Abstract
People with Parkinson’s disease (PWP) face significant gaps in care. Limited neurologist access, infrequent clinic visits, and inadequate symptom measurement culminate in suboptimal therapy and high morbidity. Quantitative Digitography (QDG) provides validated, digital metrics of the three cardinal motor signs in Parkinson’s disease (PD) in real-time from 30 seconds of a mobility task on a digitography device and can be used remotely or in clinical settings. This study demonstrates the feasibility and clinical relevance of 30-day remote QDG monitoring. Participants showed excellent compliance and found the system easy to use. The QDG Mobility Score demonstrated meaningful correlation with Activities of Daily Living (ADLs), captured motor complexities across a broad PD duration, and tracked motor changes from small therapy adjustments. QDG offers providers and PWP an accessible, objective, and real-time tool to remotely monitor motor symptoms, optimize treatment, and address care gaps created by infrequent clinic visits and subjective symptom assessment.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) presents a complex healthcare challenge. Among the >1.2 million Americans living with PD1, 40% lack access to a neurologist2,3,4. Three fundamental limitations undermine current care: infrequent, brief in-person clinic visits, a complex clinical examination, and a lack of widely available remote monitoring systems. People with PD (PWP) are usually evaluated in person every three to 6 months5. As PD is a progressive disease, the treatment plan set by the neurologist at one visit may become subtherapeutic by the next, which leaves PWP to adjust their medications themselves; a practice that results in unstable dopamine levels and swings from under- to over-treatment. This results in an increased incidence of falls, fractures, and neuropsychiatric complications such as confusion and hallucinations, the complications of which can lead to death6,7,8. Furthermore, the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)9 motor examination (Part III) is comprehensive but subjective and variable within and among raters10. Most health care providers are neither trained nor have time to administer it during short visits, and PWP lack objective, comprehensive motor monitoring in between11. The reliance on subjective recall rather than objective data about symptoms between visits can also lead to suboptimal medication management and treatment adjustments12. There is a need for remote, objective monitoring technologies that fill the majority of the PWP journey, which occurs outside of clinical visits13. This will provide a more comprehensive approach to symptom tracking and more frequent adjustments of therapy, which will facilitate optimal disease management14.
Quantitative Digitography (QDG) solves this critical unmet need by providing validated, quantitative metrics of the three cardinal motor signs in PD, remotely and in real-time15,16,17,18,19. From a brief 30-second mobility task, consisting of alternating pressing and releasing tensioned engineered levers, QDG delivers high-resolution motor metrics that correlate with the MDS-UPDRS III scores and sub-scores, track symptom progression, and demonstrate sensitivity to adjustments in therapy16,17,19,20. These individual metrics are combined into the QDG Mobility Score (0–100 scale), a composite measure that represents overall movement proficiency by integrating performance across speed, frequency, amplitude, and rhythmicity, normalized against age-matched healthy controls, where scores ≥92 indicate normal performance21. The integrated QDG system improves the assessment and management of PD by enabling point of care remote monitoring and results available in the electronic health record (EHR) in real time21, allowing clinicians flexibility in testing schedules and to optimize PWP-specific treatment plans in between in-person visits. The QDG system has been granted Breakthrough Device Designation by the United States Food and Drug Administration.
In this prospective cohort study, we demonstrate the feasibility and clinical relevance of remote digital monitoring using the QDG system in people with movement disorders, when referred by neurologists. The primary outcome was compliance with performing one test per day for at least 16/30 days of remote monitoring, which is the minimum requirement for reimbursement for the existing remote monitoring codes22. Secondary outcomes included adherence with testing once and/or twice per day, user experience, correlation between the QDG Mobility Score and the participant’s perception of the impact of their motor function on Activities of Daily Living (ADLs), and QDG’s sensitivity to detect motor changes after small adjustments in therapy. QDG technology has been extensively studied and validated in the research setting16,17,18,19,20,21,23; this study is the first long-term remote monitoring study in PWP’s home environment using the QDG platform.
Results
Participant demographics
Thirty participants (23 males) provided informed consent and entered the study; twenty-nine were referred by neurologists at Stanford Movement Disorders Clinic and one was recruited from outside of Stanford (Table 1). One participant was lost to follow-up, and four participants partially completed or exited the study early due to technical difficulties or schedule conflicts (Supplementary Fig. S1). Twenty-five participants completed the full 30-day protocol and were included in the final analysis. Participants were referred for multiple clinical purposes, ranging from medication response monitoring to pre-diagnostic assessment. The cohort also captured a broad range of PD duration, from people undiagnosed but with suspected Parkinsonism to those with clinically established PD of long duration and with motor fluctuations. The cohort exhibited well-controlled symptoms on therapy with medications and/or DBS (Table 1).
QDG mobile application, task setup, and execution
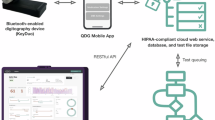
Figure 1 represents the participant interface with the QDG remote system. Participants set up a QDG account and inputted their anti-parkinsonian medication schedule and DBS model and settings (if applicable) (Fig. 1a). They were then trained on setting up the QDG system (Fig. 1b). This included finding an optimal location where they could perform the task in a seated position with their forearm flexed at roughly 90 degrees; the device was placed on a flat, stable surface with the depressions on the levers aligned with the fingertips when the wrist was supported, such that the wrist was in a neutral position. They established a Bluetooth connection between the KeyDuo and QDG Mobile App, initiated a test, and confirmed whether they were on or off medication and/or DBS when applicable. Execution of the task was cued by an instructions screen, which prompted participants to position themselves for their first test (Fig. 1c). Participants were told to fully press and release their index and middle fingers in an alternating fashion, tapping as fast and as consistently as possible. Auditory “Go” and “Stop” cues were given through the app to start and stop QDG, respectively. Testing always started with the right hand followed by the left (Fig. 1c). Upon saving both tests and returning to the mobile app home screen, data was automatically sent to a secure cloud service for analysis.
All participants were able to set up the system correctly at the first week check-in; this included hardware connection, therapy screen navigation, and task initiation. All participants achieved complete task proficiency by the second week, despite occasional initial challenges with Bluetooth connectivity.
Compliance, adherence, and user experience
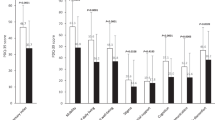
Participants demonstrated excellent compliance with remote QDG testing, with 100% of participants completing at least one test a day for 16/30 days (Fig. 2a). This 16/30-day threshold represents the minimum requirement for reimbursement under existing remote monitoring codes22. Participants maintained adherence rates of 96.2% (N = 25) for completion of one test per day and 82.2% (N = 24) for two tests per day (Fig. 2b). Only PD participants on dopaminergic medication were asked to perform two tests per day.
a Compliance with a test performed on at least 16/30 days; b Adherence to testing once-daily (N = 25) and twice-daily (N = 24), c User reported difficulty ratings with performing QDG (0 = extremely easy, 10 = extremely difficult). For visualization purposes, responses were categorized as Extremely Easy (0–2), Moderately Easy (3–5), Moderately Difficult (6–8), or Extremely Difficult (9–10); d Spearman correlation between QDG Mobility Scores and MDS-UPDRS Part II scores (ρ = −0.61 [95% CI: −0.88, −0.16], p = 0.004, n = 20); confidence intervals were calculated using bootstrapping.
Participants reported various strategies to facilitate scheduled testing. These included setting alarms, incorporating tests into written schedules, and aligning testing with medication schedules. Participants who found twice-per-day testing moderately difficult primarily reported travel schedules and Bluetooth connectivity issues as barriers to full adherence.
QDG Mobility Score correlated with MDS-UPDRS II (ADL) score
In the PD cohort, the QDG Mobility Score (averaged across hands, and across weeks) demonstrated a high, significant correlation with participants’ MDS-UPDRS II, averaged over 4 weeks; ρ = −0.61, 95% CI: [−0.88, −0.16], p = 0.004, N = 20, Fig. 2d. Higher QDG Mobility Scores reflected better abilities in daily living tasks (lower MDS-UPDRS II scores, Fig. 2d).
QDG test-retest reliability
Post-hoc test-retest reliability analysis of the QDG Mobility Score in 19 participants with PD demonstrated excellent measurement consistency with intraclass correlation coefficient (ICC)s >0.90 across all analyses (Fig. S2, Supplementary Materials).
QDG reflects time course of PD
There was a wide range of disease duration among the participants with diagnosed or suspected PD, who were referred for participation (pre-diagnosis to 20 years, Table 1). Figure 3 demonstrates representative QDG traces from the more and less affected sides from five participants with different durations of disease.
QDG traces and Mobility Scores (MS) from the more and less affected sides of representative participants with different durations of PD (−1 month, 0 months, 5, 10, 15, and 20 years). Each QDG trace displays the amplitude of lever strikes (millimeters) over time (seconds); the blue and red strikes represent the index and middle fingers on right hand traces (vice versa on left hand). A normal MS (>92) is in green and abnormal in red. The –1- and 0-month traces are off therapy, while 5- to 20-year traces are on therapy.
The top two traces are from the same participant, who was referred for remote monitoring on the day of initial presentation of intermittent left-hand tremor, only when walking. The initial QDG test revealed an abnormal Mobility Score (MS) of 87 (normal > 92) on the left (more affected, MA) hand, and normal MS (100) on the right (less affected, LA) hand. One month later, on the day of their PD clinical diagnosis, the same participant’s performance of the MA hand was worse (MS = 53), demonstrating progression of disease in the month prior to the clinical diagnosis, whereas the performance remained normal in the LA hand (MS = 100). In addition to asymmetry, there was evidence of differing performances between the two fingers (blue and red strikes) on the MA hand at the 2nd test (time of diagnosis) of this participant, which we have labeled “finger dissociation.” QDG traces from a representative participant with PD for 5 years, on therapy, showed asymmetry of performance between hands; the MS was 55 on the MA and 95 on the LA hands, respectively. There was evidence of the sequence effect in both fingers only on the MA side after 17 s of QDG. After 10 years of PD, a representative trace (on therapy) demonstrated abnormal performance on both sides, although still asymmetric (MS = 45|74). The sequence effect occurred on the MA side after only 7 s of QDG and led to a freezing event at 12 s shown by the pause in movement of the red finger strike at the bottom of the lever press. After this, the performance was more irregular in amplitude and frequency. Freezing and the sequence effect were also evident on the LA side. For the representative participant with a 15-year disease duration, on therapy, the performance was worse bilaterally with less difference between sides (MS = 30|39). Finger dissociation and the sequence effect were evident bilaterally and occurred early, and there was lower amplitude tapping in general. Lastly, the representative participant tested after 20 years of PD demonstrated the lowest MS (MS = 4|11), low amplitude QDG bilaterally with prominent finger dissociation, irregular amplitude and frequency, and very early reduction in amplitude that resulted in freezing bilaterally (red strikes).
QDG tracks small, patient-initiated adjustments of medication
Figure 4 demonstrates the change in the QDG MS after the addition (Fig. 4a) or reduction (Fig. 4b) of one tablet of immediate release carbidopa/levodopa (CD/LD 25/100) during remote monitoring.
a Participant 1 [adapted from ref. 21] and b Participant 2’s more affected left-hand scores. Green circles indicate normal values, red circles abnormal values. Dashed lines mark therapy adjustments.
The MS on the MA side of a participant with early-stage PD improved from abnormal (71.9 ± 13.2) to normal (92.6 ± 6.5) after the addition of one tablet of CD/LD 25/100 mg on day 17 to a regimen of three doses a day plus Sinemet-CR at night (Fig. 4a, dashed line). The improvement was evident on the same day and the day-to-day variability also decreased. In contrast, Fig. 4b demonstrates a decrease of the MS after a participant decided to stop medication altogether after the first 10 days of monitoring due to involuntary movements. The participant had advanced PD, treated with DBS (14 years post-diagnosis) and minimal medication (0.5 tablet CD/LD 25/100 mg twice a day). Their average MS of 95.0 ± 4.6 deteriorated after the medication withdrawal (77.0 ± 10.8). The progressive decrease of the MS over 3 weeks may reflect the long duration levodopa response24.
Symptom tracking and communication survey
Participants who had clinically established PD at the time of referral answered a survey focused on symptom tracking and communication at the exit interview. 84.2% (16/19) of participants responded that prior to the study, they did not have a robust monitoring method for their symptoms, and either did not track their symptoms at all, or tracked them mentally. One participant recorded symptoms with a diary, and one tracked tremor using the Apple Watch. However, the majority (57.9%, 11/19) wished they had a more objective way to communicate their symptoms or change in symptoms with their clinician. With respect to standard of care, 52.6% (10/19) of participants were assessed by their neurologist using the MDS-UPDRS III no more than twice annually, with 15.8% (3/19) of participants seeing their neurologist no more than once per year.
Discussion
Remote monitoring using the QDG system demonstrated 100% compliance with the Centers for Medicare & Medicaid Services (CMS) remote monitoring reimbursement requirement to perform one test a day for at least 16 out of 30 days22. There were strong test adherence rates of 96.2% for once-daily and 82.2% for twice-daily testing over the 30-day period. All participants were able to set up and complete the task correctly at their first weekly check-in, and 96% of participants rated once-daily testing as easy. Referrals came from Movement Disorders specialists, based on individual neurologists’ prescriptions and need for adjunctive information to their clinical assessments for a range of movement disorders diagnoses and monitoring objectives. Among the participants with PD, QDG reflected symptom severity and asymmetry throughout a broad range of disease durations, from pre-diagnosis through 20 years post-diagnosis. The QDG Mobility Score was highly correlated with the PWP’s reported ADL impairment, and QDG’s high-resolution metrics were sensitive to small medication adjustments.
The clinical impact of QDG monitoring was highlighted through several key findings. First, the strong correlation between the QDG Mobility Score and MDS-UPDRS II validates its real-world relevance. The Mobility Score provides a sensitive quantification of motor symptoms that also meaningfully reflects a patient’s functional status and the impact of those symptoms on everyday activities. Next, the excellent test-retest reliability of the QDG Mobility Score demonstrated in this analysis (ICC > 0.90, Fig. S2) establishes the measurement precision necessary for clinical monitoring applications. Moreover, QDG measured and conveyed the motor complexities of PD over a broad range of disease duration. Pre-diagnosis, QDG captured high asymmetry between the MA and LA hands, a valuable insight that could aid clinicians in diagnosis, and documented progression of disease severity even before diagnosis, which would not have been noticed or documented without remote monitoring in between visits. It is well documented that the sequence effect in gait (progressive shortening of stride length) leads to Freezing of Gait and Freezing of Upper Limb movement (FOUL)15,25,26,27,28. In this study QDG demonstrated that the sequence effect also led to FOUL; it was more severe and occurred earlier in the task at longer durations of disease.
Daily monitoring with QDG revealed critical therapeutic windows typically missed in routine care – from quantifying immediate motor improvement after a single tablet of CL/LD in early-stage PD, to capturing the gradual decline when a DBS participant abruptly stopped a single tablet of CD/LD. To our knowledge, QDG represents the only quantitative point of care remote monitoring system to capture the motor effect of small adjustments in CD/LD dosing in early and advanced PD, while achieving the high compliance needed for routine clinical care. QDG’s ability to detect changes in dopa-responsive motor symptoms positions it to play a similar role in PD care as continuous glucose monitoring (CGM) does for diabetes management. In both diseases, patients must adhere to strict, time-sensitive medication schedules to maintain glucose or dopamine levels respectively within defined physiological ranges. While CGM has advanced diabetes care by providing real-time feedback for insulin dosing29, a similar tool has been so far lacking in PD care. We believe that QDG multi-modal motor monitoring will ameliorate PD care by giving the provider and PWP actionable insight into their motor function, enabling healthcare providers to optimize therapy in real-time across the disease spectrum.
QDG addresses a major need for people with movement disorders: the clinician-patient gap in assessment and communication. In our PD cohort, 52.6% of participants were assessed by their neurologist using the MDS-UPDRS III no more than twice annually, and 15.8% of participants saw their neurologist no more than once per year. Although the majority of participants did not monitor their disease symptoms prior to the study, most expressed a desire for more objective ways to monitor and communicate symptoms to their healthcare providers. QDG can only provide clinically meaningful value if patients and providers adopt it. The CMS requires patients to obtain a physiological parameter (i.e., blood pressure, glucose) on at least 16/30 days for providers to gain additional revenue through remote physiological/therapeutic monitoring (RP/TM) codes22. QDG met this CMS requirement with 100% of participants testing at least 16/30 days, further strengthening its viability for widespread clinical adoption.
While telemedicine has expanded access to neurological care during the COVID-19 pandemic, the limitations of video-based assessments highlight the need for complementary quantitative tools. Although clinicians can observe motor asymmetries during video visits, visual assessment alone cannot provide the objective measurements necessary for optimizing therapy between appointments. Rigidity, a cardinal sign of PD, cannot be evaluated via video, and subtle motor changes that inform medication adjustments remain below the threshold of visual detection30,31. Recent evidence underscores these limitations. Video-based motor assessments demonstrate only moderate agreement with in-person examinations, and inter-rater reliability varies widely depending on the specific motor feature being assessed10,32.
The burden on patients also differs markedly. Telemedicine visits, though valuable for maintaining continuity of care, require scheduled appointments, technical setup, and sustained engagement throughout the consultation33. Studies report that interest in telemedicine decreases with disease severity, with only 60% of advanced patients expressing willingness to engage compared to 70% of early-stage patients34. In contrast, our participants completed QDG testing with minimal burden (30 s at their convenience) achieving 100% compliance with CMS monitoring requirements. Most importantly, telemedicine and QDG together will provide more comprehensive PD care. Video consultations maintain the essential patient-provider relationship and enable complex clinical discussions that no technology can replace35. QDG could supplement these interactions by allowing for objective continuous motor monitoring that could inform clinical decision-making.
Current wearable systems for passive monitoring could be a complementary solution to QDG monitoring; however, these face limitations that may impact their clinical utility on a daily basis. None can capture all cardinal motor symptoms of PD with one device and in real-time. Specifically, PKG and Kinesia ONE™ measure bradykinesia, tremor, and dyskinesia but not rigidity. Users are instructed to wear the sensor for at least 6 h per day and over the course of 6 days to produce reliable data36,37. The averaged retrospective data is reported asynchronously to the provider and not through the EHR. Most monitor a single wrist’s movement, and this may not reflect or capture movements involving multiple body parts. Wearable systems that require multiple simultaneous sensors, such as PDMonitor, can create usability barriers due to the complexity and inconvenience38,39,40.
More recently, smartphone-embedded sensors have enabled quantification of bradykinesia and, to a lesser degree, tremor (but not rigidity). Large-scale studies using the mPower app (n = 12,703) demonstrated correlations with clinical disease severity, while smaller studies using apps like STOP (Sentient Tracking of Parkinson’s) have shown moderate correlations with tremor and medication effects41,42. However, smartphone-based approaches face challenges including variable accelerometer sampling rates across devices, participants implementing strategies to circumvent protocols leading to compliance failures in unsupervised settings with behaviors ranging from removing phones from pockets during walking assessments to performing tasks without carrying devices41,42,43. Similarly, current task-based remote monitoring systems, while less common, present substantial limitations as demonstrated by the Parkinson’s Remote Interactive Monitoring System, which combines motor and non-motor assessments using MDS-UPDRS-based questionnaires and dual depth cameras for 3D motion tracking but faces significant barriers including accessibility challenges, excessive test duration (mean 84.2 min), lack of validation against the gold-standard MDS-UPDRS III, and high sensitivity to patient orientation relative to the cameras44.
By contrast, QDG yields validated metrics of overall PD motor severity and its cardinal motor symptoms and an early or prodromal indicator of PD16,19,45,46,47,48,49, in just 30 seconds. QDG provides crucial insight into bilateral characteristics of PD, such as asymmetry, which would be missed by single-point sensors. The QDG system’s direct integration with the EHR allows clinicians to access QDG data synchronously with their management of each patient, thus not impacting their over-capacity workflow. QDG’s objective nature further expands the scope of its accessibility amongst physicians, allowing general neurologists and primary care physicians to rapidly glean clinical insights that would typically require examination by a movement disorders specialist.
QDG is designed to function not only as a standalone assessment tool but also as a complementary technology that can integrate with existing smartphone-based monitoring approaches. The QDG system includes a QDG Mobile Application with access to the Apple HealthKit. Future developments will combine QDG with QDG health app, including gait and other movement metrics from embedded smartphone sensors, providing a multi-modal approach that leverages the strengths of both technologies while addressing their individual limitations.
While QDG focuses specifically on objective motor assessment, it does not directly capture non-motor symptoms, mood fluctuations, or sleep disturbances. However, the significant correlation with patient-reported functional outcomes (MDS-UPDRS II) demonstrates clinical relevance. Integration with comprehensive clinical assessment remains essential for holistic PD care. The inclusion criteria required the ability to “follow instructions and provide informed consent,” which may have excluded people with severe cognitive impairment and formal MMSE/MoCA screening was not performed. The cognitive demands of daily QDG use may limit feasibility in PD patients with cognitive impairment, warranting future investigation in cognitively impaired populations.
The study included participants across the spectrum of Parkinsonism and tremor (established PD, suspected Parkinsonism, tremor disorders) referred by movement disorders neurologists for various clinical purposes. This enrollment across the diagnostic spectrum reflected real-world clinical scenarios where remote monitoring may be useful during diagnostic workup periods. QDG remote monitoring was used in conjunction with standard subspecialty clinical assessment, including the MDS-UPDRS and diagnostic imaging such as the quantitative DatScan. For PD-specific clinical correlations (e.g., MDS-UPDRS II correlation), we analyzed only the clinically established PD cohort (N = 20 with complete 30-day data). This initial feasibility study included a relatively small sample size. A larger study may expand QDG compliance and usability. Post-hoc test-retest reliability analysis revealed excellent correlation (ICC > 0.90) but relatively wide limits of agreement (±24 points) on the 0–100 QDG Mobility Score scale. Early in the study research personnel traveled to participants’ homes for QDG system setup, training, and exiting. Once this was determined to be successful, the study expanded to recruit out-of-state participants, utilize remote video conferencing, and leverage device shipment to and from participants’ homes. This enabled geographical expansion of remote monitoring using the QDG system.
This feasibility study demonstrated excellent compliance, significant and meaningful correlation with ADLs, clinical utility across disease stages, and proof-of-concept evidence for medication effect detection. Future studies will investigate the critical clinical outcomes that would validate real-world utility: reduced hospitalizations, fewer emergency visits, improved long-term quality of life, improved medication adherence, overall cost savings to healthcare systems. This study informs large-scale, multicenter implementation studies examining patient-centered endpoints, healthcare utilization metrics, and long-term disease management effectiveness across diverse healthcare systems and patient populations.
These findings establish QDG as an objective, real-time, remote monitoring system for people with neurological disorders that offers a flexible set of use case scenarios for neurologists and other healthcare providers. All study participants successfully completed at least 16/30 days of testing and demonstrated high rates of adherence to both once-daily and twice-daily testing schedules for 30 days. Participants were able to effortlessly interact with the system, and 96% rated once-daily testing as easy. In addition, the QDG system generated high-resolution data that closely correlated with PWP’s self-reported ADLs and differentiated motor impairment and asymmetry from those pre-diagnosis to others up to 20 years post-diagnosis. A majority of participants expressed the desire to have a more objective way to communicate their symptoms, or change in symptoms, with their provider. The QDG system represents a critical advancement in PD care, equipping providers with a portable, accessible tool to monitor the validated set of motor symptoms remotely, optimize therapeutic regimens, and bridge the care gaps created by infrequent clinic visits and subjective symptom assessment. By integrating seamlessly into clinical workflows without adding complexity, QDG redefines the standard of care for movement disorders. Its unique ability to scale across disease stages and sense small adjustments in therapy positions QDG as an indispensable resource in the ongoing effort to improve the management of PD.
Methods
Participants
Inclusion criteria: people with suspected or clinically established PD, who were over 18 years of age, able to follow instructions, and provide informed consent. Exclusion criteria: people unable to perform the task due to pain and/or musculoskeletal injury or disease. The sample size was adapted from Goetz et al., which evaluated compliance with weekly testing of a 30-min task for 26 weeks in early-stage PD and required 50 participants50. This study evaluated compliance with once-a-day testing for at least 16/30 days. Assuming proportional testing effort, the target sample size was 30 participants.
The 30-day monitoring period was specifically chosen based on CMS reimbursement requirements, which stipulate that remote physiologic monitoring services must involve data collection on at least 16 days within a 30-day period to qualify for billing under RP/TM codes22. This design ensures clinical feasibility within existing healthcare reimbursement frameworks while demonstrating the system’s viability for routine clinical adoption
Standard protocol approvals, registrations, and patient consents
The study was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to enrollment in the study. This study was approved by the Stanford University Institutional Review Board (IRB) in accordance with recognized ethical guidelines (IRB eProtocol #60883). The study was not registered as a clinical trial, as it does not involve the testing of a health-related intervention within the scope of a regulated trial registry.
QDG system
The QDG system consists of a Bluetooth-enabled digitography device (KeyDuo), a patient-facing mobile application, a HIPAA-compliant cloud web service and customized algorithm (PRECISE), and an EHR-integrated web dashboard21. The KeyDuo comprises adjacent tensioned, engineered levers, which can sense the displacement and timing of lever motion with a sampling rate of 201 Hz and accuracy of 0.12 mm throughout the device’s range of motion. Patients interface with the QDG mobile application (operating system iOS 16.4-current) to initiate a test, enter therapy settings, and complete the QDG task. The data are transferred from the KeyDuo to the QDG mobile application using Bluetooth. The mobile application conducts a device calibration-specific raw data transformation, checks for errors, and collates medication and deep brain stimulation (DBS) settings. The data are stored in the HIPAA-compliant cloud service, where each QDG test is queued for analysis by the QDG PRECISE algorithm. The QDG at-home apparatus included a KeyDuo, iPad mini, palm rest, and cable (to power the KeyDuo via iPad). The QDG mobile application was installed on the iPad in advance.
The PRECISE algorithm analyzes KeyDuo raw data to extract press and release amplitudes and speeds, their coefficients of variation (CV = standard deviation/mean), inter-strike intervals (ISI) and ISI CV, as well as release and dwell times (durations at the top of the release and base of the press phases, respectively)17,18,19,20,21. Sub-algorithms detect strikes generated by rest or action tremor, analyze the duration, average amplitude, and frequency of tremor, and remove those strikes from the analysis of voluntary movements23.
The measures from voluntary strikes yield quantitative, validated metrics of bradykinesia, rigidity, and gait impairment18,19. QDG provides four validated metrics of bradykinesia: tapping frequency, press amplitude, press speed, and press amplitude variability (press amplitude Coefficient of Variation (CV)), which captures the deterioration of press amplitude over time, known as the sequence effect. The validated QDG rigidity metric is the release speed18, and the metric for gait impairment and freezing behavior is arrhythmicity19. The algorithm also quantifies the number and duration of freezing episodes during the trial, termed percent freezing.
Metrics are averaged across the 30-s trial for each finger, averaged between fingers for each hand, and used to calculate the QDG Mobility Score. The QDG Mobility Score (ranging from 0 to 100) is a statistically derived composite score that represents overall motor performance by statistically weighting QDG voluntary movement metrics and normalizing them against age-matched healthy controls from a normative dataset of 42 healthy individuals (age 60.0 ± 9.0 years)21. The normal performance threshold of ≥92 was established as the 75th percentile of the healthy control distribution, where scores ≥92 represent performance within the expected range of neurologically healthy21. A higher score represents better performance. A separate Tremor Severity Score (ranging from 0–100) is calculated based on percent duration and average amplitude of tremor strikes during the task; a higher score represents greater tremor severity21.
QDG output metrics are transmitted back to the web service and are displayed through an interactive SMART-on-FHIR Dashboard, a protocol for interoperability across EHR systems. The dashboard displays single test results, medication schedule, and metric data over any time range. The dashboard was available on the web portal and was embedded in the EHR, allowing health care providers to access QDG data within the PWP’s chart in real-time.
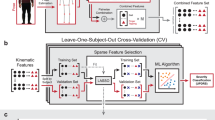
To evaluate the test-retest reliability of the QDG Mobility Score, we conducted a post-hoc analysis using data from 20 of the 25 participants who had multiple QDG assessments during baseline clinic visit and at-home setup visit periods. One participant was excluded due to insufficient valid test-retest pairs, resulting in n = 19 participants for analysis. The first QDG assessments per hand were analyzed to evaluate measurement consistency under standardized conditions.
Reliability was assessed using ICC with 95% confidence intervals calculated via bootstrap resampling (1000 iterations). Four analyses were performed: (1) overall combining all measurement pairs (n = 38), (2) right hand-specific analysis (n = 19), (3) left hand-specific analysis (n = 19), and (4) combined analysis averaging both hands per participant (n = 19). Agreement was evaluated using Bland-Altman plots, with limits of agreement calculated as mean difference ±1.96 standard deviations. ICC values ≥ 0.75 indicated excellent reliability, 0.60–0.74 good, 0.40–0.59 fair, and <0.40 poor reliability.
Experimental protocol
Figure 5 outlines the study protocol and flow. Participants were screened for the study inclusion and exclusion criteria over the phone.
At the initial clinic visit, participants received an overview of the study purpose and protocol, including participant responsibilities, and provided written informed consent. Participants completed baseline and test-retest QDG mobility tasks and underwent MDS-UPDRS III assessment by a certified rater.
Research team members initially traveled to participants’ homes to assist with setup and training on use of the QDG system. As the study progressed, setup and training expanded to include a remote option via video conferencing. The research team first reviewed the QDG user guide with the participant and trained them on mobile app navigation. Participants were then asked to set up and execute the QDG mobility task on their own to ensure proper use of the system. Once a participant-initiated QDG mobility test had been successfully completed, research personnel confirmed the participants’ testing schedule based on the recommendation of the referring clinician. PWP on medication were instructed to complete two tests per day, once in their worst “off state” and once in their best “on state,” whereas participants who did not take medication were asked to test once a day. The participants then reported their baseline “motor experiences of daily living” using the MDS-UPDRS II (ADL) scale. The 30-day remote testing period commenced after this visit.
Routine check-ins were conducted either in-home or via video conferencing once per week for 4 weeks with research team members (Fig. 5). At each check-in, the participant completed questionnaires regarding potential adverse events (Custom Adverse Event Questionnaire), testing adherence and usability (In-Home Usability Testing and User Feedback Questionnaire), and the MDS-UPDRS II. Additionally, research personnel observed participants’ execution of the QDG mobility task to confirm that they maintained correct task performance and retrained participants as needed.
Compliance and adherence were measured objectively through automatic system logging on the QDG web-dashboard, which recorded the date, time, and completion status of each test. The compliance ratio, displayed on the dashboard, was calculated as the number of days with a completed test divided by the total number of possible test days in the current month. Adherence to once-daily and twice-daily testing schedules was measured as the mean compliance ratio for once-per-day and twice-per-day testing, respectively, across the cohort. These objective metrics were verified during weekly check-ins through structured questionnaires documenting any missed tests, technical difficulties, or scheduling conflicts.
Upon completion of the remote monitoring period, research team members visited the participant’s home to complete their final check-in, which included routine weekly check-in items as well as a PD History Questionnaire, Design Feedback Questionnaire, Exit Interview, and Symptom Tracking and Communication Survey. Further along in the study, the exit visit could be completed over video conferencing if the participant lived further away.
Questionnaires
The MDS-UPDRS II is a 13-item questionnaire, where each item is rated on a scale of 0–4 (higher number is more severe). Total MDS-UPDRS II scores were averaged across the 4 weekly check-ins for each participant.
The In-home Usability Testing and User Feedback Questionnaire assesses QDG device testing, setup, connectivity, task performance, and participant feedback on device usability and functionality. Participants were scored on accurate execution of QDG set-up and task performance. Number of observations in each sub-category of task setup and execution were totaled, and percent of correct observations was reported.
In the Exit Interview, participants rated their ease of use of QDG testing once and twice per day using an 11-point Likert scale (0–10, where 0 = extremely easy and 10 = extremely difficult). For analysis and reporting, responses were dichotomized: scores <5 were classified as “easy” and scores >5 as “difficult”. For visualization purposes, responses were further categorized as Extremely Easy (0–2), Moderately Easy (3–5), Moderately Difficult (6–8), or Extremely Difficult (9–10).
The Symptom Tracking and Communication Survey examined PD patients’ symptom tracking methods, healthcare provider communication channels, and attitudes toward remote monitoring technologies. Participants were asked whether they would prefer more objective methods of symptom communication with their provider on a scale of 0–10. For reporting, responses were categorized: scores <5 were classified as “agree”, scores = 5 as “neutral”, and scores >5 as “disagree”.
Statistical analysis
Statistical analyses were performed using Python (v3.12.5) with SciPy (v1.11.3) and NumPy (v2.0.0) libraries. Spearman rank correlation was used to evaluate the relationship between QDG Mobility Scores and MDS-UPDRS Part II scores. A bootstrapping approach with 10,000 resamples was used to estimate the 95% confidence interval (CI) for the Spearman correlation coefficient. A p < 0.05 was considered significant. No correction for multiple comparisons was required since only one statistical test was run.
Data availability
The datasets used and/or analyzed during the current study may be shared (anonymized) from the corresponding author on reasonable request for research studies with a defined scientific question and plan pertaining to the use of the data. The code for this study is not publicly available but may be made available to qualified researchers upon reasonable request from the corresponding author.
Code availability
The code for this study is not publicly available but may be made available to qualified researchers upon reasonable request from the corresponding author.
References
Statistics | Parkinson’s Foundation. (accessed 19 November 2024). https://www.parkinson.org/understanding-parkinsons/statistics.
Lin, C. C. et al. Geographic variation in neurologist density and neurologic care in the United States. Neurology 96, e309–e321 (2021).
Cheng, E. M. et al. Association of specialist involvement and quality of care for Parkinson’s disease. Mov. Disord. 22, 515–522 (2007).
Willis, A. W., Schootman, M., Evanoff, B. A., Perlmutter, J. S. & Racette, B. A. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology 77, 851–857 (2011).
Effects of physician visit frequency for Parkinson’s disease treatment on mortality, hospitalization, and costs: a retrospective cohort study—PMC. (accessed 25 November 2024). https://pmc.ncbi.nlm.nih.gov/articles/PMC8672617/.
Hommel, A. L. A. J., Krijthe, J. H., Darweesh, S. & Bloem, B. R. The association of comorbidity with Parkinson’s disease-related hospitalizations. Park. Relat. Disord. 104, 123–128 (2022).
Hassan, A. et al. High rates and the risk factors for emergency room visits and hospitalization in Parkinson’s disease. Park. Relat. Disord. 19, 949–954 (2013).
Gerlach, O. H., Winogrodzka, A. & Weber, W. E. Clinical problems in the hospitalized parkinson’s disease patient: systematic review. Mov. Disord. 26, 197–208 (2011).
Goetz, C. G. et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Evers, L. J. W., Krijthe, J. H., Meinders, M. J., Bloem, B. R. & Heskes, T. M. Measuring Parkinson’s disease over time: the real-world within-subject reliability of the MDS-UPDRS. Mov. Disord. 34, 1480–1487 (2019).
Riggare, S., Scott Duncan, T., Hvitfeldt, H. & Hägglund, M. You have to know why you’re doing this”: a mixed methods study of the benefits and burdens of self-tracking in Parkinson’s disease. BMC Med. Inform. Decis. Mak. 19, 175 (2019).
Hauser, A. D., Auinger, P. & Parkinson Study Group. Determination of minimal clinically important change in early and advanced Parkinson’s disease. Mov. Disord. Wiley Online Library. (accessed 19 November 2024). https://movementdisorders.onlinelibrary.wiley.com/doi/10.1002/mds.23638 (2011).
Riggare, S. et al. Patients are doing it for themselves: a survey on disease-specific knowledge acquisition among people with Parkinson’s disease in Sweden. Health Inform. J. 25, 91–105 (2019).
Dorsey, E. R. & Bloem, B. R. The Parkinson pandemic—a call to action. JAMA Neurol. 75, 9–10 (2018).
Bronte-Stewart, H. M., Ding, L., Alexander, C., Zhou, Y., & Moore, G. P. Quantitative digitography (QDG): a sensitive measure of digital motor control in idiopathic Parkinson’s disease. Mov. Disord. Wiley Online Library. (accessed 19 November 2024). https://movementdisorders.onlinelibrary.wiley.com/doi/10.1002/1531-8257(200001)15:1%3C36::AID-MDS1008%3E3.0.CO;2-M (2000).
Taylor Tavares, A. L. et al. Quantitative measurements of alternating finger tapping in Parkinson’s disease correlate with UPDRS motor disability and reveal the improvement in fine motor control from medication and deep brain stimulation. Mov. Disord. 20, 1286–1298 (2005).
Trager, M. H. et al. Arrhythmokinesis is evident during unimanual not bimanual finger tapping in Parkinson’s disease. J. Clin. Mov. Disord. 2, 8 (2015).
Trager, M. H., Wilkins, K. B., Koop, M. M. & Bronte-Stewart, H. A validated measure of rigidity in Parkinson’s disease using alternating finger tapping on an engineered keyboard. Park. Relat. Disord. 81, 161–164 (2020).
Wilkins, K. B. et al. Quantitative Digitography Measures Motor Symptoms and Disease Progression in Parkinson’s Disease. J. Park. Dis. 12, 1979–1990 (2022).
Prabhakar, V. et al. Quantitative digitography measures fine motor disturbances in chronically treated HIV similar to Parkinson’s disease. Front. Aging Neurosci. 12, https://doi.org/10.3389/fnagi.2020.539598 (2020).
Hoffman, S. L. et al. Comprehensive real time remote monitoring for Parkinson’s disease using Quantitative DigitoGraphy. npj Park. Dis. 10, 1–13 (2024).
Billing for remote patient monitoring | Telehealth.HHS.gov. (accessed 22 November 2024). https://telehealth.hhs.gov/providers/best-practice-guides/telehealth-and-remote-patient-monitoring/billing-remote-patient.
Gala, A. S. et al. The digital signature of emergent tremor in Parkinson’s disease. npj Park. Dis. 10, 1–11 (2024).
Anderson, E. & Nutt, J. The long-duration response to levodopa: Phenomenology, potential mechanisms and clinical implications. Park. Relat. Disord. 17, 587–592 (2011).
Kehnemouyi, Y. M., Petrucci, M. N., Wilkins, K. B., Melbourne, J. A. & Bronte-Stewart, H. M. The Sequence Effect Worsens Over Time in Parkinson’s Disease and Responds to Open and Closed-Loop Subthalamic Nucleus Deep Brain Stimulation. J. Park. Dis. 13, 537–548 (2023).
Nieuwboer, A. et al. Upper limb movement interruptions are correlated to freezing of gait in Parkinson’s disease. Eur. J. Neurosci. 29, 1422–1430 (2009).
Syeda, H. B. et al. Objective markers of finger tapping in idiopathic Parkinson’s disease with freezing of gait (4468). Neurology 94, 4468 (2020).
Heremans, E. et al. When motor control gets out of hand: speeding up triggers freezing in the upper limb in Parkinson’s disease. Park. Relat. Disord. 64, 163–168 (2019).
Rodbard, D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diab. Technol. Ther. 19, S25–S37 (2017).
Abdolahi, A., Scoglio, N., Killoran, A., Dorsey, E. R. & Biglan, K. M. Potential reliability and validity of a modified version of the Unified Parkinson’s Disease Rating Scale that could be administered remotely. Park. Relat. Disord. 19, 218–221 (2013).
Cubo, E., Hassan, A., Bloem, B. R. & Mari, Z. MDS-telemedicine study group. implementation of telemedicine for urgent and ongoing healthcare for patients with Parkinson’s disease during the COVID-19 pandemic: new expectations for the future. J. Park. Dis. 10, 911–913 (2020).
Tarolli, C. G. et al. Feasibility, reliability, and value of remote video-based trial visits in Parkinson’s disease. J. Park. Dis. 10, 1779–1786 (2020).
Beck, C. A. et al. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology 89, 1152–1161 (2017).
Joo, J. Y. et al. A survey of perspectives on telemedicine for patients with Parkinson’s disease. J. Mov. Disord. 17, 89–93 (2024).
van den Bergh, R., Bloem, B. R., Meinders, M. J. & Evers, L. J. W. The state of telemedicine for persons with Parkinson’s disease. Curr. Opin. Neurol. 34, 589–597 (2021).
Powers, R. et al. Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson’s disease. Sci. Transl. Med. 13, eabd7865 (2021).
Joshi, R. et al. PKG Movement recording system use shows promise in routine clinical care of patients with Parkinson’s disease. Front. Neurol. 10, https://doi.org/10.3389/fneur.2019.01027 (2019).
Moreau, C. et al. Overview on wearable sensors for the management of Parkinson’s disease. npj Park. Dis. 9, 1–16 (2023).
Espay, A. J. et al. Technology in Parkinson’s disease: challenges and opportunities. Mov. Disord. 31, 1272–1282 (2016).
Fisher, J. M., Hammerla, N. Y., Rochester, L., Andras, P. & Walker, R. W. Body-worn sensors in Parkinson’s disease: evaluating their acceptability to patients. Telemed. J. E Health 22, 63–69 (2016).
Omberg, L. et al. Remote smartphone monitoring of Parkinson’s disease and individual response to therapy. Nat. Biotechnol. 40, 480–487 (2022).
Kuosmanen, E. et al. Smartphone-based monitoring of Parkinson disease: quasi-experimental study to quantify hand tremor severity and medication effectiveness. JMIR Mhealth Uhealth 8, e21543 (2020).
Moreta-de-Esteban, P., Martín-Casas, P., Ortiz-Gutiérrez, R. M., Straudi, S. & Cano-de-la-Cuerda, R. Mobile applications for resting tremor assessment in Parkinson’s disease: a systematic review. J. Clin. Med. 12, 2334 (2023).
Bridges, B., Taylor, J. & Weber, J. T. Evaluation of the Parkinson’s remote interactive monitoring system in a clinical setting: usability study. JMIR Hum. Factors 11, e54145 (2024).
Agostino, R., Berardelli, A., Currà, A., Accornero, N. & Manfredi, M. Clinical impairment of sequential finger movements in Parkinson’s disease. Mov. Disord. 13, 418–421 (1998).
Agostino, R. et al. Impairment of individual finger movements in Parkinson’s disease. Mov. Disord. 18, 560–565 (2003).
Koop, M. M., Shivitz, N. & Brontë-Stewart, H. Quantitative measures of fine motor, limb, and postural bradykinesia in very early stage, untreated Parkinson’s disease. Mov. Disord. 23, 1262–1268 (2008).
Roalf, D. R. et al. Quantitative assessment of finger tapping characteristics in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease. J. Neurol. 265, 1365–1375 (2018).
Mollica, M. A. et al. Early detection of subtle motor dysfunction in cognitively normal subjects with amyloid-β positivity. Cortex 121, 117–124 (2019).
Goetz, C. G. et al. Testing objective measures of motor impairment in early Parkinson’s disease: feasibility study of an at-home testing device. Mov. Disord. (accessed 16 December 2024). Wiley Online Library. https://movementdisorders.onlinelibrary.wiley.com/doi/10.1002/mds.22379 (2009).
Acknowledgements
The authors would like to thank all research participants who generously contributed their time to this study. We would also like to thank Simon Revlock and Christopher Coriz for their contribution to QDG hardware design; Paul Schmiedmayer, Vishnu Ravi, and Warren Hiemstra for their software development work; and Morgan Hunt for project management. This work was funded by Stanford Medicine Catalyst, Wu Tsai Neurosciences Institute at Stanford University, and the Debbie and Andy Rachleff Foundation.
Author information
Authors and Affiliations
Contributions
A.S.N and S.K. are co-first authors. A.S.N.: investigation—performed the experiments, collected and analyzed data; data curation—managed and maintained data and database; visualization—creation and presentation of data; formal analysis—application of statistical, mathematical, and computational techniques to analyze study data; writing—original draft and critical review and editing. S.K.: investigation—performed the experiments, collected and analyzed data; data curation—managed and maintained data and database; visualization—creation and presentation of data; formal analysis—application of statistical, mathematical, and computational techniques to analyze study data; writing—original draft and critical review and editing. L.P.: conceptualization—development and evolution of overall research goals and aims; investigation—performed the experiments, collected and analyzed data; data curation—managed and maintained data and database; writing—critical review and editing. K.W.D.: investigation—performed the experiments, collected and analyzed data; data curation—managed and maintained data and database; visualization—creation and presentation of data; formal analysis—application of statistical, mathematical, and computational techniques to analyze study data; writing—original draft and critical review and editing. A.K.A.: investigation—performed the experiments, collected and analyzed data; data curation—managed and maintained data and database; visualization—creation and presentation of data; writing—original draft and critical review and editing. A.S.G.: development and validation of methodology; software—programming, software development; designing computer programs; implementation of the computer code and supporting algorithms; testing of existing code components; investigation—performed the experiments, collected and analyzed data; data curation—managed and maintained data and database; formal analysis—application of statistical, mathematical, and computational techniques to analyze study data; writing—critical review and editing. K.B.W.: conceptualization—development and evolution of overall research goals and aims; writing—critical review and editing. S.L.H: conceptualization—development and evolution of overall research goals and aims; development and validation of methodology; project administration—management and coordination of research activity and execution; writing—critical review and editing. M.S.F.: writing—critical review and editing H.Z.: writing—critical review and editing G.C.: writing—critical review and editing B.P.: writing—critical review and editing H.B.S.: conceptualization—formulation and development of overall research goals and aims and manuscript; design, development and validation of methodology; provision of project materials; supervision—responsible for oversight and leadership of the research activity planning and execution; project administration—management and coordination of research activity and execution; funding acquisition- acquisition of the financial support for the project leading to this publication; writing original draft, critical review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Negi, A.S., Karjagi, S., Parisi, L. et al. Remote real time digital monitoring fills a critical gap in the management of Parkinson’s disease. npj Parkinsons Dis. 11, 239 (2025). https://doi.org/10.1038/s41531-025-01101-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01101-0