Abstract
Triple-negative breast cancer (TNBC) is a heterogeneous disease with variable response to neoadjuvant systemic therapy (NAST). Patients with pathologic complete response (pCR) following NAST have improved survival. Our goal was to establish readily accessible imaging biomarker to identify which TNBC patients will have pCR. Building on prior favorable results in the literature, we hypothesized that manually measured tumor volume changes at DCE-MRI may predict pCR early during NAST. This prospective study included 287 stage I-III TNBC patients who underwent DCE-MRI at baseline, after two and four cycles of NAST, with pCR accessed at surgery (NCT02276443). Tumor volume and percentage tumor volume reduction were calculated, and their correlation with pCR was evaluated. Our study showed that manually extracted tumor volume changes from DCE-MRI early during NAST were able to predict pCR with high accuracy and can serve as a clinically relevant imaging biomarker for prediction of NAST response in TNBC patients.
Similar content being viewed by others
Introduction
Triple-negative breast cancer (TNBC) accounts for ~20% of all breast cancers and is characterized by the lack of expression of estrogen receptor (ER), progesterone receptor (PR), and epidermal growth factor receptor 2 expression (HER-2). NAST is the standard of care for early-stage TNBC and offers in vivo evaluation of the tumor’s chemosensitivity, as well as downstaging, which allows more conservative surgery. TNBC is a heterogenous disease with variable clinical outcomes and response to neoadjuvant systemic therapy (NAST). Pathologic complete response (pCR) to NAST at surgery is a clinically useful predictor for improved disease-free and overall survival1,2,3. The rate of pCR to NAST regimens in TNBC is variable ranging from 18% to 65% and differs by TNBC subtype4,5.
Breast MRI is emerging as the imaging modality of choice to monitor breast cancer response to treatment because of its reproducibility and high soft tissue contrast resolution6. Currently, tumor size on dynamic contrast-enhanced MRI (DCE-MRI) is the primary method to monitor treatment response. In the ACRIN 6657/I-SPY trial, functional tumor volume measured by DCE-MRI was more accurate than the longest tumor diameter or clinical exam at predicting pCR; area under the curve (AUC) of 0.75, 0.64, and 0.68, respectively7. Functional tumor volume is defined as the subset of enhancing tumor voxels that demonstrate enhancement above a certain preset percent enhancement threshold. A more recent study by Panthi et al. confirmed that functional tumor volume is superior to the longest tumor diameter in predicting pCR8. However, acquisition of functional tumor volumes requires dedicated software and postprocessing that can be time consuming and may not be readily available in clinical practice, especially in the community practice setting. Golshan et al. demonstrated the utility of MRI tumor response by investigating complete MRI response and the change in longest tumor diameter as predictors of pCR9. Building on these prior results, we hypothesized that early changes in tumor volume based on three-dimensional manual MRI measurements might be more reliable than the longest tumor diameter, and a more readily obtainable imaging biomarker than functional tumor volume changes at identifying responders versus nonresponders to NAST. Additionally, our study investigates the possibility of predicting pCR at an earlier timepoint during NAST. If successful, it can allow triage of responders to standard-of-care NAST or to de-escalation trials, and triage of nonresponders to novel clinical trials or targeted treatments. To our knowledge, this novel yet simple approach has not been demonstrated previously in the literature. As such, the purpose of this study was to determine if changes in tumor volume based on manual DCE-MRI measurements between baseline and after two and/or four cycles of NAST can be used as a clinically relevant, early noninvasive biomarker to predict pCR and guide treatment for these patients.
Results
Patients
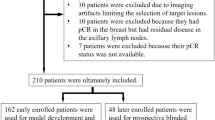
The clinical and tumor characteristics of the 287 patients included in the analysis are summarized in Table 1. Following NAST, 132 patients (132 of 287, 46%) achieved pCR, 64 (44.1%) in the discovery cohort and 68 (47.9%) in the validation cohort (Supplementary Data 1). NAST consisted of doxorubicin/cyclophosphamide (AC) for four cycles every 2 weeks followed by paclitaxel weekly for 12 doses. DCE-MRI after two and four cycles of AC was used for imaging response assessment (Fig. 1). Figure 2 shows a representative example of a patient with excellent treatment response following two and four cycles of NAST, with pCR confirmed by surgical pathology.
MRI at baseline (A), after two cycles (B), and four cycles (C) of AC shows an oval, heterogeneously enhancing mass with associated clip marker (arrows). MRI shows concentric shrinkage and tumor volume reduction of 83% after two cycles, and 96% after four cycles. Pathology after the completion of NAST confirmed pathologic complete response.
Tumor volume reduction after two cycles of NAST
In the discovery cohort, tumor volume reduction > 80% after two cycles of NAST predicted pCR with a PPV of 82% (18 of 22 patients had pCR) (Fig. 3a). The results in the validation cohort confirmed these findings: tumor volume reduction > 80% predicted pCR with a PPV of 83% (29 of 35 patients had pCR) (Fig. 3c).
PPV was 82% in the discovery cohort (A) and 83% in the validation cohort (C) at tumor volume reduction > 80% (vertical line). NPV was 91% in the discovery cohort (B) and 84% in the validation cohort (D) at tumor volume reduction < 55% (vertical line). The dotted lines represent 95% confidence intervals. Area under the curve (AUC) 0.82 (0.74-0.90, p < 0.0001) in the discovery cohort and 0.81(0.73-0.89, p < 0.0001) in the validation cohort.
In the discovery cohort, tumor volume reduction <55% after two cycles of NAST predicted residual disease with an NPV of 91% (39 of 43 patients had residual disease) (Fig. 3b). In the validation cohort, tumor volume reduction < 55% predicted residual disease with an NPV of 84% (31 of 37 patients had residual disease) (Fig. 3d).
The AUC for tumor volume reduction to predict pCR after two cycles of NAST was 0.82 (0.74-0.90) in the discovery cohort and 0.81(0.73-0.89) in the validation cohort (p < 0.0001) (Fig. 4a). For a subset of 90 patients, the AUCs for tumor volume reduction and for functional tumor volume reduction after two cycles of NAST were comparable (p = 0.67, Table 2) and were 0.80 (0.70-0.89) and 0.79 (0.69-0.88), respectively.
AUC for tumor volume reduction to predict pCR after two cycles of NAST was 0.82 (0.74-0.90) in the discovery cohort and 0.81(0.73-0.89) in the validation cohort (p < 0.0001) (A). AUC for tumor volume reduction to predict pCR after four cycles of NAST was 0.82 (0.75–0.90) in the discovery cohort and 0.79 (0.71-0.86) in validation cohort (p < 0.0001) (B).
Tumor volume reduction after four cycles of NAST
In the discovery cohort, tumor volume reduction > 90% after four cycles of NAST predicted pCR with a PPV of 80% (37 of 46 patients had pCR) (Fig. 5a). In the validation cohort, tumor volume reduction > 90% predicted pCR with a PPV of 75% (45 of 60 patients had pCR) (Fig. 5c).
PPV was 80% in the discovery cohort (A) and 75% in the validation cohort (C) at tumor volume reduction >90%. NPV was 84% in the discovery cohort (B) and 81% in the validation cohort (D) at tumor volume reduction <80% (vertical line). The dotted lines represent 95% confidence intervals. AUC 0.82 (0.75–0.90, p < 0.0001) in the discovery cohort; AUC 0.79 (0.71–0.86, p < 0.001) in the validation cohort.
In the discovery cohort, tumor volume reduction <80% after 4 cycles of NAST predicted residual disease with an NPV of 84% (46 of 55 patients had residual disease) (Fig. 5b). In the validation cohort, tumor volume reduction < 80% predicted residual disease with an NPV of 81% (29 of 36 patients had residual disease) (Fig. 5d).
The AUC to predict pCR after four cycles of NAST was 0.82 (0.75–0.90) in the discovery cohort and 0.79 (0.71-0.86) in validation cohort (p < 0.0001) (Fig. 4b). For a subset of 90 patients, the AUCs for tumor volume reduction and for functional tumor reduction after four cycles of NAST were comparable (p = 0.85, Table 2) and were 0.81 (0.73–0.90) and 0.82 (0.73–0.91), respectively.
Inter-reader variability
ICCs were calculated for all DCE-MRI timepoints using measurements from both readers in the study. An ICC value closer to 1 means better reproducibility, and values > 0.81 indicate excellent reproducibility10. The ICC (95% CI) was 0.951 (0.939–0.961) for baseline measurements, 0.987 (0.983-0.990) for measurements after two cycles, and 0.996 (0.995-0.997) for measurements after four cycles, indicating excellent inter-reader reproducibility.
Discussion
Our analysis of this large prospective cohort of patients with TNBC showed that early DCE-MRI tumor volume changes can differentiate responders from non-responders and serve as a clinically relevant noninvasive predictor of pCR just after two or four cycles of NAST with AUC of 0.80. Tumor volume reduction greater than 80% after two cycles of NAST was predictive of pCR with high PPV of 80%. Less than 55% tumor volume reduction early after two cycles of NAST initiation was predictive of residual disease with NPV of 84%. The percentage tumor volume reduction thresholds shown in our study can serve as a practical, clinically applicable model in patients with TNBC undergoing NAST. Patients who achieve tumor volume reduction > 80% after two cycles of NAST, or greater than 90% after four cycles of NAST have a high likelihood of pCR, potentially triaging them to standard-of-care NAST or to investigational de-escalation therapies and sparing them unnecessary toxicity and cost. Similarly, tumor volume reduction < 55% after two cycles, or less than 80% after four cycles suggests chemoresistance to standard NAST, allowing these patients the opportunity to be considered for investigational treatments, and biomarker-directed novel targeted treatment regimens to improve the likelihood of pCR.
The clinical utility of using early DCE-MRI response to tailor NAST therapy was demonstrated in a retrospective study by Fatayer et al. in which DCE-MRI was performed after two cycles of NAST11. Tumor response was measured using the modified RECIST criteria and all molecular subtypes of breast cancer were included. Partial response was defined as 50% reduction in the diameter of the index tumor mass, or 25–50% reduction with improvement of the enhancement curve; minimal response was defined as reduction in tumor diameter of 25–50% without improvement in the enhancement curve. Of 72 patients with minimal response on DCE-MRI after two NAST cycles, 62 (86%) were switched to a different regimen, with improvement in tumor response seen in 74.2% following this change11. The utility of early DCE-MRI response to predict pCR was shown by Golshan et al.9. In their prospective study of 519 patients with TNBC, DCE-MRI response was assessed after 12 weeks of paclitaxel-based therapy using percentage reduction in the largest lesion diameter. Complete MRI response was defined as resolution of all target lesions, while partial response was defined as reduction in largest tumor diameter by 50% or greater. Complete DCE-MRI response at 12 weeks of paclitaxel-based therapy was seen in 116 patients (22%), of whom 91 (78.5%) achieved pCR at final pathology. Partial DCE-MRI response was seen in 166 (32%), of whom 95 (57.2%) had pCR. The PPV of pCR based on complete DCE-MRI response after 12 weeks of NAST was 79%. Our study builds on these results in that it attempts to predict pCR at earlier timepoints, after two and four cycles of NAST, to allow for more time for risk-adapted therapy. Additionally, our study identified percentage tumor volume reduction values that identified which early partial responders were likely to achieve pCR. Furthermore, our study differed from these studies in that we used tumor volume and tumor volume reduction, instead of percent difference in largest single target lesion diameter.
The clinical utility of functional tumor volumes was demonstrated by the I-SPY 1 Trial, which demonstrated that breast DCE-MRI was more accurate than clinical examination at predicting pCR7. Functional tumor volume is the number of pixels within the tumor that reach a minimum predefined threshold of signal enhancement and requires specialized software12. Hylton et al. showed that the use of functional tumor volumes was more accurate than the use of the longest tumor diameter for pCR prediction. In their study, functional tumor volume after one cycle of NAST was the most predictive of pCR with AUC of 0.707. Functional tumor volume after one cycle of NAST also had the strongest predictive performance for recurrence-free survival12. Recurrence-free survival was also shown in a retrospective study by Gampenrieder et al. to be significantly associated with tumor response based on DCE-MRI13.
In a similar study by Henderson et al., enhancing tumor volume was shown to be a comparable or a more useful model than functional tumor volume at predicting pCR: sensitivity of 81% and specificity 91.8%, compared to 80% and 76.8% for functional tumor volume14. Enhancing tumor volume, defined as the total number of pixels that enhance, is a another measure of tumor volume and does not inadvertently exclude foci of tumor that do not meet a predefined threshold. In our study, an a simpler form of volume estimation was performed by measuring the tumor dimensions in all three planes during the early phase of DCE-MRI images. These results are promising as this method of volume estimation is more accessible to radiologists and does not require dedicated software. The excellent interreader reproducibility shown in our study further supports the clinical utility of manual volume measurements and volume reduction calculations.
Our findings are consistent with our earlier study using B scale ultrasound to estimate tumor volume reduction and to predict pCR; in that study tumor volume reduction >80% after two cycles of AC was predictive of pCR15. However, although ultrasound is an inexpensive and widely available tool, breast MRI is more reproducible across centers and demonstrates less inter-reader variability and operator dependency14. Breast MRI is the most sensitive breast imaging modality and is superior to physical examination, mammography, and sonography at estimating tumor volume and tumor response when compared to surgical pathology16. Studies have shown that breast MRI is more accurate and sensitive than conventional imaging for assessing treatment response after conclusion of NAST6,17,18,19,20,21,22,23,24,25. Newly developed American College of Radiology appropriateness criteria as well as the guidelines from the European Society of Breast Imaging recommend MRI as the modality of choice for tumor response assessment26.
With the introduction of immunotherapy in the treatment of early TNBC following the KEYNOTE-522 trial, it has become evident that not all patients benefit from this treatment4,27. Although the patients receiving pembrolizumab had higher rates of pCR and improved disease-free survival than the control arm, 64.8% versus 51.2%, patients who received this regimen had higher incidence of treatment-related adverse events, including death4,27. Therefore, development of noninvasive imaging biomarkers that allow identification of responders versus nonresponders early during NAST is of even greater importance. If these findings are confirmed in additional datasets, this model could identify patients who could be spared immunotherapy and avoid its associated toxicities if projected to have a high chance of pCR on standard chemotherapy alone.
The main novelty of our study is in using changes in tumor volume based on three dimensional measurements from standard of care DCE-MRI performed early during NAST as a clinically relevant noninvasive biomarker for pCR prediction. This model could be easily obtained and adapted in daily clinical practice without the need of specialized software for post processing or additional analysis. High inter-reader reproducibility of DCE-MRI tumor measurements further supports the use of this model across different practices. Furthermore, a subset analysis confirmed that the AUC for tumor volume reduction is comparable to the AUC of functional tumor volume reduction.
The main limitation of our study is that it was performed at a single institution. This study included only patients with TNBC; therefore, results cannot be directly applied to other subtypes of breast cancer. Our prospective protocol finished enrollment before US Food and Drug Administration approval of immunotherapy for TNBC; therefore, only patients undergoing chemotherapy were included in this study. We are evaluating the applicability of these results to an immunotherapy cohort in an ongoing prospective clinical trial.
DCE-MRI tumor volume reduction early during NAST is a useful tool for predicting pCR in TNBC. Our study proposes a novel, clinically relevant noninvasive imaging biomarker that can be easily used for predicting pCR and non-PCR status as early as after two cycles of NAST. These findings may allow patients with a high likelihood of pCR to avoid toxicities of a complete chemotherapeutic regimen, including those of immunotherapy. Excellent responders may be triaged to de-escalation treatment strategies, including shortening of NAST regimens and early surgery, while nonresponders can be triaged to clinical trials for treatment escalation and targeted therapy. Clinical application of these results will require multi-institutional prospective trials including other breast cancer molecular subtypes and different treatment regimens.
Methods
Patients
Patients were enrolled in the ARTEMIS (A Robust TNBC Evaluation Framework to Improve Survival) prospective clinical trial for patients with stage I to III TNBC (NCT02276443). The study was approved by the Institutional Review Board at the University of Texas MD Anderson Cancer Center. Informed consent was obtained from all patients before study enrollment. The study was performed in accordance with all relevant ethical regulations and those specified under the Declaration of Helsinki.
A total of 326 patients with stage I-III TNBC who underwent standard of care NAST and were monitored during therapy using serial DCE-MRI imaging were evaluated for this analysis (Fig. 1). Of 326 patients, 39 were excluded because they had inflammatory breast cancer (3 patients); achieved pCR following experimental targeted therapies (14 patients); had MRI artifact or diffuse disease making tumor response difficult to assess (12 patients); or lacked MRI for both time points: after two cycles and after four cycles of NAST (10 patients). Our study cohort therefore consisted of 287 patients, who were randomly assigned in a 1:1 ratio to a discovery cohort (145 patients) and a validation cohort (142 patients) (Supplementary Data 1).
Chemotherapy
NAST consisted of standard of care doxorubicin/cyclophosphamide (AC) for four cycles every 2 weeks followed by paclitaxel weekly for 12 doses. Imaging response was assessed using DCE-MRI after two and four cycles of AC (Fig. 2). After completion of NAST, patients underwent either mastectomy or breast conserving surgery, and axillary lymph node surgery. Residual disease or pCR was assessed following surgery.
Histopathology
Core needle biopsy of the index tumor was performed in all patients before treatment. ER, PR, HER-2 and Ki-67 results were obtained via immunohistochemical staining of the biopsy specimen. TNBC was defined as tumor in which <10% of invasive tumor cells had positive nuclear staining for ER and PR receptors. HER-2 negativity was defined according to the American Society of Clinical Oncology/College of American Pathologists guidelines28,29. Surgical specimens were examined by dedicated breast pathologists. pCR was defined as no residual invasive disease (ypT0 or ypTis) in the breast and the resected nodal tissue (ypN0).
MRI and tumor volumes
Patients were imaged with DCE-MRI at baseline, after two cycles and four cycles of NAST (GE 3 Tesla MR750w whole body scanner (Waukesha, WI) with bilateral 8-channel phased array RF coil). Imaging was performed in the prone, feet first position. A single bolus of gadobutrol (Gadovist, Bayer Health Care) was injected (0.1 mL/kg at 2 mL/second followed by saline flush). At least one pre-contrast phase was obtained. The imaging protocol included a T2-weighted series and DCE-MRI series based on differential subsampling with Cartesian ordering (DISCO) sequence. Typical MRI scan parameters used for DISCO acquisition were as follows: field of view = 34 × 34 cm, slice thickness = 3.0 mm, slice spacing = −1.5 mm, flip angle = 12°, repetition time = 7.6 ms, echo time 1/echo time 2 = 1.1/2.3 ms, total acquisition time = 7 min, matrix = 320 × 320, number of acquired slices = 60 to 115, in-plane spatial resolution = 0.6−0.8 mm, temporal resolution of DISCO series = 8−15.5 s, receiver bandwidth = ±166.7 kHz, and number of excitations = 0.69.
Tumor volumes were calculated using manual measurements in the anterior-posterior, craniocaudal, and transverse dimension (width, height, length,) of the enhancing index lesion obtained during the early phase of DCE-MRI (acquired at 60 s after contrast injection) using the ellipse volume equation:
Tumors were independently measured by two fellowship-trained breast imaging radiologists with 10 and 16 years of experience, respectively, to allow evaluation of inter-reader variability. Percentage tumor volume reduction was calculated after two and four cycles of NAST with respect to the volume at baseline, using the following formula:
Statistical analysis
Receiver operating characteristic (ROC) analysis and assessment of AUC were used to evaluate the predictability of pCR based on the percentage reduction in tumor volume between baseline and after two cycles of NAST and between baseline and after four cycles of NAST. ROC curves were plotted with sensitivity on the y-axis and 1-specificity on the x-axis. Positive predictive value (PPV) and negative predictive value (NPV) curve analyses with 95% confidence intervals were plotted, along with Youden index calculations. Combination of the highest PPV with best Youden’s index was used to identify the specific tumor volume reduction threshold that could predict the occurrence of pCR and highest NPV and Youden’s index combination for the presence of residual disease, after two and four cycles of NAST. Patient age was compared between the discovery and validation cohorts using a Wilcoxon rank-sum test. Categorical variables were compared between the two cohorts using either chi-square tests or Fisher exact tests. The interreader variability in volumetric measurements was assessed by calculating the intraclass correlation coefficients (ICCs). A p-value <0.05 was considered statistically significant. All statistical analyses were carried out using R (version 3.6.1, R Development Core Team, Vienna, Austria). For a subset of 90 patients that were included in both, this cohort and the cohort from Panthi et al.8, the AUCs for the percentage changes in functional tumor volumes were compared with those for tumor volume reduction using DeLong’s test.
Data availability
Data are available from the corresponding author upon request.
References
Symmans, W. F. et al. Assessment of residual cancer burden and event-free survival in neoadjuvant treatment for high-risk breast cancer: an analysis of data from the I-SPY2 randomized clinical trial. JAMA Oncol 7, 1654–1663 (2021).
von Minckwitz, G. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30, 1796–1804 (2012).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
Lee, J. S., Yost, S. E. & Yuan, Y. Neoadjuvant treatment for triple negative breast cancer: recent progresses and challenges. Cancers (Basel) 12, 1404 (2020).
Scheel, J. R. et al. MRI, Clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 trial. AJR Am. J. Roentgenol. 210, 1376–1385 (2018).
Hylton, N. M. et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—results from ACRIN 6657/I-SPY TRIAL. Radiology 263, 663–672 (2012).
Panthi, B. et al. Assessment of response to neoadjuvant systemic treatment in triple-negative breast cancer using functional tumor volumes from longitudinal dynamic contrast-enhanced MRI. Cancers (Basel) 15, 1025 (2023).
Golshan, M. et al. Early assessment with magnetic resonance imaging for prediction of pathologic response to neoadjuvant chemotherapy in triple-negative breast cancer: results from the phase III BrighTNess trial. Eur. J. Surg. Oncol. 46, 223–228 (2020).
McGraw, K. O. & Wong, S. P. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1, 30–46 (1996).
Fatayer, H. et al. Serial MRI scans help in assessing early response to neoadjuvant chemotherapy and tailoring breast cancer treatment. Eur. J. Surg. Oncol. 42, 965–972 (2016).
Hylton, N. M. et al. Neoadjuvant chemotherapy for breast cancer: functional tumor volume by mr imaging predicts recurrence-free survival-results from the ACRIN 6657/CALGB 150007 I-SPY 1 TRIAL. Radiology 279, 44–55 (2016).
Gampenrieder, S. P. et al. Radiologic complete response (rCR) in contrast-enhanced magnetic resonance imaging (CE-MRI) after neoadjuvant chemotherapy for early breast cancer predicts recurrence-free survival but not pathologic complete response (pCR). Breast Cancer Res. 21, 19 (2019).
Henderson, S. A. et al. Breast cancer: influence of tumour volume estimation method at MRI on prediction of pathological response to neoadjuvant chemotherapy. Br. J. Radiol. 91, 20180123 (2018).
Adrada, B. E. et al. Early ultrasound evaluation identifies excellent responders to neoadjuvant systemic therapy among patients with triple-negative breast cancer. Cancer 127, 2880–2887 (2021).
Shin, H. J. et al. Comparison of mammography, sonography, MRI and clinical examination in patients with locally advanced or inflammatory breast cancer who underwent neoadjuvant chemotherapy. Br. J. Radiol. 84, 612–620 (2011).
Kim, M. J. et al. Evaluation with 3.0-T MR imaging: predicting the pathological response of triple-negative breast cancer treated with anthracycline and taxane neoadjuvant chemotherapy. Acta Radiol. 56, 1069–1077 (2015).
Croshaw, R., Shapiro-Wright, H., Svensson, E., Erb, K. & Julian, T. Accuracy of clinical examination, digital mammogram, ultrasound, and MRI in determining postneoadjuvant pathologic tumor response in operable breast cancer patients. Ann. Surg. Oncol. 18, 3160–3163 (2011).
Marinovich, M. L. et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J. Natl. Cancer Inst. 105, 321–333 (2013).
Reig, B. et al. Breast MRI for evaluation of response to neoadjuvant therapy. Radiographics 41, 665–679 (2021).
Mann, R. M., Cho, N. & Moy, L. Breast MRI: state of the art. Radiology 292, 520–536 (2019).
Mann, R. M., Kuhl, C. K., Kinkel, K. & Boetes, C. Breast MRI: guidelines from the European society of breast imaging. Eur. Radiol. 18, 1307–1318 (2008).
De Los Santos, J. F. et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational breast cancer research consortium trial 017. Cancer 119, 1776–1783 (2013).
Chen, C. A. et al. Complete breast MRI Response to neoadjuvant chemotherapy and prediction of pathologic complete response. J. Breast Imag. 1, 217–222 (2019).
Kim, J. et al. Prediction of pathologic complete response on MRI in patients with breast cancer receiving neoadjuvant chemotherapy according to molecular subtypes. Eur. Radiol. 32, 4056–4066 (2022).
Expert Panel on Breast, I. et al. ACR apropriateness criteria((R)) monitoring response to neoadjuvant systemic therapy for breast cancer. J. Am. Coll. Radiol. 14, S462–S475 (2017).
Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 386, 556–567 (2022).
Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. Arch. Pathol. Lab Med. 138, 241–256 (2014).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J. Clin. Oncol. 36, 2105–2122 (2018).
Acknowledgements
This study has received funding from the National Institutes of Health/ National Cancer Institute (Cancer Center Support Grant P30 CA016672). The study was supported by the University of Texas MD Anderson Cancer Center Breast Cancer Moonshot Program, Robert D. Moreton Distinguished Chair Funds in Diagnostic Radiology, and the Cancer Prevention and Research Institute of Texas Multi-Investigator Research Award (RP16710-C1-CPRIT). This work was supported in part by generous philanthropic contributions to the Moon Shots Program of The University of Texas MD Anderson Cancer, the Shari Sella Memorial Fund (to D.T.), the Winterhof Fund (to D.T.), the Gayle Monroe Kuoni Breast Medical Oncology Research Endowment (to D.T.), the Still Water Foundation (to D.T.), the Suzanne Potter ARTEMIS Fund (to C.Y.), the Amelia Handegan Fund (to C.Y.), and a Conquer Cancer Career Development Award supported by Fleur Fairman (2020CDABC-5423266503, to C.Y.). Dr. Yam was additionally supported by a Conquer Cancer Career Development Award supported by Fleur Fairman, the 2018 Gianni Bonadonna Breast Cancer Research Fellowship (Conquer Cancer Foundation), the Allison and Brian Grove Endowed Fellowship for Breast Medical Oncology, and the Susan Papizan Dolan Fellowship in Breast Oncology. The authors would like to thank Stephanie Deming, Research Medical Library, The University of Texas MD Anderson Cancer Center, for editing the manuscript.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to this manuscript as follows: Conceptualization: M.G., G.R., B.A., C.Y. Formal analysis: J.S., P.W., G.R., M.G., B.A., J.M., J.B.S. Investigation: M.G., B.A., R.M., S.P., F.P., M.P., R.C., T.M., G.W., J.L.P., D.L., A.K., Z.X., J.W., J.L., V.V., K.H., L.H., B.A., A.T. Supervision: G.R., J.M., D.T., W.Y., S.M., C.Y.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests or non-financial interests. The author would like to declare non-related financial interests: G.J.W. is an editor of UpToDate. T.W.M. is a medical consultant for Merit Medical Inc. and Hologic Inc. D.T. receives support from Novartis, Pfizer, GlaxoSmithKline, Immunomedics, and Oncopep. C.Y. receives research support to the institution from Amgen, GlaxoSmithKline, Merck, and Genetech. W.Y. receives textbook royalties from Elsevier. J.M. has the following relationships: Research support: GE Healthcare, Siemens Healthineers. Intellectual Property Licensing: GE Healthcare, Siemens Healthineers. Consultant: C4 Imaging’. G.M.R. receives research support from GE Healthcare. All other authors declare that they have nothing to disclose. There are no family members who present a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guirguis, M.S., Adrada, B.E., Yam, C. et al. Early tumor volume reduction by breast DCE MRI predicts pathologic complete response to neoadjuvant therapy in triple negative breast cancer. npj Breast Cancer 11, 129 (2025). https://doi.org/10.1038/s41523-025-00839-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41523-025-00839-3