Abstract
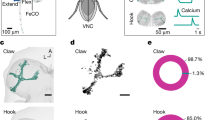
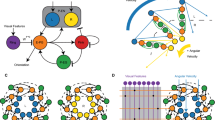
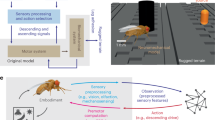
Many animals possess mechanosensory neurons that fire when a limb nears the limit of its physical range, but the function of these proprioceptive limit detectors remains poorly understood. Here, we investigate a class of proprioceptors on the Drosophila leg called hair plates. Using calcium imaging in behaving flies, we find that a hair plate on the fly coxa (CxHP8) detects the limits of anterior leg movement. By reconstructing CxHP8 axons in an electron microscopy dataset, we found that they are wired to excite posterior leg movement and inhibit anterior leg movement. Consistent with this connectivity, optogenetic activation of CxHP8 neurons elicited posterior postural reflexes, while silencing altered the swing-to-stance transition during walking. Finally, we use comprehensive reconstruction of peripheral morphology and downstream connectivity to predict the function of other hair plates distributed across the fly leg. Our results suggest that each hair plate is specialized to control specific sensorimotor reflexes that are matched to the joint limit it detects. They also illustrate the feasibility of predicting sensorimotor reflexes from a connectome with identified proprioceptive inputs and motor outputs.
Similar content being viewed by others
Data availability
The calcium imaging, optogenetic, and treadmill kinematic silencing datasets generated in this study have been deposited in the Dryad database under https://doi.org/10.5061/dryad.fxpnvx153. All datasets are publicly available. FANC connectome data were analyzed from the CAVE materialization version 840, timestamp 2024-01-17T08:10:01.179472. FlyWire connectome data were analyzed from the public release version 783. Any additional information required to reanalyze the data is available from the lead contact upon request.
Code availability
Code for analyzing and visualizing hair plate connectivity in the EM dataset, calcium activity of CxHP8 neurons, kinematics during optogenetic experiments, and walking kinematics and posture during treadmill experiments is located on GitHub (https://github.com/Prattbuw/Hair_Plate_Paper/releases/tag/v1.0.0).
References
Burgess, P. R. & Clark, F. J. Characteristics of knee joint receptors in the cat. J. Physiol. 203, 317–335 (1969).
Pringle, J. W. S. Proprioception in Insects: III. The Function of the Hair Sensilla at the Joints. J. Exp. Biol. 15, 467–473 (1938).
Tuthill, J. C. & Azim, E. Proprioception. Curr. Biol. 28, R194–R203 (2018).
Proske, U. & Gandevia, S. C. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 92, 1651–1697 (2012).
Grigg, P., Finerman, G. A. & Riley, L. H. Joint-position sense after total hip replacement. J. Bone Joint Surg. Am. 55, 1016–1025 (1973).
Clark, F. J., Horch, K. W., Bach, S. M. & Larson, G. F. Contributions of cutaneous and joint receptors to static knee-position sense in man. J. Neurophysiol. 42, 877–888 (1979).
Bässler, U. Sensory control of leg movement in the stick insect Carausius morosus. Biol. Cybernetics 25, 61–72 (1977).
Wong, R. K. & Pearson, K. G. Properties of the trochanteral hair plate and its function in the control of walking in the cockroach. J. Exp. Biol. 64, 233–249 (1976).
Pearson, K. G., Wong, R. K. & Fourtner, C. R. Connexions between hair-plate afferents and motoneurones in the cockroach leg. J. Exp. Biol. 64, 251–266 (1976).
Azevedo, A. et al. Connectomic reconstruction of a female Drosophila ventral nerve cord. Nature 631, 360–368 (2024).
Takemura, S. et al. A connectome of the male Drosophila ventral nerve cord. ELife 13, https://doi.org/10.7554/eLife.97769.1.sa2 (2024).
Winding, M. et al. The connectome of an insect brain. Science 379, eadd9330 (2023).
Dorkenwald, S. et al. Neuronal wiring diagram of an adult brain. Nature 634, 124–138 (2024).
Phelps, J. S. et al. Reconstruction of motor control circuits in adult Drosophila using automated transmission electron microscopy. Cell 184, 759–774 (2021).
Zheng, Z. et al. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 174, 730–743.e22 (2018).
Schlegel, P. et al. Whole-brain annotation and multi-connectome cell typing of Drosophila. Nature 634, 139–152 (2024).
Scheffer, L. K. et al. A connectome and analysis of the adult Drosophila central brain. Elife 9, e57443 (2020).
Eckstein, N. et al. Neurotransmitter classification from electron microscopy images at synaptic sites in Drosophila melanogaster. Cell 187, 2574–2594 (2024).
Jenett, A. et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2, 991–1001 (2012).
Meissner, G. W. et al. A searchable image resource of Drosophila GAL4 driver expression patterns with single neuron resolution. ELife 12, e80660 (2023).
Lee, S.-Y. J., Dallmann, C. J., Cook, A., Tuthill, J. C. & Agrawal, S. Divergent neural circuits for proprioceptive and exteroceptive sensing of the Drosophila leg. Nat. Commun. 16, 4105 (2025).
Marin, E. C. et al. Systematic annotation of a complete adult male Drosophila nerve cord connectome reveals principles of functional organisation. ELife 13, https://doi.org/10.7554/eLife.97766.1.sa3 (2024).
Agrawal, S. et al. Central processing of leg proprioception in Drosophila. Elife 9, e60299 (2020).
Chen, C. et al. Functional architecture of neural circuits for leg proprioception in Drosophila. Curr. Biol. 31, 5163–5175 (2021).
Dallmann, C. J. et al. Selective presynaptic inhibition of leg proprioception in behaving Drosophila. Nature 647, 445–453 (2025).
Mamiya, A. et al. Biomechanical origins of proprioceptor feature selectivity and topographic maps in the Drosophila leg. Neuron 111, 3230–3243 (2023).
Mamiya, A., Gurung, P. & Tuthill, J. C. Neural coding of leg proprioception in Drosophila. Neuron 100, 636–650 (2018).
Cheng, L. E., Song, W., Looger, L. L., Jan, L. Y. & Jan, Y. N. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 67, 373–380 (2010).
Phillis, R., Statton, D., Caruccio, P. & Murphey, R. K. Mutations in the 8 kDa dynein light chain gene disrupt sensory axon projections in the Drosophila imaginal CNS. Development 122, 2955–2963 (1996).
Smith, S. A. & Shepherd, D. Central afferent projections of proprioceptive sensory neurons in Drosophila revealed with the enhancer-trap technique. J. Comp. Neurol. 364, 311–323 (1996).
Akitake, B. et al. Coordination and fine motor control depend on Drosophila TRPγ. Nat. Commun. 6, 7288 (2015).
Chockley, A. S. et al. Subsets of leg proprioceptors influence leg kinematics but not interleg coordination in Drosophila melanogaster walking. J. Exp. Biol. 225, jeb244245 (2022).
Isakov, A. et al. Recovery of locomotion after injury in Drosophila melanogaster depends on proprioception. J. Exp. Biol. 219, 1760–1771 (2016).
Mendes, C. S., Bartos, I., Akay, T., Márka, S. & Mann, R. S. Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster. ELife Sci. 2, e00231 (2013).
Pratt, B. G., Lee, S.-Y. J., Chou, G. M. & Tuthill, J. C. Miniature linear and split-belt treadmills reveal mechanisms of adaptive motor control in walking Drosophila. Curr. Biol. 34, 4368–4381 (2024).
McKelvey, E. G. Z. et al. Drosophila females receive male substrate-borne signals through specific leg neurons during courtship. Curr. Biol. 31, 3894–3904 (2021).
Mendes, C. S., Rajendren, S. V., Bartos, I., Márka, S. & Mann, R. S. Kinematic responses to changes in walking orientation and gravitational load in Drosophila melanogaster. PLOS ONE 9, e109204 (2014).
Godesberg, V., Bockemühl, T. & Büschges, A. Natural variability and individuality of walking behavior in Drosophila. J. Exp. Biol. 227, jeb247878 (2024).
Shanbhag, S. R., Singh, K. & Naresh Singh, R. Ultrastructure of the femoral chordotonal organs and their novel synaptic organization in the legs of Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int. J. Insect Morphol. Embryol. 21, 311–322 (1992).
Schubiger, G. Anlageplan, Determinationszustand und Transdeterminationsleistungen der männlichen Vorderbeinscheibe vonDrosophila melanogaster. Wilhelm Roux’ Arch. für Entwickl. der Org. 160, 9–40 (1968).
Hodgkin, H. M. & Bryant, P. J. Scanning electron microscopy of the adult of Drosophila melanogaster. Genet. Biol. Drosophila 2 (1979).
Kuan, A. T. et al. Dense neuronal reconstruction through X-ray holographic nano-tomography. Nat. Neurosci. 1–7 https://doi.org/10.1038/s41593-020-0704-9 (2020).
Oh, S. M., Jeong, K., Seo, J. T. & Moon, S. J. Multisensory interactions regulate feeding behavior in Drosophila. Proc. Natl. Acad. Sci. USA 118, e2004523118 (2021).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281 (2018).
Karashchuk, P. et al. Anipose: A toolkit for robust markerless 3D pose estimation. Cell Rep. 36, 109730 (2021).
Lesser, E. et al. Synaptic architecture of leg and wing premotor control networks in Drosophila. Nature 631, 369–377 (2024).
Azevedo, A. W. et al. A size principle for recruitment of Drosophila leg motor neurons. ELife 9, e56754 (2020).
Wendler, G. Körperhaltung bei der Stabheuschrecke: Ihre Beziehung zur Schwereorientierung und Mechanismen ihrer Regelung. Verhandlungen der Deutschen Zoologischen Gesellschaft 214, 219 (1972).
Theunissen, L. M., Vikram, S. & Dürr, V. Spatial co-ordination of foot contacts in unrestrained climbing insects. J. Exp. Biol. 217, 3242–3253 (2014).
Newland, P. L., Watkins, B., Emptage, N. J. & Nagayama, T. The structure, response properties and development of a hair plate on the mesothoracic leg of the locust. J. Exp. Biol. 198, 2397–2404 (1995).
Kuenzi, F. & Burrows, M. Central connections of sensory neurones from a hair plate proprioceptor in the thoraco-coxal joint of the locust. J. Exp. Biol. 198, 1589–1601 (1995).
BrÄunig, P. & Hustert, R. Actions and interactions of proprioceptors of the locust hind leg coxo-trochanteral joint. J. Comp. Physiol. A 157, 83–89 (1985).
BrÄunig, P. & Hustert, R. Actions and interactions of proprioceptors of the locust hind leg coxo-trochanteral joint. J.Comp. Physiol. A 157, 73–82 (1985).
Haskell, P. T. Hair receptors in locusts: function of certain prothoracic hair receptors in the desert locust. Nature 183, 1107–1107 (1959).
French, A. S. & Wong, R. K. S. The responses of trochanteral hair plate sensilla in the cockroach to periodic and random displacements. Biol. Cybernet. 22, 33–38 (1976).
French, A. S. & Sanders, E. J. The mechanism of sensory transduction in the sensilla of the trochanteral hair plate of the cockroach, Periplaneta americana. Cell and Tissue Res. 198, 159–174 (1979).
Gebehart, C., Schmidt, J. & Büschges, A. Distributed processing of load and movement feedback in the premotor network controlling an insect leg joint. J. Neurophysiol. 125, 1800–1813 (2021).
Cruse, H., Dean, J. & Suilmann, M. The contributions of diverse sense organs to the control of leg movement by a walking insect. J.Comp. Physiol. A 154, 695–705 (1984).
Wang-Chen, S. et al. NeuroMechFly v2: simulating embodied sensorimotor control in adult Drosophila. Nat. Methods 21, 2353–2362 (2024).
Vaxenburg, R. et al. Whole-body physics simulation of fruit fly locomotion. Nature 643, 1312–1320 (2025).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Maitin-Shepard, J. et al. google/neuroglancer: Zenodo https://doi.org/10.5281/zenodo.5573294 (2021).
Dorkenwald, S. et al. CAVE: Connectome Annotation Versioning Engine. Nat. Methods 22, 1112–1120 (2025).
Harris, R. M., Pfeiffer, B. D., Rubin, G. M. & Truman, J. W. Neuron hemilineages provide the functional ground plan for the Drosophila ventral nervous system. ELife 4, e04493 (2015).
Lacin, H. et al. Neurotransmitter identity is acquired in a lineage-restricted manner in the Drosophila CNS. Elife 8, https://doi.org/10.7554/elife.43701 (2019).
Liu, W. W. & Wilson, R. I. Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. PNAS https://doi.org/10.1073/pnas.1220560110 (2013)
Guizar-Sicairos, M., Thurman, S. T. & Fienup, J. R. Efficient subpixel image registration algorithms. Opt. Lett. 33, 156–158 (2008).
Moore, R. J. D. et al. FicTrac: A visual method for tracking spherical motion and generating fictive animal paths. J. Neurosci. Methods 225, 106–119 (2014).
DeAngelis, B. D., Zavatone-Veth, J. A. & Clark, D. A. The manifold structure of limb coordination in walking Drosophila. ELife 8, e46409 (2019).
Wosnitza, A., Bockemühl, T., Dübbert, M., Scholz, H. & Büschges, A. Inter-leg coordination in the control of walking speed in Drosophila. J. Exp. Biol. 216, 480–491 (2013).
Strauss, R. & Heisenberg, M. Coordination of legs during straight walking and turning in Drosophila melanogaster. J. Comp. Physiol. A 167, 403–412 (1990).
Acknowledgements
We thank members of the Tuthill and Brunton Labs for technical assistance and feedback on the manuscript. We thank Seok Jun Moon for sharing the CxHP8 split-GAL4 driver line (R48A07 AD: R20C06 DBD). B.G.P. was supported by an NSF Graduate Research Fellowship (Fellow ID: 2018261272). C.J.D. was supported from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) project 432196121. Other support was provided by National Institutes of Health grants R01NS102333, R01NS128785, and U19NS104655, a Searle Scholar Award, a Klingenstein-Simons Fellowship, a Pew Biomedical Scholar Award, a McKnight Scholar Award, a Sloan Research Fellowship, the New York Stem Cell Foundation, and a UW Innovation Award to J.C.T. J.C.T. is a New York Stem Cell Foundation – Robertson Investigator.
Author information
Authors and Affiliations
Contributions
B.G.P. and J.C.T. conceived the study and wrote the manuscript. I.S. created the gorgeous Blender model of a fruit fly with hair plates, as well as Supplementary Video 2 B.G.P. and A.S. performed confocal imaging of hair plates. B.G.P. and A.C. proofread the hair plate axons and their downstream and upstream partners in FANC. C.J.D. collected and processed the calcium imaging data for CxHP8. G.M.C. and S.W.B. collected optogenetic activation and silencing datasets for CxHP8. B.G.P. analyzed the connectivity, calcium imaging, and optogenetic datasets. B.G.P. collected and analyzed the kinematics and posture of flies walking in the treadmill setup. A.A. provided valuable feedback about the connectivity of hair plates onto motor circuits.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pratt, B.G., Dallmann, C.J., Chou, G.M. et al. Proprioceptive limit detectors contribute to sensorimotor control of the Drosophila leg. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69333-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69333-z