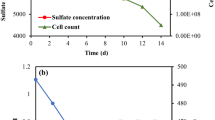
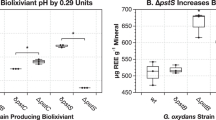
Abstract
The growing demand for rare earth elements (REEs) in clean energy and high-tech industries underscores the need for sustainable recovery methods and a reliable supply of processing chemicals. Here, we establish a microbial platform using the acid-tolerant yeast Issatchenkia orientalis SD108 to produce bio-oxalic acid for REE recovery. By introducing an oxaloacetate cleavage pathway and applying metabolic engineering, the engineered strain produces 39.53 g·L-1 oxalic acid at pH 4.0 in fed-batch fermentation. The crude fermentation broth, used without purification, efficiently precipitates over 99% neodymium (Nd), 99% dysprosium (Dy), and 98% lanthanum (La) from individual REE chloride solutions. Recovery from a low-grade ore leachate achieves over 99% total recovery. X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) confirm that REE oxalates precipitated with bio-oxalic acid closely resemble those obtained using commercial oxalic acid. Techno-economic analysis (TEA) and life cycle assessment (LCA) further demonstrate that bio-oxalic acid can be produced at a competitive price of $1.79·kg-1 while reducing carbon intensity (CI) by 112% to 63.5% with and without electricity displacement, respectively, relative to the fossil-based benchmark. These results highlight bio-oxalic acid as a green, economically viable alternative to synthetic oxalate for sustainable REE recovery.
Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper, its Supplementary Information files, and the Source Data file. Source data are provided with this paper.
Code availability
Python scripts for BioSTEAM and the biomanufacturing facility design as well as analyses can be found on Github at https://github.com/BioSTEAMDevelopmentGroup/Bioindustrial-Park/tree/master/biorefineries/oxalic64.
References
Tyler, G. Rare earth elements in soil and plant systems-A review. Plant Soil 267, 191–206 (2004).
Gielen, D. & Lyons, M. Critical materials for the energy transition: Rare earth elements. International Renewable Energy Agency, Abu Dhabi, 1-48 (2022).
Zhanheng, C. Global rare earth resources and scenarios of future rare earth industry. J. Rare Earths 29, 1–6 (2011).
Han, K. N. Characteristics of precipitation of rare earth elements with various precipitants. Minerals 10, 178 (2020).
Chi, R. & Xu, Z. A solution chemistry approach to the study of rare earth element precipitation by oxalic acid. Metall. Mater. Trans. B 30, 189–195 (1999).
Nawab, A., Yang, X. & Honaker, R. Parametric study and speciation analysis of rare earth precipitation using oxalic acid in a chloride solution system. Miner. Eng. 176, 107352 (2022).
Silva, R. G., Morais, C. A., Teixeira, L. V. & Oliveira, ÉD. Selective precipitation of high-quality rare earth oxalates or carbonates from a purified sulfuric liquor containing soluble impurities. Min. Metall. Explor. 36, 967–977 (2019).
Amenaghawon, A. N. et al. A comprehensive review of recent advances in the applications and biosynthesis of oxalic acid from bio-derived substrates. Environ. Res., 118703 (2024).
Precedence Research. Oxalic Acid Market Size, Share, and Trends 2024 to 2034. https://www.precedenceresearch.com/oxalic-acid-market (2024).
Schuler, E., Demetriou, M., Shiju, N. R. & Gruter, G. J. M. Towards sustainable oxalic acid from CO2 and biomass. ChemSusChem 14, 3636–3664 (2021).
Liu, H. et al. Recent advances and perspectives on production of value-added organic acids through metabolic engineering. Biotechnol. Adv. 62, 108076 (2023).
Grąz, M. Role of oxalic acid in fungal and bacterial metabolism and its biotechnological potential. World J. Microbiol. Biotechnol. 40, 178 (2024).
Strasser, H., Burgstaller, W. & Schinner, F. High-yield production of oxalic acid for metal leaching processes by Aspergillus niger. FEMS Microbiol. Lett. 119, 365–370 (1994).
Mandal, S. K. & Banerjee, P. C. Submerged production of oxalic acid from glucose by immobilized Aspergillus niger. Process Biochem 40, 1605–1610 (2005).
Bohlmann, J., Cameselle, C., Nunez, M. & Lema, J. Oxalic acid production by Aspergillus niger: Part II: Optimisation of fermentation with milk whey as carbon source. Bioprocess Eng 19, 337–342 (1998).
Brown, K., Harrison, J. & Bowers, K. Production of oxalic acid from Aspergillus niger and whey permeate. Water Air Soil Pollut 229, 1–10 (2018).
Rymowicz, W. & Lenart, D. Oxalic acid production from lipids by a mutant of Aspergillus niger at different pH. Biotechnol. Lett. 25, 955–958 (2003).
Paul, G., Priede, M. & Thomas, C. Relationship between morphology and citric acid production in submerged Aspergillus niger fermentations. Biochem. Eng. J. 3, 121–129 (1999).
Kheirkhah, T., Neubauer, P. & Junne, S. Controlling Aspergillus niger morphology in a low shear-force environment in a rocking-motion bioreactor. Biochem. Eng. J. 195, 108905 (2023).
Tan, S.-I., Liu, Z., Tran, V. G., Martin, T. A. & Zhao, H. Issatchenkia orientalis as a platform organism for cost-effective production of organic acids. Metab. Eng. (2025).
Li, Q.-Z. et al. Recovery processes of organic acids from fermentation broths in the biomass-based industry. J. Microbiol. Biotechnol. (2016).
Xiao, H., Shao, Z., Jiang, Y., Dole, S. & Zhao, H. Exploiting Issatchenkia orientalis SD108 for succinic acid production. Microb. cell fact. 13, 1–11 (2014).
Wu, Z.-Y. et al. Metabolic engineering of low-pH-tolerant non-model yeast, Issatchenkia orientalis, for production of citramalate. Metab. Eng. Commun. 16, e00220 (2023).
Tran, V. G. et al. An end-to-end pipeline for succinic acid production at an industrially relevant scale using Issatchenkia orientalis. Nat. Commun. 14, 6152 (2023).
Park, H. J. et al. Low-pH production of d-lactic acid using newly isolated acid tolerant yeast Pichia kudriavzevii NG7. Biotechnol. Bioeng. 115, 2232–2242 (2018).
Xi, Y. et al. Metabolic engineering of the acid-tolerant yeast Pichia kudriavzevii for efficient L-malic acid production at low pH. Metab. Eng. 75, 170–180 (2023).
Cao, M. et al. A genetic toolbox for metabolic engineering of Issatchenkia orientalis. Metab. Eng. 59, 87–97 (2020).
Tran, V. G., Cao, M., Fatma, Z., Song, X. & Zhao, H. Development of a CRISPR/Cas9-based tool for gene deletion in Issatchenkia orientalis. Msphere 4, 00345–00319 (2019).
Fatma, Z., Tan, S.-I., Boob, A. G. & Zhao, H. A landing pad system for multicopy gene integration in Issatchenkia orientalis. Metab. Eng. 78, 200–208 (2023).
Kobayashi, K., Hattori, T., Honda, Y. & Kirimura, K. Oxalic acid production by citric acid-producing Aspergillus niger overexpressing the oxaloacetate hydrolase gene oahA. J. Ind. Microbiol. Biotechnol. 41, 749–756 (2014).
Akamatsu, Y. & Shimada, M. Partial purification and characterization of glyoxylate oxidase from the brown-rot basidiomycete Tyromyces palustris. Phytochemistry 37, 649–653 (1994).
Munir, E., Yoon, J. J., Tokimatsu, T., Hattori, T. & Shimada, M. A physiological role for oxalic acid biosynthesis in the wood-rotting basidiomycete Fomitopsis palustris. Proc. Natl. Acad. Sci. USA. 98, 11126–11130 (2001).
Li, W. et al. Biosynthesis of plant hemostatic dencichine in Escherichia coli. Nat. Commun. 13, https://doi.org/10.1038/s41467-022-33255-3 (2022).
Watanabe, T. et al. Oxalate efflux transporter from the brown rot fungus Fomitopsis palustris. Applied and environmental microbiology 76, 7683–7690 (2010).
Yang, Z. et al. Cloning, Expression Analysis, and Functional Characterization of Candidate Oxalate Transporter Genes of HbOT1 and HbOT2 from Rubber Tree (Hevea brasiliensis). Cells 11, 3793 (2022).
Qin, N. et al. Flux regulation through glycolysis and respiration is balanced by inositol pyrophosphates in yeast. Cell 186, 748–763 e715 (2023).
Xiao, Z. & Zhang, W. Review of allanite: Properties, occurrence and mineral processing technologies. Green Smart Min. Eng. (2024).
Research Nester. Oxalic Acid Market Size & Share, by Application (Bleaching/Cleaning, Pharmaceuticals, Water Treatment, Textile Dyeing, Metal Leaching, Others) - Global Supply & Demand Analysis, Growth Forecasts, Statistics Report 2025-2037. https://www.researchnester.com/reports/oxalic-acid-market/7493 (2025).
U.S. Department of Agriculture, Agricultural Marketing Service. Oxalic acid dihydrate https://www.ams.usda.gov/rules-regulations/organic/petitioned-substances/oxalic-acid-dihydrate (2025).
Cameselle, C., Bohlmann, J., Nunez, M. & Lema, J. Oxalic acid production by Aspergillus niger: Part I: Influence of sucrose and milk whey as carbon source. Bioprocess Eng 19, 247–252 (1998).
Walaszczyk, E., Podgórski, W., Janczar-Smuga, M. & Dymarska, E. Effect of medium pH on chemical selectivity of oxalic acid biosynthesis by Aspergillus niger W78C in submerged batch cultures with sucrose as a carbon source. Chem. Pap. 72, 1089–1093 (2018).
Musiał, I., Cibis, E. & Rymowicz, W. Designing a process of kaolin bleaching in an oxalic acid enriched medium by Aspergillus niger cultivated on biodiesel-derived waste composed of glycerol and fatty acids. Appl. Clay Sci. 52, 277–284 (2011).
André, A. et al. Biotechnological conversions of bio-diesel derived waste glycerol into added-value compounds by higher fungi: production of biomass, single cell oil and oxalic acid. Ind. Crops Prod. 31, 407–416 (2010).
Betiku, E., Emeko, H. A. & Solomon, B. O. Fermentation parameter optimization of microbial oxalic acid production from cashew apple juice. Heliyon 2 (2016).
Alibaba.com. High-quality oxalic acid CAS 144-62-7. https://www.alibaba.com/product-detail/High-quality-Oxalic-acid-CAS-144_1600467879003.html?spm=a2700.7724857.0.0.737f3dddErELOM (2025).
https://ecoinvent.org/ (2025). ecoinvent. ecoinvent.
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Gietz, R. D., Schiestl, R. H., Willems, A. R. & Woods, R. A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11, 355–360 (1995).
Havel, J., Moreno, C., Hrdlička, A. & Valiente, M. Spectrophotometric determination of rare earth elements by flow injection analysis based on their reaction with xylenol orange and cetylpyridinium bromide. Talanta 41, 1251–1254 (1994).
Cortes-Pena, Y., Kumar, D., Singh, V. & Guest, J. S. BioSTEAM: a fast and flexible platform for the design, simulation, and techno-economic analysis of biorefineries under uncertainty. ACS Sustain. Chem. Eng. 8, 3302–3310 (2020).
Cortés-Peña, Y. Thermosteam: BioSTEAM’s premier thermodynamic engine. J. Open Source Softw. 5, 2814 (2020).
Lynas Rare Earths. U.S. project update. https://lynasrareearths.com/u-s-project-update/ (2025).
MP Materials. Mountain Pass. https://mpmaterials.com/mountain-pass (2025).
ISO. ISO 14040:2006(en) Environmental management — Life cycle assessment — Principles and framework. https://www.iso.org/obp/ui/#iso:std:iso:14040:ed-2:v1:en (2006).
ISO. ISO 14044: Environmental Management, Life Cycle Assessment, Requirements, and Guidelines. https://www.iso.org/standard/38498.html (2006).
California Air Resources Board. LCFS Pathway Certified Carbon Intensities. https://ww2.arb.ca.gov/resources/documents/lcfs-pathway-certified-carbon-intensities (2025).
Canadian Fuels Association. Clean Fuel Regulations. https://www.canadianfuels.ca/industry-facts/low-carbon-fuels/clean-fuel-regulations (2025).
Government of Canada. Clean Fuel Regulations (SOR 2022-140). laws-lois.justice.gc.ca/eng/regulations/SOR-2022-140/page-6.html#h-1359334 (2022).
European Commission Joint Research Centre. Renewable Energy - Recast to 2030 (RED II). https://joint-research-centre.ec.europa.eu/welcome-jec-website/reference-regulatory-framework/renewable-energy-recast-2030-red-ii_en (2025).
Ahlgren, S. & Di Lucia, L. Indirect land use changes of biofuel production–a review of modelling efforts and policy developments in the European Union. Biotechnol. Biofuels 7, 35 (2014).
Lee, H. et al. IPCC, 2023: Climate change 2023: Synthesis report, summary for policymakers. Contribution of working groups i, II and III to the sixth assessment report of the intergovernmental panel on climate change [core writing team, H. Lee and J. Romero (Eds.)]. IPCC, Geneva, Switzerland. (2023).
Ecoinvent. ecoinvent cutoff system model, version 3.8. https://ecoquery.ecoinvent.org/3.8/cutoff (2025).
Argonne National Laboratory. GREET model. https://greet.anl.gov/ (2025).
Yoel et al. Bio-based Oxalic Acid Production in Issatchenkia orientalis Enables Sustainable Rare Earth Recovery. BioSTEAMDevelopmentGroup/Bioindustrial-Park: Jan2026, https://doi.org/10.5281/zenodo.18234694 (2026).
Acknowledgements
This work was funded by the DARPA Environmental Microbes as a Bioengineering Resource (EMBER) program (contract DE-AC52-07NA27344) (R.H., Y.J., and H.Z.). Distribution Statement A. Approved for public release: distribution is unlimited. The views, opinions, and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government. Work at LLNL was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DEAC52-07NA27344 (LLNL-JRNL-2014570). The online tool BioRender (biorender.com) was used to create Fig. 1 and Fig. 2a. We thank Vinh G. Tran from University of Illinois Urbana-Champaign for discussion about the metabolic engineering design, Keith D. Morrison from Lawrence Livermore National Laboratory for his assistance with X-ray diffraction data collection, and Forrest Dills form University of Kentucky for his assistance for precipitation test.
Author information
Authors and Affiliations
Contributions
J.L. and H.Z. conceived and designed the study. J.L. performed all experiments related to the metabolic engineering and fermentation. Z.D., Y.J. and D.P. performed the precipitation test, structural and chemical analyses via X-ray diffraction and Fourier transform infrared spectroscopy. A.J. and R.H. performed the precipitation test using the allanite leachate as substrate. W.G. and S.B. performed biomanufacturing facility design, modeling, techno-economic analysis, and life cycle assessment. S.T. and Z.Z. assisted with the construction of plasmids. J.L., W.G., Z.D., S.B., J.G. and H.Z. wrote the manuscript with input from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declared no competing interests.
Peer review
Peer review information
Nature Communications thanks Baskar Gurunathan, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, J., Guo, W., Dong, Z. et al. Bio-based oxalic acid production in Issatchenkia orientalis enables sustainable rare earth recovery. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68957-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68957-5