Abstract
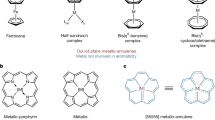
Metallocene research has influenced the development of organometallic chemistry, and the metal–nonmetal inverted half-sandwich structure (inverse metallocene) was very recently discovered. However, additional instances are required to propose the concept inverse metallocene and it remains uncertain whether metal five-membered ring structures analogous to the cyclopentadienyl anion structure exist. Herein we report the synthesis of a series of palladium analogs Pd8(PPh)2(PPh3)2(Ph2P=O)(S-Adm)5 (Pd8–P), Pd6(PPh)(PPh3)(S-Adm)6 (Pd6), Pd5(PPh)(S-Adm)4[(Ph2P)2O] (Pd5–O), and Pd5(PPh)(S-Adm)4[(Ph2P)2CH2] (Pd5–C), identify their fundamental metal building block named five-membered Pd aromatic ring, and reveal their conjugation‒photothermy correlation. In particular, we report an average NIR-II photothermal conversion efficiency per metal atom of 14.7% and the stability of the five-membered Pd aromatic ring, as illustrated by the fact that Pd5–C maintained photothermy performance for >10 heating–cooling cycles even after the ligands were removed. We further demonstrate the great potential of inverse palladocenes in areas such as laser shielding, high-temperature degradation, ignition, and temperature/light control. These results declare the research and application start of a type of materials named inverse palladocenes.
Similar content being viewed by others
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers 2500802 for Pd5–C, 2500805 for Pd5–O, 2500806 for Pd6, 2500807 for Pd8–P, and 2500808 for (Ph2P=O)2, respectively. These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif. Checkcif file for Pd5–C, Pd5–O, Pd6, Pd8–P, and (Ph2P=O)2 CIF files and Supplementary Movie 1 are given as Supplementary Dataset. All data supporting the findings of this study are available within the article and its Supplementary Information files. All data are available from the corresponding author upon request. Source data are provided with this paper.
References
Kealy, T. J. & Pauson, P. L. A new type of organo-iron compound. Nature 168, 1039–1040 (1951).
Wilkinson, G., Rosenblum, M., Whiting, M. C. & Woodward, R. B. The structure of iron bis-cyclopentadienyl. J. Am. Chem. Soc. 74, 2125–2126 (1952).
Miller, S. A., Tebboth, J. A. & Tremaine, J. F. Dicyclopentadienyliron. J. Chem. Soc. 114, 632–635 (1952).
Werner, H. At least 60 years of ferrocene: the discovery and rediscovery of the sandwich complexes. Angew. Chem. Int. Ed. 51, 6052–6058 (2012).
Consiglio, G. & Morandini, F. Half-sandwich chiral ruthenium complexes. Chem. Rev. 87, 761–778 (1987).
Green, M. L. H. & Ng, D. K. P. Cycloheptatriene and -enyl complexes of the early transition metals. Chem. Rev. 95, 439–473 (1995).
Arndt, S. & Okuda, J. Mono(cyclopentadienyl) complexes of the rare-earth metals. Chem. Rev. 102, 1953–1976 (2002).
Goodwin, C. A. P. et al. Isolation and electronic structures of derivatized manganocene, ferrocene and cobaltocene anions. Nat. Chem. 13, 243–248 (2021).
Siemeling, U. Chelate complexes of cyclopentadienyl ligands bearing pendant O-donors. Chem. Rev. 100, 1495–1526 (2000).
van Staveren, D. R. & Metzler-Nolte, N. Bioorganometallic chemistry of ferrocene. Chem. Rev. 104, 5931–5986 (2004).
Theys, R. D., Dudley, M. E. & Hossain, M. M. Recent chemistry of the η5-cyclopentadienyl dicarbonyl iron anion. Coord. Chem. Rev. 253, 180–234 (2009).
Patra, M. & Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 1, 0066 (2017).
Mas-Roselló, J., Herraiz, A. G., Audic, B., Laverny, A. & Cramer, N. Chiral cyclopentadienyl ligands: design, syntheses, and applications in asymmetric catalysis. Angew. Chem. Int. Ed. 60, 13198–13224 (2021).
Kharitonov, V. B., Muratov, D. V. & Loginov, D. A. Cyclopentadienyl complexes of group 9 metals in the total synthesis of natural products. Coord. Chem. Rev. 471, 214744 (2022).
You, Q. et al. Pd8 nanocluster with nonmetal-to-metall- ring coordination and promising photothermal conversion efficiency. Angew. Chem. Int. Ed. 63, e202313491 (2024).
Murahashi, T. et al. Discrete sandwich compounds of monolayer palladium sheets. Science 313, 1104–1107 (2006).
Sunada, Y., Haige, R., Otsuka, K., Kyushin, S. & Nagashima, H. A ladder polysilane as a template for folding palladium nanosheets. Nat. Commun. 4, 2014 (2013).
Li, S., Li, N.-N., Dong, X.-Y., Zang, S.-Q. & Mak, T. C. W. Chemical flexibility of atomically precise metal clusters. Chem. Rev. 124, 7262–7378 (2024).
Yao, Q. et al. Molecule-like synthesis of ligand-protected metal nanoclusters. Nat. Rev. Mater. 10, 89–108 (2025).
Jadzinsky, P. D., Calero, G., Ackerson, C. J., Bushnell, D. A. & Kornberg, R. D. Structure of a thiol monolayer–protected gold nanoparticle at 1.1 Å resolution. Science 318, 430–433 (2007).
Desireddy, A. et al. Ultrastable silver nanoparticles. Nature 501, 399–402 (2013).
Narouz, M. R. et al. N-heterocyclic carbene-functionalized magic-number gold nanoclusters. Nat. Chem. 11, 419–425 (2019).
Li, Y. et al. Double-helical assembly of heterodimeric nanoclusters into supercrystals. Nature 594, 380–384 (2021).
Li, Q. et al. Mechanical nanolattices printed using nanocluster-based photoresists. Science 378, 768–773 (2022).
Ma, F., Abboud, K. A. & Zeng, C. Precision synthesis of a CdSe semiconductor nanocluster via cation exchange. Nat. Synth. 2, 949–959 (2023).
Wang, X. et al. Ligand-protected metal nanoclusters as low-loss, highly polarized emitters for optical waveguides. Science 381, 784–790 (2023).
Yonesato, K. et al. Surface-exposed silver nanoclusters inside molecular metal oxide cavities. Nat. Chem. 15, 940–947 (2023).
Shi, W.-Q. et al. Near-unity NIR phosphorescent quantum yield from a room-temperature solvated metal nanocluster. Science 383, 326–330 (2024).
Pei, X.-L. et al. Single-gold etching at the hypercarbon atom of C-centred hexagold(I) clusters protected by chiral N-heterocyclic carbenes. Nat. Commun. 15, 5024 (2024).
Tang, L. et al. Structure and optical properties of an Ag135Cu60 nanocluster incorporating an Ag135 buckminsterfullerene-like topology. Nat. Synth. 4, 506–513 (2025).
Jung, H. S. et al. Organic molecule-based photothermal agents: an expanding photothermal therapy universe. Chem. Soc. Rev. 47, 2280–2297 (2018).
Fang, L. et al. Sandwich-kernelled AgCu nanoclusters with golden ratio geometry and promising photothermal efficiency. Angew. Chem. Int. Ed. 62, e202305604 (2023).
Yan, T., Su, M., Wang, Z. & Zhang, J. Second near-infrared plasmonic nanomaterials for photoacoustic imaging and photothermal therapy. Small 19, 2300539 (2023).
Gu, W. et al. Concomitant near-infrared photothermy and photoluminescence of rod-shaped Au52(PET)32 and Au66(PET)38 synthesized concurrently. Angew. Chem. Int. Ed. 63, e202407518 (2024).
Wang, R. et al. Atomically precise nanometer-sized Pt catalysts with an additional photothermy functionality. Angew. Chem. Int. Ed. 63, e202402565 (2024).
Tang, J. et al. Selective hydrogenation of alkyne by atomically precise Pd6 nanocluster catalysts: Accurate construction of the coplanar and specific active sites. ACS Catal. 14, 2463–2472 (2024).
Ambreen, A. et al. Single thiolate replacement of metal nanoclusters. Sci. China Chem. 67, 523–528 (2024).
Zhuang, S. et al. Thiolated, reduced palladium nanoclusters with resolved structures for the electrocatalytic reduction of oxygen. Angew. Chem. Int. Ed. 61, e202208751 (2022).
Liu, Q. & Zhao, L. Low valent palladium clusters: synthesis, structures and catalytic applications. Chin. J. Chem. 38, 1897–1908 (2020).
Bastiansen, O. & Cyvin, S. J. Molecular vibrations and standard deviation of interatomic distances in benzene. Nature 180, 980–981 (1957).
Ingraham, K. A., Remy, C. D. & Rouse, E. J. The role of user preference in the customized control of robotic exoskeletons. Science 7, eabj3487 (2022).
Liao, Y. et al. Theoretical study on the optoelectronic properties of electron-withdrawing substituted diethynylfluorenyl gold(I) complexes. J. Phys. Chem. A 110, 13036–13044 (2006).
Gleiter, R. & Gebhard, H. Aromaticity and Other Conjugation Effects (Wiley-VCH, 2012).
Chen, W.-X. et al. Capturing aromatic Cr5 pentagons in large main-group molecular cages. Nat. Synth. 4, 471–478 (2025).
Rienmüller, J. et al. Isolation of a planar π-aromatic Bi5− ring in a cobalt-based inverse-sandwich-type complex. Nat. Chem. 17, 547–555 (2025).
Hunter, C. A. & Sanders, J. K. M. The nature of π‒π interactions. J. Am. Chem. Soc. 112, 5525–5534 (1990).
Johnson, G. et al. Nanocluster superstructures assembled via surface ligand switching at high temperature. Nat. Synth. 2, 828–837 (2023).
Li, H., Kang, X. & Zhu, M. Superlattice assembly for empowering metal nanoclusters. Acc. Chem. Res. 57, 3194–3205 (2024).
Zhao, H. et al. Assembly of air-stable copper(I) alkynide nanoclusters assisted by tripodal polydentate phosphoramide ligands. Nat. Synth. 3, 517–526 (2024).
Zhai, X.-J. et al. Hierarchical assembly of Ag40 nanowheel ranging from building blocks to diverse superstructure regulation. Nat. Commun. 15, 9155 (2024).
Li, Y. et al. Self-assembly of chiroptical ionic co-crystals from silver nanoclusters and organic macrocycles. Nat. Chem. 17, 169–176 (2025).
Patil, A. O., Heeger, A. J. & Wudl, F. Optical properties of conducting polymers. Chem. Rev. 88, 183–200 (1988).
Friend, R. H. et al. Electroluminescence in conjugated polymers. Nature 397, 121–128 (1999).
Rumi, M. et al. Structure−property relationships for two-photon absorbing chromophores: bis-donor diphenylpolyene and bis(styryl)benzene derivatives. J. Am. Chem. Soc. 122, 9500–9510 (2000).
Zhuang, S. et al. Kernel homology in gold nanoclusters. Angew. Chem. Int. Ed. 57, 15450–15454 (2018).
Li, Y. et al. Atomically precise Au42 nanorods with longitudinal excitons for an intense photothermal effect. J. Am. Chem. Soc. 144, 12381–12389 (2022).
Feng, L. et al. Long-pursued structure of Au23(S-Adm)16 and the unexpected doping effects. Acta Phys. Chim. Sin. 40, 2305029 (2024).
Wu, Z., Suhan, J. & Jin, R. One-pot synthesis of atomically monodisperse, thiol-functionalized Au25 nanoclusters. J. Mater. Chem. 19, 622–626 (2009).
Breitwieser, K. et al. Pd8(PDip)6: cubic, unsaturated, zerovalent. Adv. Sci. 11, 2400699 (2024).
Cook, A. W., Hrobarik, P., Damon, P. L., Wu, G. & Hayton, T. W. A ketimide-stabilized palladium nanocluster with a hexagonal aromatic Pd7 core. Inorg. Chem. 59, 1471–1480 (2020).
Hooper, T. N. et al. The partial dehydrogenation of aluminium dihydrides. Chem. Sci. 10, 8083–8093 (2019).
Neese, F. Software update: the ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 8, e1327 (2018).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 111, 6158–6170 (1999).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Glendening, E. D., Landis, C. R. & Weinhold, F. NBO 7.0: new vistas in localized and delocalized chemical bonding theory. J. Comput. Chem. 40, 2234–2241 (2019).
Zubarev, D. Y. & Boldyrev, A. I. Developing paradigms of chemical bonding: adaptive natural density partitioning. Phys. Chem. Chem. Phys. 10, 5207–5217 (2008).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Becke, A. D. & Edgecombe, K. E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 92, 5397–5403 (1990).
Michalak, A., Mitoraj, M. & Ziegler, T. Bond orbitals from chemical valence theory. J. Phys. Chem. A. 112, 1933–1939 (2008).
Mitoraj, M. P., Michalak, A. & Ziegler, T. A combined charge and energy decomposition scheme for bond analysis. J. Chem. Theory Comput. 5, 962–975 (2009).
te Velde, G. et al. Chemistry with ADF. J. Comput. Chem. 22, 931–967 (2001).
Van Lenthe, E. & Baerends, E. J. Optimized slater-type basis sets for the elements 1-118. J. Comput. Chem. 24, 1142–1156 (2003).
van Lenthe, E., van Leeuwen, R., Baerends, E. J. & Snijders, J. G. Relativistic regular two-component hamiltonians. Int. J. Quantum Chem. 57, 281–293 (1996).
Johnson, E. R. et al. Revealing noncovalent interactions. J. Am. Chem. Soc. 132, 6498–6506 (2010).
Contreras-García, J. et al. NCIPLOT: a program for plotting noncovalent interaction regions. J. Chem. Theory Comput. 7, 625–632 (2011).
Humphrey, W., Dalke, A. & Schulten, K. V. M. D. Visual molecular dynamics. J. Mol. Graphics 14, 33–38 (1996).
Perdew, J. P., Burke, K. & Emzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Andrae, D., Häußermann, U., Dolg, M., Stoll, H. & Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta. 77, 123–141 (1990).
Bergner, A., Dolg, M., Küchle, W., Stoll, H. & Preuß, H. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys. 80, 1431–1441 (1993).
Petersson, G. A. et al. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 89, 2193–2218 (1988).
Petersson, G. A. & Al-Laham, M. A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 94, 6081–6090 (1991).
Pritchard, B. P., Altarawy, D., Didier, B., Gibson, T. D. & Windus, T. L. New basis set exchange: an open, up-to-date resource for the molecular sciences community. J. Chem. Inf. Model. 59, 4814–4820 (2019).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 32, 1456–1465 (2011).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Gaussian 16 Revision C. 01 (Gaussian Inc., 2016).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 22471275, 22403096, 21925303, 21829501, 22033005, 22171267, 21771186, 21222301, 21171170 and 21528303), to Q.Y., J.L., and Z.W., the National Key Research and Development Program of China (No. 2022YFA1503900), the Guangdong Provincial Key Laboratory of Catalysis (No. 2020B121201002), the NSFC Center for Single-Atom Catalysis (22388102) to J.L., and the Anhui Provincial Natural Science Foundation 2408085QB040 to Q.Y. Computational resources were supported by the Center for Computational Science and Engineering at Southern University of Science and Technology and the CHEM high-performance supercomputer cluster (CHEM-HPC) located at department of chemistry, SUSTech.
Author information
Authors and Affiliations
Contributions
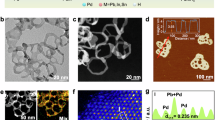
Z.W and J.L. conceived the initial idea and managed the overall project. Q.Y. designed and performed the experiments. X.-L.J. performed the theoretical calculations and conducted the bonding analysis under the supervision of J.L. Q.Y. performed the electronic transition analyses using TD-DFT calculations. Y.Z. collected and analyzed the XPS, DPV, SEM, and TEM. data with the assistance of W.G., Q.Y., and Z.W. wrote the paper with the assistance of X.-L.J. and J.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Tokuhisa Kawawaki, Qingming Shen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
You, Q., Jiang, XL., Zhao, Y. et al. Inverse palladocenes. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68955-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68955-7