Abstract
Iron dysregulation is known to play a critical role in the pathogenesis and progression of Parkinson’s disease (PD). The specific patterns of regional iron accumulation in PD and in idiopathic rapid eye movement sleep behavior disorder (iRBD), which is considered the prodromal stage of PD, remain incompletely characterized. In this study, we employed an advanced sub-voxel quantitative susceptibility mapping technique (APART-QSM), which separates paramagnetic from diamagnetic sources, thereby enabling in vivo iron quantification based specifically on paramagnetic susceptibility measurement. We applied APART-QSM to 36 healthy controls, 54 patients with iRBD, and 52 patients with early-stage PD (mean disease duration: 5.8 ± 3.6 years; Hoehn and Yahr stage ≤3). Fifteen participants from each group underwent follow-up assessments ranging from 6 to 48 months. Our findings identified novel extrapyramidal and pyramidal regions exhibiting spatial and temporal changes of paramagnetic susceptibility in iRBD and PD. Moreover, paramagnetic susceptibility values were found to serve as potential image biomarkers, reflecting tremor severity (tremor subscore of Movement Disorder Society-Unified Parkinson’s Disease Rating Scale III, MDS-UPDRS III) and non-tremor severity (rigidity, bradykinesia, and postural instability subscores of MDS-UPDRS III), with the ability to predict disease progression and track phenoconversion from iRBD to PD.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by rigidity, bradykinesia, postural instability, and tremor1. PD has a prodromal phase that can extend several years before the overt clinical symptoms of Parkinsonism appear2,3. This phase is often marked by idiopathic rapid eye movement sleep behavior disorder (iRBD), a specific type of parasomnia characterized by dream-enacting behaviors and loss of muscle atonia during rapid eye movement sleep phases3,4. Longitudinal studies have demonstrated that more than 80% of patients with iRBD eventually convert to an α-synucleinopathy, such as PD, dementia with Lewy bodies (DLB), or multiple system atrophy (MSA), over years to decades5,6. Notably, ~14–21% of individuals progress to PD within five years7,8. Evidence from diverse imaging techniques indicates that iRBD can exhibit neural alterations characteristic of prodromal PD. Specifically, free-water values in the posterior substantia nigra (pSN) were significantly elevated in iRBD patients compared with HC, but remained lower than those observed in PD9. Longitudinally, iRBD patients exhibited a significant increase in pSN free-water values over an average follow-up of 29 months relative to HC9. In another resting-state functional magnetic resonance imaging (fMRI), iRBD patients exhibit basal ganglia network dysfunction indistinguishable from early PD10. Furthermore, a longitudinal 18Ffluorodeoxyglucose-positron emission tomography (FDG-PET) study demonstrates that progressive abnormalities in PD-related metabolic networks in iRBD patients may serve as a strong predictor of phenoconversion timing11. Collectively, these findings underscore the critical need for non-invasive imaging biomarkers that can facilitate early differential diagnosis and enable monitoring of disease phenoconversion from iRBD to PD.
It is widely recognized that iron deposition plays a critical role in the progression of PD12,13,14,15. Various abnormalities in iron metabolism have been identified in PD patients, including alterations in iron distribution, transport, and storage12,13. These disruptions can lead to cellular oxidative stress, ferroptosis, and the aggregation of α-synuclein14,15, collectively accelerating the degeneration of dopaminergic neurons. Previous studies utilizing quantitative susceptibility mapping (QSM) have demonstrated its utility in detecting susceptibility differences between iRBD and PD patients16,17,18. Alterations in QSM values have been reported in the brainstem and basal ganglia of iRBD and PD patients compared to healthy controls16,17,18. Although prior studies have demonstrated an increase in cerebrospinal fluid iron levels during a one-year follow-up in PD patients19, recent longitudinal studies have largely failed to identify significant changes in QSM values of PD over time20,21, with only one study reporting alterations in the ventral substantia nigra after a three-year follow-up22. Notably, there are currently no longitudinal QSM studies targeting iRBD patients.
Increases in conventional QSM values are generally interpreted as reflecting elevated metal content, with iron accounting for the dominant effect, while other metals such as copper and manganese contribute to a much smaller extent to the overall paramagnetic susceptibility23. However, this interpretation has limitations, as magnetic susceptibility in the human brain is influenced not only by iron but also by other diamagnetic substances24,25,26, which often coexist within the same spatial locations in the brain and can attenuate the paramagnetic susceptibility effects, potentially leading to underestimation of true iron content changes. Iterative magnetic susceptibility source separation-quantitative susceptibility mapping (APART-QSM) is a sub-voxel QSM technique that incorporates GRE image information to separate paramagnetic and diamagnetic susceptibility sources27. It employs a more comprehensive complex signal model with a voxel-specific magnitude decay kernel, enhancing its ability to provide a more specific quantification of both types of magnetic susceptibilities28,29,30. The paramagnetic susceptibility maps provide a more precise measure of iron concentration by accounting for the effect of diamagnetic components, whereas the diamagnetic susceptibility maps predominantly capture protein deposition, demyelination, and calcium. Three recent studies have applied the APART-QSM technique to various neuropathologies, including multiple sclerosis, epilepsy, and Alzheimer’s disease31,32,33. This advanced method has demonstrated superior capability in distinguishing overlapping susceptibility sources that often confound the conventional QSM technique. Two of these investigations utilized post-mortem brain analyses and validated the accuracy of paramagnetic susceptibility separation for quantifying iron concentrations31,33. These histologically confirmed results highlight the technique’s reliability in minimizing the influence of diamagnetic components and accurately detecting subtle changes in iron concentrations.
Given the prominent involvement of the motor system in the phenoconversion from iRBD to PD4,34, our study employs the APART-QSM technique to quantify the spatial distribution and longitudinal changes of paramagnetic and diamagnetic components within the motor system across cohorts of healthy controls (HC), iRBD, and PD patients. Susceptibility values were measured in key regions of both the extrapyramidal and pyramidal motor systems. Group classification performance was assessed by receiver operating characteristic (ROC) curve analysis. Furthermore, we examined the relationship between paramagnetic susceptibility values and dopaminergic dysfunction in iRBD patients, and assessed their potential in reflecting tremor and non-tremor symptom severity, as well as predicting disease progression in PD patients.
Results
Demographic and clinical information
The demographic and clinical characteristics of HC, iRBD, and PD participants were systematically delineated in Table 1. Comparative analyses revealed no significant disparities in sex (P = 0.112) and age (P = 0.120) across groups. The HC group demonstrated a higher mini-mental state examination (MMSE) score compared to the PD group (P = 0.019), while there was no significant difference in Montreal cognitive assessment (MoCA) score among participants (P = 0.363). The iRBD group exhibited a higher rapid eye movement sleep behavior disorder screening questionnaire (RBDSQ) score relative to both the HC and PD groups (P < 0.001), and the PD group recorded an elevated RBDSQ score in comparison to the HC group (P < 0.001).
Susceptibility values in the motor system and correlation with clinical symptoms
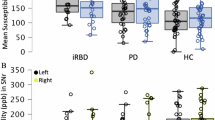
For clarity, all susceptibility values in the Results are expressed in parts per million (ppm). Within the extrapyramidal system, paramagnetic susceptibility values in the substantia nigra pars compacta (SNc) differed significantly among the three participant groups [F(2, 139) = 18.39, P < 0.001, PFDR < 0.001; Fig. 1A]. Tukey’s post hoc analysis revealed significantly elevated paramagnetic substances in the PD group (mean ± SD: 0.127 ± 0.023) compared to both the iRBD (0.115 ± 0.026, P = 0.011, PFDR = 0.030) and HC (0.100 ± 0.016, P < 0.001, PFDR < 0.001) groups, with a significant difference also observed between the iRBD and HC groups (P < 0.001, PFDR = 0.006). Increased diamagnetic susceptibility was detected in the iRBD (0.013 ± 0.003, P < 0.001, PFDR = 0.006) and PD (0.014 ± 0.003, P < 0.001, PFDR < 0.001) compared to HC (0.011 ± 0.002). Furthermore, QSM values were significantly elevated in the PD group (0.114 ± 0.021) compared to both iRBD (0.104 ± 0.024, P = 0.010, PFDR = 0.030) and HC (0.090 ± 0.014, P < 0.001, PFDR < 0.001), with a significant difference also present between the iRBD and HC groups (P < 0.001, PFDR = 0.006).
Paramagnetic, diamagnetic, and quantitative susceptibility values in A SNc. B RN. C aPu. D aCau. E GPi. F Precentral gyrus. Paramagnetic and quantitative susceptibility (left Y-axis) and diamagnetic susceptibility (right Y-axis) values are plotted on separate axes to account for differences in scale. HC healthy control, iRBD idiopathic REM sleep behavior disorder, PD Parkinson’s disease, SNc substantia nigra pars compacta, RN red nucleus, aPu aterior putamen, aCau aterior caudate, GPi internal globus pallidus, ppm parts per million, Para paramagetic susceptibility value, Dia diamagetic susceptibility value, QSM quantitative susceptibility mapping. Error bars represent the standard error. ***P < 0.001; **P < 0.01; *P < 0.05.
Besides, significant group differences in paramagnetic susceptibility were observed in red nucleus (RN) [F(2, 139) = 5.929, P = 0.003, PFDR = 0.006; Fig. 1B]. APART-QSM analysis indicated elevated paramagnetic and diamagnetic values in the iRBD (Paramagnetic: 0.107 ± 0.022, P = 0.003, PFDR = 0.010; Diamagnetic: 0.012 ± 0.003, P = 0.017, PFDR = 0.041) and PD (Paramagnetic: 0.103 ± 0.022, P = 0.022, PFDR = 0.039; Diamagnetic: 0.012 ± 0.003, P = 0.014, PFDR = 0.028) groups compared to HC (Paramagnetic: 0.090 ± 0.022; Diamagnetic: 0.010 ± 0.002). Similarly, QSM values were also significantly elevated in the iRBD (0.097 ± 0.019, P = 0.002, PFDR = 0.007) and PD (0.093 ± 0.019, P = 0.024, PFDR = 0.040) groups compared to HC (0.081 ± 0.019). No significant differences were found between the iRBD and PD groups in any of these values.
Moreover, paramagnetic susceptibility in the anterior putamen (aPu) also differed significantly across groups [F(2, 139) = 5.847, P = 0.004, PFDR = 0.006; Fig. 1C]. Post hoc comparisons indicated that the PD group exhibited significantly higher paramagnetic susceptibility (0.042 ± 0.009) compared to both iRBD (0.038 ± 0.004, P = 0.004, PFDR = 0.037) and HC (0.037 ± 0.005, P = 0.024, PFDR = 0.036). Diamagnetic components were also increased in the iRBD (0.013 ± 0.003, P = 0.012, PFDR = 0.037) and PD (0.013 ± 0.003, P = 0.024, PFDR = 0.040) groups relative to HC (0.011 ± 0.002). However, QSM values in the aPu did not significantly differ across the three groups [F(2, 139) = 0.803, P = 0.450, PFDR = 0.450].
In the anterior caudate (aCau), diamagnetic susceptibility was elevated in PD (0.009 ± 0.002, P = 0.002, PFDR = 0.005) groups compared to HC (0.007 ± 0.001; Fig. 1D). No significant group differences were observed for QSM values in this region [F(2, 139) = 2.055, P = 0.132, PFDR = 0.149].
No significant group differences were detected in paramagnetic, diamagnetic, or QSM values within the posterior putamen or posterior caudate.
In the internal globus pallidus (GPi), paramagnetic susceptibility was significantly higher in the PD group (0.091 ± 0.018) compared to both iRBD (0.08 ± 0.021, P = 0.004, PFDR = 0.037) and HC (0.075 ± 0.016, P < 0.001, PFDR < 0.001; Fig. 1E). Diamagnetic susceptibility did not differ significantly across groups [F(2, 139) = 2.815, P = 0.063, PFDR = 0.082]. QSM values were significantly elevated in the PD group (0.075 ± 0.016) compared to iRBD (0.067 ± 0.019, P = 0.021, PFDR = 0.039) and HC (0.064 ± 0.014, P < 0.001, PFDR < 0.001) in this region.
By contrast, no significant group differences were detected in the external globus pallidus (GPe) for paramagnetic susceptibility, diamagnetic susceptibility, or QSM values.
Regarding the pyramidal system, significant group differences in paramagnetic susceptibility were observed in the precentral gyrus [F(2, 139) = 32.416, P < 0.001, PFDR < 0.001; Fig. 1F]. Both paramagnetic and diamagnetic susceptibility values were significantly higher in the PD group (Paramagnetic: 0.022 ± 0.002; Diamagnetic: 0.013 ± 0.002) compared to iRBD (Paramagnetic: 0.019 ± 0.002, P < 0.001, PFDR < 0.001; Diamagnetic: 0.012 ± 0.002, P = 0.018, PFDR = 0.041) and HC (Paramagnetic: 0.018 ± 0.003, P < 0.001, PFDR < 0.001; Diamagnetic: 0.010 ± 0.002, P < 0.001, PFDR < 0.001). However, QSM values in the precentral gyrus did not differ significantly across groups [F(2, 139) = 0.917, P = 0.402, PFDR = 0.426].
In the cortical spinal tract (CST), diamagnetic susceptibility was elevated in the iRBD (0.044 ± 0.008, P = 0.010, PFDR = 0.034) and PD (0.043 ± 0.009, P = 0.004, PFDR = 0.032) groups compared to HC (0.038 ± 0.008), while QSM values were reduced in PD (−0.036 ± 0.009, P = 0.017, PFDR = 0.037) groups relative to HC (−0.031 ± 0.008). Group differences in susceptibility values were evaluated using analysis of covariance (ANCOVA), with age and sex included as covariates.
Multivariate logistic regression was performed, incorporating all regions that exhibited paramagnetic positivity in differentiating iRBD and PD from HC, including the SNc, RN, aPu, GPi, and precentral gyrus. Predicted probabilities derived from the model were then used for pairwise group comparisons, which demonstrated distinct discriminatory capacities. The classification between PD and HC achieved an area under the curve (AUC) of 0.934, with a sensitivity of 82.7% and specificity of 94.5%. The discrimination between iRBD and HC achieved an AUC of 0.761, with a sensitivity of 66.7% and a specificity of 80.6%. Differentiation between PD and iRBD yielded an AUC of 0.872, with a sensitivity of 78.8% and specificity of 88.9% (Fig. 2A). Collectively, these results indicate high performance for distinguishing PD from HC and differentiating PD from iRBD, with moderate performance for iRBD from HC.
A Group discrimination illustrated by ROC curves based on multivariate logistic regression incorporating paramagnetic-positive regions. Correlations between paramagnetic susceptibility values in B SNc and C aPu with the non-tremor part of MDS-UPDRS III. D Correlations between paramagnetic susceptibility values in GPi with the tremor part of MDS-UPDRS III. E Correlations between paramagnetic susceptibility values in the precentral gyrus with the non-tremor part of MDS-UPDRS III. The shaded area denotes the 95% confidence interval of the correlation. AUC area under curve, PD Parkinson’s disease, iRBD idiopathic rapid eye movement sleep behavior disorder, HC healthy control, MDS-UPDRS III movement disorder society-unified Parkinson’s disease rating scale III, SNc substantia nigra pars compacta, aPu anterior putamen, GPi internal globus pallidus, ppm parts per million, Para paramagetic susceptibility value.
In the iRBD cohort (n = 54), partial Pearson’s correlation analysis adjusted for age and sex revealed no significant association between paramagnetic susceptibility values and RBDSQ score. In contrast, among PD patients (n = 52), after subdividing the movement disorder society-unified Parkinson’s disease rating scale III (MDS-UPDRS III) motor scores into non-tremor components (rigidity, bradykinesia, and postural instability) and tremor components, partial correlation analysis identified a positive correlation between the non-tremor subscore and paramagnetic susceptibility values in the SNc (R2 = 0.225, P < 0.001, PFDR = 0.003; Fig. 2B) and aPu (R2 = 0.236, P < 0.001, PFDR = 0.004; Fig. 2C). Additionally, paramagnetic susceptibility values in the GPi were positively correlated with the tremor subscore (R2 = 0.122, P = 0.013, PFDR = 0.043; Fig. 2D). Furthermore, in the precentral gyrus, a significant positive correlation was observed between paramagnetic susceptibility values and the non-tremor subscore (R2 = 0.115, P = 0.016, PFDR = 0.040; Fig. 2E).
Longitudinal susceptibility value analyses in the iRBD group
Fifteen healthy controls and fifteen iRBD patients were followed longitudinally for 12 to 48 months. The demographic and clinical characteristics of these participants are summarized in Table 2. For regions that exhibited alterations in iRBD at baseline, longitudinal changes were assessed using a linear mixed-effects model adjusted for age and sex. In the SNc of iRBD patients (Fig. 3A), the Group x Time interaction revealed a significant increase in paramagnetic susceptibility values over time compared to the HC group (estimates = 3.042E-04, t = 2.955, P = 0.006, PFDR = 0.037), along with an increase in diamagnetic susceptibility (estimates = 7.590E-05, t = 2.588, P = 0.015, PFDR = 0.045). However, the observed changes in QSM values did not remain statistically significant after adjustment. (estimates = 1.773E-04, t = 2.057, P = 0.050, PFDR = 0.098). No significant longitudinal changes were observed in susceptibility values in the RN, aPu, aCau, or precentral gyrus. In a subset of 48 iRBD patients who underwent dopamine transporter (DAT) imaging scan, partial Pearson’s correlation analysis adjusted for age and sex was performed to evaluate the relationship between paramagnetic susceptibility values and DAT-specific binding ratio (SBR) in the putamen and caudate. A significant negative correlation was found between paramagnetic susceptibility in the SNc and DAT SBR in both the putamen (R2 = 0.131, P = 0.013, PFDR = 0.018) and the caudate (R2 = 0.149, P = 0.008, PFDR = 0.032; Fig. 3B). Additionally, paramagnetic susceptibility in the precentral gyrus was positively correlated with DAT SBR in the caudate (R2 = 0.124, P = 0.017, PFDR = 0.022), but not with the putamen (R2 = 0.047, P = 0.147, PFDR = 0.147; Fig. 3C). No other significant associations were identified.
A The mean paramagnetic, diamagnetic, and quantitative susceptibility value of SNc for the HC and iRBD group at baseline and follow-up. B Correlations between paramagnetic susceptibility values in SNc with SBR of putamen and caudate. C Correlations between paramagnetic susceptibility values in the precentral gyrus with SBR of putamen and caudate. The shaded area denotes the 95% confidence interval of the correlation. HC healthy control, iRBD idiopathic REM sleep behavior disorder, PD Parkinson’s disease, Para paramagetic susceptibility value, Dia diamagetic susceptibility value, QSM quantitative susceptibility mapping, SNc substantia nigra pars compacta, ppm parts per million, SBR specific binding ratio.
Longitudinal susceptibility value analyses in the PD group
Fifteen healthy controls and fifteen PD patients were also followed up for 6 to 48 months. The demographic and clinical characteristics of these participants are presented in Table 2. For regions that exhibited significant differences between PD and HC groups in the cross-sectional cohort, a Group × Time interaction analysis adjusted for age and sex was conducted to assess longitudinal changes. In the SNc of PD patients, there were faster elevations in both paramagnetic (Group x Time: estimates = 2.758E-04, t = 3.327, P = 0.002, PFDR = 0.019) and diamagnetic (Group x Time: estimates = 5.500E-04, t = 2.915, P = 0.007, PFDR PFDR = 0.026) susceptibility components. (Fig. 4A), and follow-up imaging also showed an increase in QSM values over time (Group x Time, estimates = 2.422E-04, t = 3.295, P = 0.003, PFDR = 0.019). Besides, in the aPu and precentral gyrus, there are faster increases in both paramagnetic (Group x Time, aPu: estimates = 1.202E-04, t = 2.639, P = 0.013, PFDR = 0.031; precentral gyrus: estimates = 3.560E-05, t = 2.749, P = 0.010, PFDR = 0.031) and diamagnetic (Group x Time, aPu: estimates = 4.587E-05, t = 2.610, P = 0.014, PFDR = 0.031; precentral gyrus: estimates = 7.048E-05, t = 3.136, P = 0.004, PFDR = 0.019) components during follow-up, while Group x Time interaction in QSM values in the aPu (estimates = 6.300E-05, t = 1.287, P = 0.208, PFDR = 0.284; Fig. 4B) and precentral gyrus (estimates = −1.648E-05, t = −0.750, P = 0.459, PFDR = 0.459; Fig. 4C) did not increase over time.
The mean paramagnetic, diamagnetic, and quantitative susceptibility value of A SNc. B aPu. C precentral gyrus for HC and PD groups at baseline and follow-up. D Partial correlations between paramagnetic susceptibility values in the precentral gyrus with non-tremor symptom prognosis. E Partial correlations between paramagnetic susceptibility values in aPu with non-tremor symptom progression. The shaded area denotes the 95% confidence interval of the correlation. HC healthy control, PD Parkinson’s disease, Para paramagetic susceptibility value, Dia, diamagetic susceptibility value, QSM quantitative susceptibility mapping, SNc substantia nigra pars compacta, aPu anterior putamen, ppm parts per million, MDS-UPDRS III movement disorder society-unified Parkinson’s disease rating scale part III.
Subsequently, we assessed whether baseline paramagnetic susceptibility values in the PD group were predictive of clinical progression. Partial Pearson’s correlation analysis, with age, sex and follow-up duration included as covariates, showed that higher baseline paramagnetic susceptibility in the precentral gyrus was associated with greater progression in non-tremor symptoms among PD patients (R2 = 0.449, P = 0.012, PFDR = 0.018; Fig. 4D). In addition, linear regression analysis was also conducted to examine the relationship between changes in paramagnetic susceptibility and changes in clinical symptom scores over time. This analysis revealed that an increase in paramagnetic susceptibility in the aPu was positively associated with worsening non-tremor symptoms in PD patients during follow-up (R2 = 0.693, P < 0.001, PFDR = 0.001; Fig. 4E).
Discussion
In this study, we applied APART-QSM to effectively detect both transverse and longitudinal iron changes in individuals with iRBD and PD. We observed elevated paramagnetic susceptibility values in the SNc and RN in both iRBD and PD groups, consistent with increased QSM values. In contrast, increased paramagnetic susceptibility was detected in the aPu of PD patients, accompanied by elevated diamagnetic substances, resulting in no significant overall change in QSM. Within the GPi, the increase in paramagnetic substances was consistent with the corresponding rise in QSM. Notably, we report for the first time a significant increase in both paramagnetic and diamagnetic susceptibility in the precentral gyrus of PD patients, despite the absence of significant alterations in QSM values. Furthermore, paramagnetic susceptibility levels in the SNc, aPu, and precentral gyrus were significantly associated with the severity of non-tremor symptoms in PD. In contrast, levels in the GPi were associated with tremor symptoms. Although no significant longitudinal changes in QSM values were observed in the SNc of iRBD patients, APART-QSM revealed progressive increases in both paramagnetic and diamagnetic susceptibility components. The paramagnetic increases in SNc and precentral gyrus were significantly associated with dopaminergic degeneration based on basal ganglia SBR during the iRBD stage. As for the follow-up PD group, increases were observed in paramagnetic susceptibility and QSM within the SNc, whereas in the aPu and precentral gyrus, only paramagnetic and diamagnetic components increased over time, without a significant change in total QSM. Importantly, baseline paramagnetic susceptibility in the precentral gyrus predicted non-tremor symptom severity at follow-up in PD, while increasing paramagnetic values in the aPu were significantly associated with non-tremor symptom progression.
Our results demonstrate that the novel APART-QSM technique effectively distinguishes between paramagnetic and diamagnetic susceptibility sources, thereby may improve the sensitivity of brain iron assessment. Previous studies have identified increased QSM values in the SNc and RN of iRBD patients, and in the SNc, RN, and GPi of PD patients35,36. APART-QSM enables us to determine that both paramagnetic and diamagnetic components drive the QSM increase in the SNc and RN, whereas in the GPi, the increase is primarily attributed to the paramagnetic component. Two studies comparing clinical PD patients with older adults found QSM increases in the putamen but no changes in the caudate17,37, whereas another study reported no QSM changes in the putamen or caudate head in PD16, only one study reported increased QSM values in the caudate head in PD patients with mild cognitive impairment38. To address these inconsistencies, we subdivided the putamen and caudate structures into anterior and posterior parts based on structural connectivity profiles and observed that, although the overall QSM change in the aPu was negative in our study, both paramagnetic and diamagnetic susceptibility values increased in the aPu of PD patients. This suggests that iron changes indeed occur, specifically in the anterior region, which may sometimes be masked by the increase of diamagnetic components. We also found a slight increase in diamagnetic content in the aCau of PD patients, without significant iron changes or association with any clinical symptom severity in PD. In addition, previous cortical imaging studies did not report QSM changes in the precentral gyrus39,40, and our study is the first to use APART-QSM to show that the precentral gyrus exhibits iron interference compared to HC, which may be obscured by diamagnetic increases in PD. Currently, there are no longitudinal QSM studies in iRBD patients. We first demonstrate that there were progressive increases in both paramagnetic and diamagnetic components in SNc over time, though no significant QSM changes were observed during follow-up. In the longitudinal analysis of PD, two previous QSM follow-up studies did not detect significant QSM changes in the basal ganglia system20,21, and only one study reported an increase in QSM values in the ventral part of the substantia nigra over three years compared to HC22. Another study, which stratified patients into de novo, mild, and moderate PD stages, demonstrated a progressive increase in R2* values in the aPu41. In our follow-up analysis, we also found QSM increases in the SNc but no detectable QSM changes in the aPu or precentral gyrus. However, by applying APART-QSM, we identified paramagnetic iron changes in these regions, accompanied by concurrent increases in diamagnetic components. Together, these results suggest that APART-QSM has an improved ability to minimize diamagnetic interference and to detect paramagnetic iron changes with higher sensitivity, thereby revealing additional regions of iron deposition not identified in conventional QSM studies.
Our study also reports that the precentral gyrus presents as a useful image biomarker. Results showed that paramagnetic substances were increased in the precentral gyrus of PD patients and showed continuous progression during PD follow-up, while no changes were found in the iRBD group. In the meantime, the paramagnetic susceptibility value in this area was correlated with caudate SBR in iRBD, and it can predict longitudinal non-tremor progression in PD, reflecting its potential to indicate dopaminergic degeneration and motor symptom aggravation. Since nigrostriatal deficits detected by DAT imaging are currently regarded as the most robust imaging predictors for iRBD future phenoconversion42, we propose that the precentral gyrus may serve as a potential imaging biomarker for marking the transition from iRBD to PD, thereby facilitating the early recognition of incident PD cases for inclusion in disease-modifying treatment trials. Previous studies have also found that iRBD patients who later converted to clinically defined synucleinopathies demonstrated baseline cortical thinning in the precentral gyrus43, which also underscores the importance of this region as a sensitive early indicator of disease conversion. Iron chelators, such as deferiprone, have previously been proposed as a strategy to reduce excess free iron in the brain, thereby alleviating oxidative stress, inhibiting ferroptosis, and improving motor function. However, current evidence on iron chelation therapy remains inconclusive. One trial showed that early treatment with deferiprone reduced substantia nigra iron content and slowed disease progression44. Another study reported iron reductions in the dentate and caudate nuclei but limited effects in the substantia nigra and no significant clinical improvement45. A third trial in drug-naïve patients demonstrated that, despite lowering nigrostriatal iron, deferiprone worsened symptoms and was associated with more adverse events46. These findings suggest that iron dysregulation in PD is more complex than simple overload, and that indiscriminate iron reduction may induce neurotoxicity, considering the essential roles of iron in neurotransmitter synthesis and mitochondrial function. APART-QSM may facilitate future investigations into the detection of phenoconversion from iRBD to PD, and, by enabling the identification of de novo PD patients, could support the early evaluation of candidate disease-modifying interventions47, such as iron chelation therapy.
In previous studies investigating symptom correlation, four studies did not find correlations between QSM values and MDS-UPDRS III scores16,18,20,40, only two studies revealed a significant association between higher MDS-UPDRS III scores and increased QSM in the right caudal putamen and left SNc36,37. Considering the variability between susceptibility values and motor symptoms, we subdivided the MDS-UPDRS III scores into tremor and non-tremor components, because previous studies have found that tremor-dominant PD subtypes may show different patterns of motor impairment and distinct cortical and subcortical morphological alterations compared to akinetic-rigid or postural instability gait difficulty PD subtypes48,49. One small-sample study investigated regional brain iron deposition differences between akinetic/rigid-dominant (PD-AR) and tremor-dominant (PD-TD) PD subtypes utilizing susceptibility-weighted imaging. Still, it did not further explore the relationship between iron accumulation level and subtype-specific symptom severity50. Our study demonstrated that paramagnetic components in the SNc, aPu, and precentral gyrus are associated with the non-tremor components of MDS-UPDRS III, while paramagnetic components in the GPi are associated with the tremor components. Thus, we identified symptom-specific imaging biomarkers. And this regional specificity of associations resonates with recent literature emphasizing that tremor and non-tremor symptoms in PD may be linked to partially distinct pathophysiological mechanisms51,52. One study has reported that while rigidity and bradykinesia correlate negatively with contralateral striatal DAT binding, resting tremor amplitude shows a positive association with increased ipsilateral DAT binding, indicating that tremor does not exhibit a stable correlation with contralateral dopaminergic loss52. Similarly, microelectrode recordings in PD patients have demonstrated oscillatory activity at tremor frequency in neurons of the ventral GPi and subthalamic nucleus (STN), which suggests that intrinsic oscillatory properties of the GPi, and STN may play a key role in tremor generation51. Moreover, the frequent use of the GPi as a DBS target for drug-refractory tremor further underscores its central role in tremor pathophysiology in PD53,54. Taken together, these evidence supports the notion that Parkinsonian tremor cannot be fully accounted for by nigrostriatal dopaminergic denervation alone, but rather arises from a distinct functional neurocircuit. Our longitudinal analysis further validated that the progression of paramagnetic susceptibility in the aPu is associated with the worsening of non-tremor motor symptoms during follow-up. Though the posterodorsal putamen has traditionally been associated with sensorimotor processing in PD patients, emerging evidence highlights the involvement of the aPu in motor function as well. One structural imaging study has reported that larger local volumes of the aPu in PD patients are associated with higher motor reserve55, underscoring the potential role of aPu in motor regulation. Our findings further support this notion by demonstrating that increased iron accumulation in the aPu is specifically associated with the progression of motor symptom severity. In all, these structures represent promising targets for future individualized, symptom-driven therapies. Notably, we adopted bilateral averaging during the ROI evaluation, as our aim was to assess overall changes in paramagnetic susceptibility of the motor system. This approach has also been applied in several previous QSM studies56,57, since it can enhance robustness by reducing noise from side-to-side variability and minimizing the influence of individual anatomical differences. Nevertheless, such averaging may also mask hemispheric asymmetry, which can be clinically relevant in early PD.
Paramagnetic susceptibility values have been validated in two recent studies for their accuracy in quantifying iron concentrations through post-mortem brain analyses. In post-mortem Alzheimer’s disease brains, paramagnetic susceptibility measured by APART-QSM showed a strong positive correlation with iron-positive areas identified by Perls’ Prussian blue staining enhanced with diaminobenzidine (DAB)33. Similarly, in epilepsy patients, paramagnetic susceptibility values demonstrated a significant linear correlation with iron concentrations measured by laser ablation–inductively coupled plasma–time-of-flight mass spectrometry, as well as with iron deposition patterns confirmed by Perls/DAB histological staining31. These findings highlight the paramagnetic susceptibility as a more sensitive biomarker of iron-related pathology in PD. A recent study has applied APART-QSM to PD patients with and without RBD, demonstrating that paramagnetic susceptibility values provide superior diagnostic performance over conventional QSM at the regional SNc level58. Our study further complements this work by adopting a longitudinal design to characterize the temporal trajectory of iron accumulation from iRBD to PD, and by assessing the potential of paramagnetic susceptibility as a marker to reflect symptom severity, disease progression and phenocoversion from iRBD to PD. In contrast, the histological significance of the diamagnetic component remains less clear. In post-mortem Alzheimer’s disease brains, diamagnetic susceptibility values were highly correlated with Aβ-positive areas measured by immunohistochemistry in the hippocampus, middle frontal gyrus, and several other cortical regions, likely due to the high diamagnetic susceptibility of pathological protein deposits33. Based on this, we hypothesize that the increase in diamagnetic values observed in iRBD and PD may be primarily driven by α-synuclein accumulation. One case series of post-mortem neuropathological examinations in iRBD patients reported severe neuronal α-synuclein deposition in the SNc59, aligning with the observed elevation in diamagnetic components in this region. However, only isolated neuronal α-synuclein was detected in the RN, which does not correspond to the diamagnetic increase we observed in this nucleus59. As for PD, both historical and recent pathological research has demonstrated increased α-synuclein burden in the substantia nigra, RN, putamen, caudate, and primary motor cortex in PD60,61,62, corresponding to the regions with observed diamagnetic increases. Notably, no significant Lewy body burden was reported in the GP, which also matches our findings62,63. However, we did not observe a significant diamagnetic difference between iRBD and PD in the SNc, RN, putamen, or caudate, which challenges the assumption of α-synuclein accumulation during the conversion from iRBD to PD. There was another ex vivo experiment using macaque brains, revealing that diamagnetic susceptibility values showed a strong correlation with myelin distribution, as confirmed by Luxol fast blue staining in major white matter tracts27. Studies have reported significantly higher fractional anisotropy (FA) in the corticospinal tract in both iRBD and PD64,65, consistent with the observed elevation in diamagnetic components in this tract. However, most studies have reported decreased FA in the substantia nigra and striatum, suggesting impaired myelin integrity66. It is also possible that α-synuclein accumulation and myelin structure alterations simultaneously contribute to the observed diamagnetic changes. Therefore, further pathological studies specifically investigating the biological basis of diamagnetic susceptibility values are necessary to elucidate our findings.
Elevated brain iron levels were found to contribute to the selective vulnerability of dopaminergic neurons in animal studies67. Cytoplasmic iron increase promotes the non-enzymatic oxidation of dopamine, leading to the formation of reactive o-quinones and 6-hydroxydopamine, both of which exert direct cytotoxic effects by modifying proteins, damaging mitochondria, and generating reactive oxygen species67. Consequently, dopaminergic terminals undergo progressive degeneration, which is reliably reflected by reductions in the SBR in molecular imaging studies68,69. The SBR is derived from the ratio of ligand uptake in striatal regions of interest, relative to a reference region with negligible dopamine transporter expression, thereby providing a semiquantitative measure of presynaptic dopamine transporter availability68,69. A recent research found that QSM values in several clusters within the left substantia nigra pars reticulata and compacta in iRBD were negatively correlated with ipsilateral SBR in the posterior putamen18. Our study similarly demonstrated that paramagnetic susceptibility values in the SNc of iRBD patients are inversely associated with SBR of the putamen and caudate. Given that APART-QSM has revealed an increase in paramagnetic components within the SNc during the progression of iRBD, these findings collectively support the utility of SNc paramagnetic susceptibility as a reliable biomarker for tracking dopaminergic neurodegeneration during the prodromal phase of PD patients.
This study also has several limitations. First, the small sample size of the longitudinal cohort may limit the generalizability of our findings. Nevertheless, this study serves as a valid preliminary investigation, and future replication in a larger cohort would strengthen the validity of our results. Second, the study focused exclusively on comparing iRBD with PD; however, iRBD is recognized as a prodromal stage for various synucleinopathies, including PD, dementia with Lewy bodies, and multiple system atrophy34,70. Therefore, future research should compare iRBD, PD, MSA, and DLB using APART-QSM techniques to achieve a more comprehensive understanding of these disorders. Third, we did not establish the pathological significance of the diamagnetic component, which requires further pathological validation to support our hypothesis. Another limitation of this study is that within the PD cohort, 30 patients scored above the RBDSQ cut-off of 671, indicative of comorbid RBD symptoms, while 22 patients did not. This heterogeneity may have introduced potential confounding effects into our analysis. Future studies should carefully account for disease subtypes in order to more precisely delineate group-specific paramagnetic alterations. Finally, symptom severity in iRBD patients was assessed solely with the RBDSQ72, which, although widely validated and convenient, offers less granularity regarding symptom dimensions and severity than instruments such as the REM sleep behavior disorder questionnaire-Hong Kong (RBDQ-HK) or the REM sleep behavior disorder symptom severity scale (RBDSSS)73,74,75. Future studies incorporating these more detailed scales may better elucidate the relationship between paramagnetic susceptibility and specific iRBD phenotypes.
In conclusion, this study provides compelling evidence that the advanced APART-QSM technique enables a precise characterization of iron accumulation change in both iRBD and PD groups. We identified novel brain regions exhibiting iron burden during the progression of iRBD and PD. The paramagnetic susceptibility values may serve as potential biomarkers reflecting tremor or non-tremor symptom severity, predicting disease progression and phenoconversion from iRBD to PD.
Methods
Participants
The study was conducted at Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine, from August 2020 to January 2025, and received ethical approval from the institution’s ethics committee. Informed consent was obtained in written form from all participants. The cohort consisted of 116 subjects, comprising 36 HC, 54 iRBD patients, and 52 PD patients. A subset of 15 healthy controls, 15 iRBD patients, and 15 PD patients were reassessed after a follow-up period ranging from 6 to 48 months. Enrollment criteria included: (i) age between 55 and 80 years; (ii) MMSE score of 24 or higher, indicating no dementia; (iii) absence of prior intracranial surgery or traumatic brain injury; (iv) no psychiatric disorders; (v) no alcohol use disorder; and (vi) no history of other neurological disorders. HCs were recruited from various communities in Shanghai and among social workers at Ruijin Hospital. These participants exhibited no symptoms of iRBD and were screened by two neurologists to confirm the absence of any motor symptoms or neurological diseases. Patients with iRBD, exhibiting characteristic symptoms of the disorder, underwent video-polysomnography utilizing a Compumedics E-Series Electroencephalography/Polysomnography Recording System (Compumedics Ltd). The diagnosis of iRBD was confirmed through both clinical assessment and polysomnographic evidence following the International Classification of Sleep Disorders-3 criteria76. These patients were also evaluated by two experienced neurologists to rule out potential Parkinsonism. Patients with PD were diagnosed based on the Movement Disorders Society’s clinical diagnostic criteria1 by at least two movement disorder specialists and were assessed using the MDS-UPDRS III and the Hoehn and Yahr stage. Demographic data and clinical symptom scores, including MDS-UPDRS III, MMSE, MOCA, and RBDSQ, were collected from all participants. Table 1 presents the demographic characteristics of the cross-sectional participants, while Table 2 summarizes the follow-up data. All subjects underwent T1-weighted and QSM magnetic resonance imaging (MRI) scans, with 48 patients with iRBD additionally undergoing dopamine transporter positron emission tomography/magnetic resonance imaging (DAT-PET/MRI) scans to further assess dopaminergic function.
MRI and PET acquisition
MRI data acquisition was performed using a 3 T Siemens scanner equipped with a 12-channel head coil, located at the Department of Radiology, Ruijin Hospital. T1-weighted images were generated through a 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence. The parameters for this sequence were as follows: 192 axial slices, voxel dimensions of 1.0 × 1.0 × 1.0 mm³, echo time (TE) of 2.96 ms, repetition time (TR) of 2300 ms, and an inversion time (TI) of 1000 ms, a flip angle of 9 degrees. QSM images were captured under the following conditions: field of view (FOV) of 240 mm², 80 slices, voxel dimensions of 0.7 × 0.7 × 2.0 mm³, with a multi-echo sequence capturing echo times at 5.87, 12.57, 19.27, and 25.97 ms, and a TR of 32 ms. Diffusion tensor imaging (DTI) images were acquired with the following parameters: FOV of 256 mm2, 74 slices, voxel dimensions of 2.0 × 2.0 × 2.0 mm3, TE of 90 ms, TR of 10,200 ms, b-values of 0 and 1000 s/mm2, and diffusion gradient directions of 30. Hybrid PET/MRI examinations were conducted using a Siemens Biograph mMR scanner (Siemens Healthcare), featuring an eight-channel phase array head coil. Patients were administered an intravenous injection of 18F-N-(3-fluoropropyl)-2β-carbon ethoxy-3β-(4-iodophenyl) nortropane (18F-FP-CIT) at a mean dosage of 3.7 MBq/kg body weight, which was provided by Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Subsequently, static 18F-FP-CIT PET data were collected in sinogram mode for 15 min. Imaging parameters included 128 slices per slab with a 0.5 mm gap, a matrix size of 344 × 344, and image reconstruction utilizing ordered subsets expectation maximization (OSEM) with 21 subsets, four iterations, and post-filtering using an isotropic full-width at half-maximum Gaussian kernel of 2 mm. Attenuation correction was meticulously executed using an advanced PET attenuation correction technique that employs a unique five-compartment model, which includes bone structures.
Image analysis
The QSM images were reconstructed from gradient-recalled echo (GRE) data using the STI-Suite (https://people.eecs.berkeley.edu/∼chunlei.liu/software.html). Specifically, the multi-echo 3D GRE phase images were first unwrapped by a Laplacian-based phase unwrapping method77. Then, the tissue phase was extracted using the V-SHARP method to avoid the effect of the background phase78. Subsequently, the STAR-QSM method was applied to reconstruct the QSM maps79. The paramagnetic and diamagnetic susceptibility maps were reconstructed using the proposed APART-QSM method27. Briefly, the APART-QSM employed a comprehensive complex data model on the raw GRE signal:
where χ_para is the paramagnetic susceptibility, χ_dia is the diamagnetic susceptibility, ϕ_res represents the time-independent residual phase, ϕ_bg represents an echo time-dependent background phase, D is the magnetic dipole kernel, and * denotes the spatial convolution. Specifically, a is the magnitude decay kernel, which is a proportionality constant between R2’ ( = R2*- R2) and absolute susceptibility sources. APART-QSM utilized an iterative algorithm to solve the optimization problem and estimate the voxel-wise parameter α-map. The estimation of an R2 map is typically acquired using a multi-echo spin-echo (SE) sequence, which can be time-consuming. To avoid the need for additional R2 acquisitions, we used a first-order approximation to estimate R2’ from R2*, as proposed in ref. 80. The reconstruction code is available at https://doi.org/10.5281/zenodo.14375619.
Subcortical regions of interest (ROI) were delineated utilizing the multi-modal-fused magnetic Susceptibility (MuSus-100) atlas81, then guided by a connectivity-based brain atlas82 together with a globus pallidus (GP) subdivision map83,84, we further subdivided the caudate nucleus into anterior and posterior caudate regions, the putamen into anterior and posterior putamen regions, and the GP into internal and external segments, as illustrated in Fig. 5, which encompasses bilateral SNc, RN (Fig. 5A), aCau, aPu, posterior putamen, GPi and GPe (Fig. 5B). Next, we registered the brain atlas to the QSM images using the advanced normalization tools (ANTs, http://picsl.upenn.edu/software/ants). Standard QSM images were first registered to individual QSM images, and the deformation fields generated by ANTs were then applied to warp the subcortical atlas ROIs into each subject’s native QSM space. The ROIs of the SNc and RN were manually adjusted to enhance spatial accuracy. Manual adjustments of the ROIs followed the principles below: (1) Corrections were performed across all contiguous slices in the axial plane to ensure well-defined nuclear boundaries; (2) The first and last slices of each nucleus were excluded; (3) ROI boundaries were delineated one pixel inside the visible edge to ensure inclusion within the nuclear structure; (4) Obvious vascular structures were excluded. To ensure inter-rater reliability, two researchers independently delineated the ROIs, followed by a consensus discussion to resolve discrepancies. For intra-rater reliability, one researcher repeated the delineation 6 weeks after the initial drawing. Finally, quantitative, paramagnetic, and diamagnetic susceptibility values were extracted from the defined ROIs above for subsequent analyses. In the analysis of the precentral gyrus, T1-weighted images and magnitude images underwent skull-stripping via brain extraction tools, then T1-weighted images were coregistered to the magnitude images and subsequently segmented into anatomical regions using FreeSurfer version 7.4.1, based on the Destrieux atlas. Then, depth-wise cortical surfaces were generated at fractional intervals of 10% from the pial surface toward the gray-white matter interface85. Quantitative, paramagnetic, and diamagnetic susceptibility values were sampled along these surfaces, ranging from 10 to 90% cortical depth. This sampling strategy was implemented to reduce the influence of boundary artifacts at both the pial surface and the gray-white matter interface. As for the CST, the mean fractional anisotropy (FA) image of all patients was skeletonized to obtain a skeletonized mask using FSL Version 6.0.7.7. The skeleton corresponding to the posterior internal capsule was selected to represent the CST. Subsequently, the CST mask was coregistered to the individual magnitude images, following the same procedure as previously described through ANTs, and susceptibility values were extracted. Furthermore, dopaminergic transporter availability within the caudate and putamen was quantitatively assessed using 18F-FP-CIT PET. Caudate and putamen served as the target ROI while the occipital cortex was utilized as the reference region. SBR were computed using the formula: [(target region/reference region) – 1]. Values obtained from the left and right sides of the brain were averaged to derive mean susceptibility values.
The ROIs included A substantia nigra pars compacta and red nucleus, B anterior putamen, posterior putamen, anterior caudate, internal and external globus pallidus, and cortical spinal tract, C precentral gyrus. Para paramagnetic susceptibility mapping, Dia diamagnetic susceptibility mapping, QSM quantitative susceptibility mapping, ppm parts per million.
Statistical analysis
Participant demographic and clinical characteristics were analyzed using χ2 tests for categorical variables and analysis of variance (ANOVA) for continuous variables. Considering that both age and sex are known to potentially affect iron deposition in the brain47,86, we incorporated them as covariates in the subsequent analyses in order to control for their confounding effects. Group differences in susceptibility values were evaluated using ANCOVA, with age and sex included as covariates. Post hoc pairwise comparisons were performed with Tukey’s adjustment for multiple testing. Pairwise group comparisons were performed using multivariate logistic regression, with positive paramagnetic areas entered as independent variables. Predicted probabilities of group membership were derived from the fitted models, and model performance was assessed by receiver operating characteristic (ROC) analysis. To examine the longitudinal effects of disease progression on susceptibility values, linear mixed-effects models were fitted using restricted maximum likelihood estimation. In these models, time was treated as a continuous variable, and we evaluated the interaction between Group and Time (Group × Time), while additionally adjusting for sex and age as covariates. Random intercepts were included for each subject to account for within-subject correlations, and the residual errors were modeled with a compound symmetry covariance structure. Associations between susceptibility values in specific ROIs and clinical symptom scores, as well as dopaminergic function, were assessed using partial Pearson’s correlations, adjusting for age and sex. All statistical analyses were conducted using R version 4.4.1. All reported P are adjusted for age and sex, and PFDR represent P value further corrected for multiple comparisons using the false discovery rate (FDR) method, with the significance threshold set at PFDR < 0.05.
Data availability
The research data supporting the findings of this study are available from the corresponding author upon reasonable request. The raw imaging data are not publicly available due to privacy and ethical considerations.
References
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303 (2021).
Berg, D. et al. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 30, 1600–1611 (2015).
Tekriwal, A. et al. REM sleep behaviour disorder: prodromal and mechanistic insights for Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 88, 445–451 (2017).
Iranzo, A. et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS ONE 9, e89741 (2014).
Yuan, Y. et al. Factors associated with phenoconversion of idiopathic rapid eye movement sleep behavior disorder: a prospective study. NPJ Parkinsons Dis. 11, 10 (2025).
Galbiati, A., Verga, L., Giora, E., Zucconi, M. & Ferini-Strambi, L. The risk of neurodegeneration in REM sleep behavior disorder: a systematic review and meta-analysis of longitudinal studies. Sleep. Med Rev. 43, 37–46 (2019).
Ye, G. et al. Predictors of conversion to α-synucleinopathy diseases in idiopathic rapid eye movement sleep behavior disorder. J. Parkinsons Dis. 10, 1443–1455 (2020).
Zhou, L. et al. Increased free water in the substantia nigra in idiopathic REM sleep behaviour disorder. Brain 144, 1488–1497 (2021).
Rolinski, M. et al. Basal ganglia dysfunction in idiopathic REM sleep behaviour disorder parallels that in early Parkinson’s disease. Brain 139, 2224–2234 (2016).
Tang, C. C. et al. Longitudinal network changes and phenoconversion risk in isolated REM sleep behavior disorder. Nat. Commun. 15, 10797 (2024).
Jiang, H., Wang, J., Rogers, J. & Xie, J. Brain iron metabolism dysfunction in Parkinson’s disease. Mol. Neurobiol. 54, 3078–3101 (2017).
Zeng, W., Cai, J., Zhang, L. & Peng, Q. Iron Deposition in Parkinson’s disease: a mini-review. Cell Mol. Neurobiol. 44, 26 (2024).
Moreau, C. et al. Iron as a therapeutic target for Parkinson’s disease. Mov. Disord. 33, 568–574 (2018).
Abeyawardhane, D. L. et al. Iron redox chemistry promotes antiparallel oligomerization of α-synuclein. J. Am. Chem. Soc. 140, 5028–5032 (2018).
Sun, J. et al. Quantitative evaluation of iron content in idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 35, 478–485 (2020).
Guan, X. et al. Altered brain iron depositions from aging to Parkinson’s disease and Alzheimer’s disease: a quantitative susceptibility mapping study. Neuroimage 264, 119683 (2022).
Varga, Z. et al. Whole brain pattern of iron accumulation in REM sleep behavior disorder. Hum. Brain Mapp. 45, e26675 (2024).
Maass, F. et al. Cerebrospinal fluid iron-ferritin ratio as a potential progression marker for Parkinson’s disease. Mov. Disord. 36, 2967–2969 (2021).
Thomas, G., Hannaway, N., Zarkali, A., Shmueli, K. & Weil, R. S. Longitudinal associations of magnetic susceptibility with clinical severity in Parkinson’s disease. Mov. Disord. 39, 546–559 (2024).
Du, G. et al. Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson’s patients. Mov. Disord. 33, 1423–1431 (2018).
Bergsland, N. et al. Ventral posterior substantia nigra iron increases over 3 years in Parkinson’s disease. Mov. Disord. 34, 1006–1013 (2019).
Chen, P., Miah, M. R. & Aschner, M. Metals and neurodegeneration. F1000Res 5, F1000 Faculty Rev-366 (2016).
Deistung, A., Schweser, F. & Reichenbach, J. R. Overview of quantitative susceptibility mapping. NMR Biomed. 30, e3569 (2017).
Duyn, J. H. & Schenck, J. Contributions to magnetic susceptibility of brain tissue. NMR Biomed. 30 (2017).
Babaei, M., Jones, I. C., Dayal, K. & Mauter, M. S. Computing the diamagnetic susceptibility and diamagnetic anisotropy of membrane proteins from structural subunits. J. Chem. Theory Comput. 13, 2945–2953 (2017).
Li, Z. et al. APART-QSM: an improved sub-voxel quantitative susceptibility mapping for susceptibility source separation using an iterative data fitting method. Neuroimage 274, 120148 (2023).
Dimov, A. V. et al. Susceptibility source separation from gradient echo data using magnitude decay modeling. J. Neuroimaging 32, 852–859 (2022).
Chen, J., Gong, N. J., Chaim, K. T., Otaduy, M. & Liu, C. Decompose quantitative susceptibility mapping (QSM) to sub-voxel diamagnetic and paramagnetic components based on gradient-echo MRI data. Neuroimage 242, 118477 (2021).
Shin, H. G. et al. χ-separation: magnetic susceptibility source separation toward iron and myelin mapping in the brain. Neuroimage 240, 118371 (2021).
Li, Z. et al. Paramagnetic susceptibility measured by magnetic resonance imaging as an in vivo biomarker for iron pathology in epilepsy. Sci. Adv. 11, eads8149 (2025).
Zhu, Z. et al. MR susceptibility separation for quantifying lesion paramagnetic and diamagnetic evolution in relapsing-remitting multiple sclerosis. J. Magn. Reson. Imaging 60, 1867–1879 (2024).
Yao, J. et al. Distinct regional vulnerability to Aβ and iron accumulation in post mortem AD brains. Alzheimers Dement. 20, 6984–6997 (2024).
Zhang, F. et al. Rapid eye movement sleep behavior disorder and neurodegenerative diseases: an update. Aging Dis. 11, 315–326 (2020).
Nepozitek, J. et al. Magnetic susceptibility changes in the brainstem reflect REM sleep without atonia severity in isolated REM sleep behavior disorder. NPJ Parkinsons Dis. 9, 112 (2023).
Zhang, D. et al. Iron accumulation in the ventral tegmental area in Parkinson’s disease. Front. Aging Neurosci. 15, 1187684 (2023).
Thomas, G. et al. Brain iron deposition is linked with cognitive severity in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 91, 418–425 (2020).
Uchida, Y. et al. Voxel-based quantitative susceptibility mapping in Parkinson’s disease with mild cognitive impairment. Mov. Disord. 34, 1164–1173 (2019).
Thomas, G. et al. Regional brain iron and gene expression provide insights into neurodegeneration in Parkinson’s disease. Brain 144, 1787–1798 (2021).
Acosta-Cabronero, J. et al. The whole-brain pattern of magnetic susceptibility perturbations in Parkinson’s disease. Brain 140, 118–131 (2017).
López-Aguirre, M. et al. Nigrostriatal iron accumulation in the progression of Parkinson’s disease. NPJ Parkinsons Dis. 11, 72 (2025).
Miglis, M. G. et al. Biomarkers of conversion to α-synucleinopathy in isolated rapid-eye-movement sleep behaviour disorder. Lancet Neurol. 20, 671–684 (2021).
Pereira, J. B. et al. Cortical thinning in patients with REM sleep behavior disorder is associated with clinical progression. NPJ Parkinsons Dis. 5, 7 (2019).
Devos, D. et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal 21, 195–210 (2014).
Martin-Bastida, A. et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci. Rep. 7, 1398 (2017).
Devos, D. et al. Trial of deferiprone in Parkinson’s disease. N. Engl. J. Med. 387, 2045–2055 (2022).
Alushaj, E. et al. Midbrain and pallidal iron changes identify patients with REM sleep behaviour disorder and Parkinson’s disease. NPJ Parkinsons Dis. 11, 84 (2025).
Li, J. et al. Cortical and subcortical morphological alterations in motor subtypes of Parkinson’s disease. NPJ Parkinsons Dis. 8, 167 (2022).
Zhang, W. et al. Wearable sensor-based quantitative gait analysis in Parkinson’s disease patients with different motor subtypes. NPJ Digit. Med. 7, 169 (2024).
Xiong, W. et al. Different iron deposition patterns in akinetic/rigid-dominant and tremor-dominant Parkinson’s disease. Clin. Neurol. Neurosurg. 198, 106181 (2020).
Dirkx, M. F. & Bologna, M. The pathophysiology of Parkinson’s disease tremor. J. Neurol. Sci. 435, 120196 (2022).
Niemi, K. J. et al. Rest tremor in Parkinson’s disease is associated with ipsilateral striatal dopamine transporter binding. Mov. Disord. 39, 2014–2025 (2024).
Au, K. et al. Globus pallidus internus (GPi) deep brain stimulation for Parkinson’s disease: expert review and commentary. Neurol. Ther. 10, 7–30 (2021).
Wong, J. K. et al. STN vs. GPi deep brain stimulation for tremor suppression in Parkinson disease: a systematic review and meta-analysis. Parkinsonism Relat. Disord. 58, 56–62 (2019).
Jeong, S. H. et al. Local striatal volume and motor reserve in drug-naïve Parkinson’s disease. NPJ Parkinsons Dis. 8, 168 (2022).
He, N. et al. Imaging iron and neuromelanin simultaneously using a single 3D gradient echo magnetization transfer sequence: combining neuromelanin, iron and the nigrosome-1 sign as complementary imaging biomarkers in early stage Parkinson’s disease. Neuroimage 230, 117810 (2021).
Ni, C. et al. Diffusion tensor image analysis along the perivascular space and quantitative susceptibility mapping in the diagnosis and severity assessment of Parkinson’s disease. Quant. Imaging Med. Surg. 15, 1411–1424 (2025).
Dong, L. et al. Paramagnetic susceptibility mapping better quantifies brain iron content in Parkinson’s disease with RBD. NPJ Parkinsons Dis. 11, 192 (2025).
Mayà, G. et al. Post-mortem neuropathology of idiopathic rapid eye movement sleep behaviour disorder: a case series. Lancet Neurol. 23, 1238–1251 (2024).
Johnson, M. L. et al. Neuropathological findings and in vivo imaging correlates of the red nucleus compared to those of the substantia nigra pars compacta in parkinsonisms. Parkinsonism Relat. Disord. 125, 107043 (2024).
Sekiya, H. et al. Discrepancy between distribution of alpha-synuclein oligomers and Lewy-related pathology in Parkinson’s disease. Acta Neuropathol. Commun. 10, 133 (2022).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Mori, F. et al. alpha-Synuclein pathology in the neostriatum in Parkinson’s disease. Acta Neuropathol. 115, 453–459 (2008).
Fu, Y. et al. Adaptive structural changes in the motor cortex and white matter in Parkinson’s disease. Acta Neuropathol. 144, 861–879 (2022).
Shih, Y. C., Tseng, W. I. & Montaser-Kouhsari, L. Recent advances in using diffusion tensor imaging to study white matter alterations in Parkinson’s disease: a mini review. Front Aging Neurosci. 14, 1018017 (2022).
Zhang, Y. & Burock, M. A. Diffusion tensor imaging in Parkinson’s disease and Parkinsonian syndrome: a systematic review. Front Neurol. 11, 531993 (2020).
Hare, D. J. & Double, K. L. Iron and dopamine: a toxic couple. Brain 139, 1026–1035 (2016).
Palermo, G. & Ceravolo, R. Molecular imaging of the dopamine transporter. Cells 8, 872 (2019).
Palermo, G., Giannoni, S., Bellini, G., Siciliano, G. & Ceravolo, R. Dopamine transporter imaging, current status of a potential biomarker: a comprehensive review. Int. J. Mol. Sci. 22, 11234 (2021).
Postuma, R. B. et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 142, 744–759 (2019).
Halsband, C., Zapf, A., Sixel-Döring, F., Trenkwalder, C. & Mollenhauer, B. The REM sleep behavior disorder screening questionnaire is not valid in de novo Parkinson’s disease. Mov. Disord. Clin. Pract. 5, 171–176 (2018).
Stiasny-Kolster, K. et al. The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov. Disord. 22, 2386–2393 (2007).
Li, S. X. et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK). Sleep. Med. 11, 43–48 (2010).
Fantini, M. L. et al. Validation of the international REM sleep behavior disorder study group symptoms severity scale (IRBD-SSS): a new tool to assess RBD clinical severity. J. Neurol. 271, 7264–7271 (2024).
Postuma, R., Fantini, M. L., Pereira, B., Choudhury, P. & Lee-Iannotti, J. Development and description of the international RBD Study group RBD symptom severity scale (P2-7.004). Neurology 98, 3980 (2022).
Sateia, M. J. International classification of sleep disorders-third edition: highlights and modifications. Chest 146, 1387–1394 (2014).
Schofield, M. A. & Zhu, Y. Fast phase unwrapping algorithm for interferometric applications. Opt. Lett. 28, 1194–1196 (2003).
Wu, B., Li, W., Guidon, A. & Liu, C. Whole brain susceptibility mapping using compressed sensing. Magn. Reson Med. 67, 137–147 (2012).
Wei, H. et al. Streaking artifact reduction for quantitative susceptibility mapping of sources with large dynamic range. NMR Biomed. 28, 1294–1303 (2015).
Lao, G. et al. Sub-voxel quantitative susceptibility mapping for assessing whole-brain magnetic susceptibility from ages 4 to 80. Hum. Brain Mapp. 44, 5953–5971 (2023).
He, C. et al. Quantitative susceptibility atlas construction in Montreal Neurological Institute space: towards histological-consistent iron-rich deep brain nucleus subregion identification. Brain Struct. Funct. 228, 1045–1067 (2023).
Fan, L. et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526 (2016).
Jiang, Y. et al. Role of the globus pallidus in motor and non-motor symptoms of Parkinson’s disease. Neural Regen. Res. 20, 1628–1643 (2025).
Pauli, W. M., Nili, A. N. & Tyszka, J. M. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci. Data 5, 180063 (2018).
Waehnert, M. D. et al. Anatomically motivated modeling of cortical laminae. Neuroimage 93, 210–220 (2014).
Persson, N. et al. Age and sex related differences in subcortical brain iron concentrations among healthy adults. Neuroimage 122, 385–398 (2015).
Acknowledgements
We sincerely thank all participants for their time, effort, and commitment to this study. This work was supported by grants from the National Natural Science Foundation of China (82230040 and 82341251). Additional support was provided by the Shanghai Natural Science Foundation (24ZR1448600), the Shanghai Research Doctor Program (SHDC2023CRT014), the Shanghai Health Commission Research Program (202440091), the Shanghai Jiao Tong University STAR Program (20220103) and the Lingang Laboratory (Grant No. LGL-3241-PDA).
Author information
Authors and Affiliations
Contributions
S.H., L.Z., and J.L. designed and conducted the study. S.H., Y.Y., N.W., and Q.Y. assisted in data collection. S.H., Z.L., and T.Z. performed the data analysis. S.H., L.Z., and Z.L. drafted the manuscript. J.L., H.W., and X.W. provided advice on data analysis and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, S., Zhou, L., Li, Z. et al. Longitudinal insights from iron accumulation in motor system of prodromal and clinical Parkinson’s disease. npj Parkinsons Dis. 12, 7 (2026). https://doi.org/10.1038/s41531-025-01223-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01223-5