths, R. R. et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 30, 1181–1197 (2016).
Ross, S. et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J. Psychopharmacol. 30, 1165–1180 (2016).
Grob, C. S. et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch. Gen. Psychiatry 68, 71–78 (2011).
Aday, J. S. et al. Preliminary safety and effectiveness of psilocybin-assisted therapy in adults with fibromyalgia: an open-label pilot clinical trial. Front. Pain Res. 6, 1–15 (2025).
Bornemann, J., Close, J. B., Spriggs, M. J., Carhart-Harris, R. & Roseman, L. Self-medication for chronic pain using classic psychedelics: a qualitative investigation to inform future research. Front. Psychiatry 12, 735427 (2021).
Bonnelle, V. et al. Analgesic potential of macrodoses and microdoses of classical psychedelics in chronic pain sufferers: a population survey. Br. J. Pain. 16, 619–631 (2022).
Glynos, N. G., Pierce, J., Davis, A. K., McAfee, J. & Boehnke, K. F. Knowledge, Perceptions, and Use of Psychedelics among Individuals with Fibromyalgia. J. Psychoact. Drugs 55, 73–84 (2023).
Ramaekers, J. G. et al. A low dose of lysergic acid diethylamide decreases pain perception in healthy volunteers. J. Psychopharmacol. 35, 398–405 (2021).
Kolbman, N. et al. Intravenous psilocybin attenuates mechanical hypersensitivity in a rat model of chronic pain. Curr. Biol. 33, R1282–R1283 (2023).
Koseli, E. et al. IUPHAR Article: Psilocybin induces long-lasting effects via 5-HT2A receptors in mouse models of chronic pain. Pharmacol. Res. 215, 107699 (2025).
Lauria, P. S. S. et al. Ayahuasca and its major component harmine promote antinociceptive effects in mouse models of acute and chronic pain. J. Ethnopharmacol. 323, 117710 (2024).
Askey, T. et al. Psilocybin ameliorates neuropathic pain-like behaviour in mice and facilitates the gabapentin-mediated analgesia. Preprint at https://doi.org/10.21203/rs.3.rs-5026806/v1 (2024).
Hammo, A., Wisser, S. & Cichon, J. Single-dose psilocybin rapidly and sustainably relieves allodynia and anxiodepressive-like behaviors in mouse models of chronic pain. Nat. Neurosci. 1–11 https://doi.org/10.1038/s41593-025-02068-0 (2025).
Rijsketic, D. R. et al. UNRAVELing the synergistic effects of psilocybin and environment on brain-wide immediate early gene expression in mice. Neuropsychopharmacology 48, 1798–1807 (2023).
Davoudian, P. A., Shao, L.-X. & Kwan, A. C. Shared and Distinct Brain Regions Targeted for Immediate Early Gene Expression by Ketamine and Psilocybin. ACS Chem. Neurosci. 14, 468–480 (2023).
Nardou, R. et al. Psychedelics reopen the social reward learning critical period. Nature 618, 790–798 (2023).
Halberstadt, A. L., Koedood, L., Powell, S. B. & Geyer, M. A. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 25, 1548–1561 (2011).
Shao, L.-X. et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109, 2535–2544.e4 (2021).
Jones, N. T. et al. Transient Elevation of Plasma Glucocorticoids Supports Psilocybin-Induced Anxiolysis in Mice. ACS Pharmacol. Transl. Sci. 6, 1221–1231 (2023).
Shahar, O. et al. Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5-Hydroxytryptophan and Psilocybin: Translational Implications. Int J. Mol. Sci. 23, 14148 (2022).
Haberzettl, R., Fink, H. & Bert, B. Role of 5-HT(1A)- and 5-HT(2A) receptors for the murine model of the serotonin syndrome. J. Pharm. Toxicol. Methods 70, 129–133 (2014).
González-Maeso, J. et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53, 439–452 (2007).
Lu, O. D. et al. A multi-institutional investigation of psilocybin’s effects on mouse behavior. 2025.04.08.647810 Preprint at https://doi.org/10.1101/2025.04.08.647810 (2025).
McCoy, E. S. et al. Development of PainFace software to simplify, standardize, and scale up mouse grimace analyses. PAIN https://doi.org/10.1097/j.pain.0000000000003187 (2022).
Matsumiya, L. C. et al. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 51, 42–49 (2012).
Skyba, D. A., Radhakrishnan, R. & Sluka, K. A. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J. Pain. 6, 41–47 (2005).
Ruskin, D. N., Sturdevant, I. C., Wyss, L. S. & Masino, S. A. Ketogenic diet effects on inflammatory allodynia and ongoing pain in rodents. Sci. Rep. 11, 725 (2021).
Heijmans, L., Mons, M. R. & Joosten, E. A. A systematic review on descending serotonergic projections and modulation of spinal nociception in chronic neuropathic pain and after spinal cord stimulation. Mol. Pain. 17, 17448069211043965 (2021).
Heifets, B. D. & Olson, D. E. Therapeutic mechanisms of psychedelics and entactogens. Neuropsychopharmacol 49, 104–118 (2024).
Glatfelter, G. C. et al. Structure–activity relationships for Psilocybin, Baeocystin, Aeruginascin, and related analogues to produce pharmacological effects in mice. ACS Pharmacol. Transl. Sci. 5, 1181–1196 (2022).
Sierra, S. et al. Sex-specific role for serotonin 5-HT2A receptor in modulation of opioid-induced antinociception and reward in mice. Neuropharmacology 209, 108988 (2022).
Mallet, C. et al. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain 139, 190–200 (2008).
Wang, X., Ratnam, J., Zou, B., England, P. M. & Basbaum, A. I. TrkB signaling is required for both the induction and maintenance of tissue and nerve injury-induced persistent pain. J. Neurosci. 29, 5508–5515 (2009).
Erkizia-Santamaría, I., Alles-Pascual, R., Horrillo, I., Meana, J. J. & Ortega, J. E. Serotonin 5-HT2A, 5-HT2c and 5-HT1A receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twitch response. Biomed. Pharmacother. 154, 113612 (2022).
Alitalo, O. et al. Linking Hypothermia and Altered Metabolism with TrkB Activation. ACS Chem. Neurosci. 14, 3212–3225 (2023).
Mogil, J. S. The translatability of pain across species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20190286 (2019).
Ayahuasca: A review of historical, pharmacological, and therapeutic aspects - Ruffell - 2023 - Psychiatry and Clinical Neurosciences Reports - Wiley Online Library. https://onlinelibrary-wiley-com.laneproxy.stanford.edu/doi/full/10.1002/pcn5.146.
Villarinho, J. G. et al. The antinociceptive effect of reversible monoamine oxidase-A inhibitors in a mouse neuropathic pain model. Prog. Neuropsychopharmacol. Biol. Psychiatry 44, 136–142 (2013).
Schindler, E. A. D. Psychedelics as preventive treatment in headache and chronic pain disorders. Neuropharmacology 215, 109166 (2022).
Jevotovsky, D. S. et al. Psilocybin and chronic neuropathic pain: a systematic review. Reg. Anesth. Pain Med. https://doi.org/10.1136/rapm-2024-105532 (2024).
Donovan, L. J. et al. Repopulated spinal cord microglia exhibit a unique transcriptome and contribute to pain resolution. Cell Rep. 43, 113683 (2024).
Cavarra, M. et al. Potential analgesic effects of psychedelics on select chronic pain conditions: A survey study. Eur. J. Pain. 28, 153–165 (2024).
Cichon, J., Sun, L. & Yang, G. Spared Nerve Injury Model of Neuropathic Pain in Mice. Bio Protoc. 8, e2777 (2018).
Wang, D. et al. Functional Divergence of Delta and Mu Opioid Receptor Organization in CNS Pain Circuits. Neuron 98, 90–108.e5 (2018).
Ruan, Y. et al. An effective and concise device for detecting cold allodynia in mice. Sci. Rep. 8, 14002 (2018).
Ram, A. et al. GPR171 Agonist Reduces Chronic Neuropathic and Inflammatory Pain in Male, But Not Female Mice. Front. Pain Res. 2, 1–13 (2021).
Sluka, K. A., Kalra, A. & Moore, S. A. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 24, 37–46 (2001).
Durst, M. S., Arras, M., Palme, R., Talbot, S. R. & Jirkof, P. Lidocaine and bupivacaine as part of multimodal pain management in a C57BL/6J laparotomy mouse model. Sci. Rep. 11, 10918 (2021).
Modi, A. D., Parekh, A. & Pancholi, Y. N. Evaluating pain behaviours: Widely used mechanical and thermal methods in rodents. Behav. Brain Res. 446, 114417 (2023).
Pereira, T. D. et al. SLEAP: A deep learning system for multi-animal pose tracking. Nat. Methods 19, 486–495 (2022).
Goodwin, N. L. et al. Simple Behavioral Analysis (SimBA) as a platform for explainable machine learning in behavioral neuroscience. Nat. Neurosci. 27, 1411–1424 (2024).
Acknowledgements
We thank the entire Heifets, Tawfik, and Malenka Labs for helpful discussions, and the NIDA Drug Supply Program for supplying psilocybin. This work was supported by a Mentored Research Award from the International Anesthesia Research Society (N.S.G.), NIH grant T32DA035165 (A.R.) NIH grant P50 DA042012 (B.D.H. and R.C.M.), funds from the Department of Anesthesiology, Perioperative & Pain Medicine at Stanford University (V.L.T and B.D.H.), and a grant from the Stanford University Wu Tsai Neurosciences Institute (R.C.M.).
Author information
Authors and Affiliations
Contributions
Nicholas S. Gregory (N.S.G.) designed and performed experiments (CFA, SNI, AIMP, laparotomy models; von Frey, cold plate, Hargreaves, thermal place preference, muscle withdrawal threshold, mouse grimace scale), analyzed the data, interpreted the results, and wrote the manuscript; Tyler E. Girard (T.E.G.) performed experiments (cold plate, marble burying, body temperature tracking), analyzed data, and contributed to the manuscript; Akila Ram (A.R.) performed experiments (morphine controls, von Frey, Hargreaves) and contributed to the manuscript; Austen B. Casey (A.B.C.) performed experiments (rearing, head twitch) and contributed to the manuscript; Robert C. Malenka (R.C.M.) analyzed data and interpreted results; Vivianne L. Tawfik (V.L.T.) conceived of the study, designed experiments, interpreted results, and revised the manuscript; Boris D. Heifets (B.D.H.) conceived of the study, designed experiments, analyzed data, interpreted results, and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.D.H. is on the scientific advisory boards of Journey Clinical and Osmind, and is a paid consultant to Arcadia Medicine, Inc, Tactogen, LLC, and Vida Ventures, LLC. R.C.M. is now on leave from Stanford, functioning as Chief Scientific Officer at Bayshore Global Management. R.C.M. is on the scientific advisory boards of MapLight Therapeutics, Bright Minds, MindMed, and Aelis Farma. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gregory, N.S., Girard, T.E., Ram, A. et al. No evidence of immediate or persistent analgesic effect from a single dose of psilocybin in three mouse models of pain. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68763-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68763-z