Abstract
We reviewed 70 years of research defining the specificity of anti-group A carbohydrate (GAC) monoclonal and polyclonal antibodies and antibodies raised against other S. pyogenes components reacting with GAC. While some rheumatic fever associated autoantibodies react with N-Acetyl-β-D-glucosamine sidechains of GAC and cross-react with tissues, these appear to be a consequence, not the cause, of autoimmunity. We propose GAC be considered further as a broadly protective group A streptococcal vaccine.
Similar content being viewed by others
Introduction
Streptococcus pyogenes or group A streptococci (GAS) infections are a growing concern due to increasing rates of invasive disease in several countries1, coupled with a substantial global burden of autoimmune sequelae2. Repeated infection with GAS can trigger acute rheumatic fever (ARF), especially among populations where GAS infections are endemic due to factors associated with socio-economic deprivation3,4. Major manifestations of ARF include rheumatic carditis and Sydenham’s chorea, both autoimmune conditions where antibodies and cellular immunity target human antigens in the heart and brain respectively5. However, even in hyper-endemic settings, only a proportion of the population appears susceptible to ARF, likely reflecting underlying host genetic susceptibility6,7. Despite association with a specific bacterial pathogen, the GAS antigen or antigens that trigger ARF remain unknown, complicating the development of a safe and effective vaccine. Moreover, because GAS exclusively infect humans, there are limitations to the animal models available to investigate the pathogenesis of rheumatic fever, including the role of GAC in the disease process8. Nonetheless, while appraisal of these models is beyond the scope of this review, they have been widely used in rheumatic fever research9, and a proportion of what is known about immune responses to GAS and its constituent antigens – including many of the issues discussed in this paper – have been based on studies in animals rather than infections in humans10.
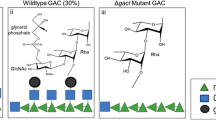
Streptococci are characterised by cell-wall anchored polysaccharides (Box 1), which in some cases can be used for serological classification11. Group A streptococci feature the Group A carbohydrate (GAC), a polymer of a 6-sugar repeat: a polyrhamnose backbone with N-acetyl glucosamine (GlcNAc) sidechains at alternating rhamnose (Fig. 1). Approximately a quarter of the GlcNAc side chains are decorated with glycerol phosphate12. Because it is the same on all GAS organisms, it makes it an ideal candidate for a comprehensive vaccine.
A GAC and (B) A Variant carbohydrate as modelled by Glycam76. Both structures have 18 repeats of the rhamnose disaccharide core - the average size of GAC isolated from GAS15. Both have the β(1, 4)linked GlcNAc that anchors the GAC to the peptidoglycan matrix77. The GAC structure is dominated by the GlcNAc linked to the core rhamnoses, Eight of the 18 GlcNAc in this structure are labelled. The green ellipse outlines one of the ~17 high affinity epitopes in this GAC identified using mAbs and other studies. It comprises 4 contiguous rhamnoses with 2 GlcNAc sidechains35. Not shown on the GAC are the glycerol phosphate attachments to some but not all GlcNAc sidechains78.
Data reported more than 50 years ago found higher levels and greater persistence of antibodies to GAC among ARF patients with carditis compared to matched controls13. More recently, this observation was replicated in a contemporary ARF endemic population, in which the authors demonstrated substantially elevated reactivity to a panel of GAS antigens including GAC also compared to matched controls14. The most likely explanation for these findings is that patients with ARF have accumulated reactivity to GAS antigens due to repeated infections, but the exaggerated response might also indicate an aberrant response to one or more of these antigens in the ARF patients. Nonetheless, extensive evidence from animal and in vitro studies with animal and human sera shows that GAC is an attractive vaccine candidate. Studies supporting its vaccine use have been recently reviewed15.
However, two areas of concern relating to GAC-based vaccines remain:
First, antibodies to the GlcNAc side chain of GAC may react with GlcNAc saccharides in human oligosaccharides16, generating autoantigens that react specifically to human GlcNAc-containing structures.
Second, antibodies to rhamnose and GlcNAc are found ubiquitously in relatively high concentrations in human sera17. These natural antibodies are constitutively expressed by germline B cells18. This class of antibody may be highly cross-reactive19, with each antibody binding to many targets, including GlcNAc. In mice, the number of germline B cells secreting anti-GlcNAc can be boosted by exposure to GAC and other antigens20. Therefore, boosting by GlcNAc in GAC may amplify these autoantibodies, potentially causing disease by binding to structures that do not contain GlcNAc.
Immunization of animals with GAS proteins (e.g., the intact M protein21 and an actin-like protein22) generates cross-reactive antibodies that recognize antigens in human tissues, especially cardiac myofibrils23. The molecular mimicry and auto-immunity associated with these antibody cross-reactions have been extensively reviewed5. Likewise, cross-reaction of T cell epitopes leading to T helper responses and infiltration of cytotoxic T cells is also well documented24,25. Although important, these protein-induced cross reactions are outside the scope of this review.
This narrative review (Box 2) examines the evidence surrounding risks of autoimmunity associated specifically with immunization with GAC. We start by
-
reviewing the important study by Goldstein et al.26,27 that examined cross-reactivity between antibodies raised against bovine heart and GAC and vice versa;
-
reviewing the immunology of the anti-GAC response including reactivity of anti-GAC antisera to human and animal tissues (i.e. by antibodies raised specifically against GAC);
-
reviewing the properties of anti-GAS cross-reactive monoclonal antibodies (mAbs) from mice and humans, some of which react with GlcNAc (i.e. antibodies raised using GAS preparations most of which lacked significant GAC content).
We conclude with an alternate hypothesis that antibodies targeting GlcNAc (and thus GAC) reflect the emergence of autoimmunity detected by GlcNAc antigen presented as a hapten, rather than GlcNAc being the immunogen.
Historical data: immunological cross-reactivity between heart and GAC – studies by Goldstein et al.26,27 and Kasp-Grochowska et al.28
In a widely quoted study that has been pivotal in defining the topic, Goldstein et al. in 1967, postulated that antibodies to glycoproteins from human heart valve cross-react with GAC26. Their study used rabbit antisera raised against bovine valve homogenates (BVH), bovine valvular structural glycoproteins (VSGP) and a chemical conjugate between GAC and a carrier protein (the hemp seed storage protein, edestin) to examine cross-reactions between heart antigens and GAC. Rabbits were vaccinated intramuscularly four times with VSGP or BVH emulsified with 0.5 mL Complete Freund’s Adjuvant (CFA). Anti-GAC sera were generated in rabbits by six intramuscular vaccinations with 15 µg/mL of GAC-edestin emulsified in 0.5 mL CFA followed by 3 iv injections of 20 µg of this conjugate. Notably, CFA contains 1 mg/mL of dried Mycobacterium tuberculosis cells and the use of large quantities of CFA for multiple vaccinations is unusual.
Antisera raised to BVH reacted by immunodiffusion with VSGP and GAC-edestin and gave bright immunofluorescence on GAS cells. Adsorption of the anti-BVH with VSGP prevented these reactions. Additionally, the results of cross-adsorption experiments with a single antiserum to GAC-edestin conjugate were reported. It gave a single immunodiffusion line when tested against VSGP or GAC-edestin and bright immunofluorescence on GAS. However, while pre-treatment of the anti-GAC antiserum with VSGP prevented the immunodiffusion reaction with VSGP, it did not prevent the immunodiffusion line with GAC-edestin nor the immunofluorescence on GAS.
A subsequent paper examined immunological relationships between GAC and a glycopeptide (“glycopeptide B”) released by partial pronase digestion of VSGP27. Glycopeptide B and GAC gave a line of identity by double diffusion, implying commonality between the antigens, but it is unclear in the paper which antisera was used.
Low concentrations of glucosamine and glucose gave partial inhibition of binding of anti-BVH antisera to glycopeptide B. Glucosamine, as well as the glycopeptide B, gave partial inhibition of binding of anti-GAC to GAC by immunodiffusion. This is unexpected for two reasons: first, the positively charged glucosamine is not a sugar found in GAC and, second, the concentration used (5 µM), was ~20 fold lower that the 100 µM KD of the anti-GAC mAb with the highest affinity for GlcNAc in the series generated by Herbst et al. (see below)29. Inhibition by GlcNAc was not reported.
Working in an independent laboratory, Kasp-Grochowska et al.28 repeated these experiments. They extracted VSGP using both the original and a variation of the extraction procedure. The VSGP prepared with the alternative procedure (VSGP-D) had a much lower hydroxyproline (i.e. collagen) content than the original method. Results were reported using individual sera from eight rabbits vaccinated with BVH and eight vaccinated with killed GAS (but not with a GAC-conjugate or pepsin treated GAS).
Importantly, the authors could not replicate the results of Goldstein et al.: they were unable to demonstrate any specific cross-reaction between BVH antisera and GAC with serum from any of the eight rabbits. Unlike the Goldstein et al. sera, the pooled anti-BVH only gave weak immunofluorescence on fixed GAS and this could not be blocked with GAC.
They showed a weak immunodiffusion line between anti-GAS sera and VSGP in all rabbits tested, but this was present in both the pre-immune sera and the immune sera. (Goldstein et al. did not report experiments to test the pre-immune sera of their rabbits.) No immunodiffusion line was seen when VGSP-D was used. Kasp-Grochowska postulated that the weak immunodiffusion line with VSGP in the pre-bleed sera was due to the solubilized collagen in the preparation.
Kasp-Grochowska et al. used CFA only for the first vaccination and used incomplete Freund’s (IFA) adjuvant (lacking M. tuberculosis cells) for subsequent vaccinations. Moreover, rabbits vaccinated with just M. tuberculosis cells gave bright immunofluorescence on fixed GAS that could only be partially reversed by pre-adsorption with either GAC or A variant polysaccharide.
As summarized in Box 3, although the Goldstein et al. papers are frequently quoted as proof of cross-reactions between anti-GAC and heart tissues, the evidence is inconsistent, could not be replicated by Kasp-Grochowska et al., and there are credible alternative explanations, including that the cross-reactivity described in these papers was elicited by M. tuberculosis. These highly influential early studies have been superseded by more defined studies using mouse and human mAbs, which are discussed below.
Immunological specificity of animal polyclonal and monoclonal anti-GAC antibodies
In 1955 McCarty and Lancefield30 showed that vaccination of rabbits with heat killed, trypsin-treated GAC-variant lacking GlcNAc or wild type streptococci (i.e., containing GlcNAc) generated antisera that strongly reacted with the homologous polysaccharide but weakly or not at all with the heterologous polysaccharides31 (Box 1). These studies suggested that GlcNAc is an important part of the rabbit response to GAC and that GlcNAc shields the polyrhamnose backbone, preventing binding of anti-rhamnose antibodies (Fig. 1).
Briles and Davie (1975) showed that immunization of mice with pepsin digested GAS generated high levels of IgM antibody32,33. By using a haemolysis plaque assay, anti-GAC antibody secreted by single B cells recognized GAC sensitized red blood cells more efficiently than GlcNAc sensitized cells. The ratio of anti-GlcNAc vs anti-GAC plaques varied substantially from mouse to mouse (range from 4% to 96%, median 27%) suggesting that anti-GAC is more than just an anti-GlcNAc response. As judged by the isoelectric focusing pattern of anti-GAC antibodies from a single animal, the immune response was highly restricted33. They concluded that “clonal commitment … does not result from competition among B cells for antigen”, findings confirmed by subsequent studies with mAbs34 and sequencing of individual V genes from GAC binding mouse B cells20.
Herbst et al.29 prepared panels of mAbs from mice immunized with pepsin-treated, heat killed streptococci with GAC or A variant carbohydrate. A few anti-GAC mAbs also bound Group E and Group L polysaccharides. There was no cross-reaction between mAbs immunized with GAS and A variant GAS or vice versa, supporting the earlier rabbit polyclonal studies of McCarty and Lancefield30. As measured by fluorescence quenching, dissociation constants (KD) for soluble GAC binding ranged from <10 nM to 700 nM and for GlcNAc from 0.1 mM to 10 mM, consistent with the Briles and Davie32 results, showing that binding to GAC involved more than just the GlcNAc. Note that the KD of the anti-GAC mAb with the highest affinity for GlcNAc (0.1 mM) is 20 times higher than the 5 µM concentration of glucosamine used in the Goldstein et al.27 study to inhibit binding of their anti-BVH antisera to GAC.
In recent data, reviewed by Pitirollo et al.35, multiple studies with polyclonal mouse and rabbit sera as well as two different anti-GAC mAbs demonstrated that GlcNAc alone was insufficient for high affinity binding. Affinity of binding to oligosaccharides increased with addition of rhamnose with the maximum affinity requiring at least a tri-saccharide and frequently the full hexasaccharide GAC repeat (Fig. 1). Results with human polyclonal anti-GAC sera were similar35. Saturation Transfer Difference-Nuclear magnetic resonance (NMR) spectroscopy indicated all four rhamnose molecules contributed to binding and that the acetyl groups were particularly important for high affinity binding35.
These studies show that GlcNAc is required, but not sufficient, for high affinity binding of defined antibodies to GAC. Importantly these studies reveal that assays based on GlcNAc-BSA, a commonly used surrogate for anti-GAC antibodies, should be interpreted with caution.
Binding of polyclonal animal anti-GAC antisera to human and animal tissues
Several studies using polyclonal anti-GAC antibodies have been conducted to investigate their binding to human or animal tissues through immunolocalization, usually using immunofluorescence. Table 1 lists all the publications that we identified that examined binding of animal polyclonal anti-GAC antibodies on sections of human or animal tissues (Box 1).
In the most comprehensive of these, Lyampert et al.36 tested sera from rabbits vaccinated with pepsin-treated GAS, adsorbing out non-GAC antibodies including anti-peptidoglycan, with A variant streptococci. The sera were tested by immunofluorescence on cryosections of: “heart tissues of man, guinea-pig, rabbit, cattle and sections of human, bovine, and rabbit heart valves … the cornea and sclera of the rabbit and mouse eye … sections of thymus (nineteen specimens) and skin (eighteen specimens) from adult humans and human embryos (15–20 weeks of gestation). Thymus and skin tissues of rabbits, guinea-pigs, mice and rats were also studied.”
The key finding as described by the authors was “The absence of fluorescence of connective tissue elements, when the antibodies were applied on sections of heart tissues, heart valves, and cornea, indicates that cross-reactions between A-polysaccharide and connective tissue antigens cannot be detected.” They found no surface immunofluorescence in any tissue examined although there was cytoplasmic fluorescence in basal epithelial cells of the skin, sclera and thymus.
These results are strikingly different to the earlier studies by Lyampert and colleagues36,37 based on vaccination of rabbits with killed and boosted with live GAS, grown through 10 passages in casein medium, which gave antibodies that strongly reacted by immunofluorescence and immunodiffusion with cardiac tissues and proteins. Similar to the adsorption studies of Kaplan and Meyeserian (1962)23, these earlier studies indicated that generation of cross-reactive antibodies by vaccination with GAS required the pepsin sensitive, i.e. protein, content of the GAS.
The Lyampert et al.36 findings based on pepsin-treated GAS are also consistent with the other studies with GAC-specific polyclonal antisera listed in Table 1, none of which found immunofluorescence on arterial smooth muscle cells, heart, brain or kidney tissues. In summary, no immunolocalization studies demonstrated reactivity of polyclonal anti-GAC antibodies with heart or brain tissues, nor any connective tissue or cell surface antigens. The only studies to show reaction of polyclonal anti-GAC antibodies with human tissue found binding limited to cytoplasmic antigens in basal epithelial cells from skin, sclera and thymus36, and one study, to nuclei38. Nonetheless, polyclonal sera derived from vaccinated animals are a crude tool with which to study a human autoimmune disease triggered by a human-specific pathogen.
Mouse monoclonal antibodies prepared with GAC containing immunogens
These antibodies were prepared by immunizing mice with pepsin or trypsin digested, killed streptococci or with GAC conjugates with or without oil-based adjuvants (e.g. IFA).
Mouse monoclonals, termed the HGAC series, were produced by Nahm and colleagues34,39,40 using vaccination of A/J and B.C20 mice with killed pepsin-treated streptococci with selection for binding to GAC. The mAbs were almost exclusively IgM and IgG3, as expected for a T independent B-1 cell type response19. The heavy chain (VH) sequence of 16 of these has been published indicatingone of two VH types (designated VH9 and VH39, after mAbs HGAC9 and HGAC39). Re-examination of these sequences using IgBLAST41 for this review, indicates all VH sequences including both the “VH9” and “VH39” variants are from the IGVH6-3 locus or a closely related sequence.
Shikhman et al.42,43 demonstrated that HGAC49 (IgMκ) bound to keratin by ELISA and western blot and showed that two other mAbs, HGAC54 and HGAC78 (IgMκ) also bound to peptides from keratin, with the highest binding to keratin peptide “b1” that encodes a GlcNAc mimotope, also recognized by wheatgerm agglutinin. HGAC49 was tested for binding to a panel of cross-reactive antigens (myosin, actin, laminin etc.) used to characterize mAbs raised by immunizing mice with GAS membranes (see below). Other than keratin, the only other antigen recognized was the M protein of GAS serotype M6.
This study suggests the possibility of cross-reactivity between the GAS M protein and GAC. The hypothesis is supported by unpublished data from the Cunningham laboratory, which show that antibodies recognizing specific M protein peptides also react with GlcNAc when it is linked to bovine serum albumin (BSA). A relationship may exist between the alpha-helical M proteins and the GAC molecule, but cross-reactivity is observed only when GlcNAc is used as a hapten bound to a protein.
Turner et al.44 established that HGAC39 (IgG3κ) and HGAC78 bound to GlcNAc covalently linked to proteins via a serine or threonine (i.e. O-GlcNAc) but not to N-Glycans. Both mAbs gave the peri-nuclear and punctate cytoplasmic immunofluorescence on rat fibroblasts and hepatocytes expected from the distribution of O-GlcNAc. Similar fluorescence was observed by Shikhman et al.42 with HGAC49 on rat heart cells.
Rook et al.45 prepared a library of more than 200 mAbs that bound to GlcNAc-BSA from mice immunized with a trypsin-treated homogenate of killed streptococci. These mAbs were screened for binding to the glycoprotein, fetuin, and fetuin digested with neuraminidase and β-galactosidase to expose oligosaccharides with terminal GlcNAc. None of these mAbs bound to untreated fetuin indicating that these anti-GlcNAc mAbs could not react with normal human GlcNAc containing oligosaccharides. Only three of the more than 200 mAbs tested bound the neuraminidase-digested fetuin: i.e. almost all the anti-GlcNAc mAbs were unable to recognize terminal GlcNAc even in digested oligosaccharides.
Two of the mAbs, GN6 and GN7 that bound digested fetuin, were tested for immunofluorescence and immunoperoxidase staining to a range of normal and diseased human tissue46. No staining was observed on sections of non-diseased connective tissue, including synovial tissue from 12 joints, fascia from six areas, subcutaneous tissue from four areas, muscle (six samples) and brain (two samples). There was staining of epithelial cells from tonsils consistent with both cytoplasmic and surface locations. Cytoplasmic staining was also observed in salivary gland epithelial cells, skin keratinocytes and the Schwann cells in myelinated nerve trunks, which the authors speculated was due to O-GlcNAc. Granular macrophages from synovial fluid from patients with rheumatoid arthritis gave intense staining possibly due to processing of GlcNAc containing cellular debris.
Russian researchers47,48,49,50,51 prepared mAbs from mice vaccinated with pepsin- or trypsin-treated GAS. Cross-reactivity to mouse, bovine or human tissues depended on the fine specificity of the mAbs. At one extreme was mAb 4D/1 that bound to pepsin-treated Group A, A variant, C and L serotype streptococci51. By immunofluorescence, 4D/1 bound to cardiac connective tissue and all layers of human foetal skin. Immunofluorescence could be partially blocked by pre-treatment with purified A, C and L polysaccharides and fully blocked only with A variant polysaccharide, which was an unexpected specificity consistent with 4D/1 being an anti-rhamnose rather than anti-GAC antibody.
Other mAbs gave a similar pattern of fluorescence to that seen earlier by Lyampert et al.36 with polyclonal anti-GAC. Fluorescence was limited to cytoplasm of skin basal epithelia cells and certain other carcinoma-derived epithelial cells49,52. In a limited set of mAbs, Bazanova et al.47 reported mAbs that gave immunofluorescence on epithelial cells also bound to both Group A, A variant, and L polysaccharides29; mAbs that only bound to GAC and not to A variant or Group L did not bind to any tissues studied.
mAbs were also generated from mice vaccinated with GAC conjugated to a polyelectrolyte (GAC-PEL)51. Two of the mAbs (BI/2 and A5/2, both IgM) gave strong immunofluorescence with cell nuclei in mouse and human tissue sections. The immunofluorescence could be inhibited by double-stranded DNA, but not denatured DNA nor GAC and they did not bind to pepsin-treated GAS, i.e. these mAbs are not directed against GAC. Their specificity is consistent with earlier results obtained from vaccinating mice with the same GAC-polyelectrolyte construct where the polyclonal sera38 gave cytoplasmic and perinuclear fluorescence (consistent with reactions to O-GlcNAc44), as well as strong nuclear fluorescence (consistent with the anti-DNA specificity of these mAbs). The only anti-nuclear reactivity seen with any of the polyclonal or monoclonal anti-GAC antibodies came from animals immunized with this GAC-PEL construct. The authors speculated that the hybridomas expressing these IgM anti-DNA mAbs may “have been obtained by polyclonal activation”, triggered by the acrylic acid and N-vinylpyrrolidone copolymer “analogous to the action of LPS”. Anti-DNA and antinuclear reactivity of GAS antibodies were found only in mice53 and it is noteworthy that anti-nuclear antibodies are not a recognised feature of ARF.
More recent studies by New et al.20, sequenced individual B cells sorted by their specificity for binding fluorescent GAC with B cells from germ-free mice, from mice with a reconstituted microbiome or from mice vaccinated with pepsin-treated GAS. They found the B cells used a limited repertoire of VH chains, predominantly IGHV6-3. They also showed that anti-GAC antibodies developed by active immunization with pepsin-treated GAS delayed development of type 1 diabetes. They proposed that anti-O-GlcNAc promoted more efficient clearing of pancreatic apoptotic cells without impacting survival in non-diabetic mice54.
In summary, some of the GAC-specific single cell antibodies or mAbs generated by vaccinating mice with protease treated GAS or GAC conjugates recognize O-GlcNAc. However, only a small proportion recognize GlcNAc in N-linked mammalian oligosaccharides and then only in partially digested or incompletely formed oligosaccharides. None have been shown to recognize normal N-linked oligosaccharides. Other than the GlcNAc mimicking peptide from keratin43, none have been shown to recognize non-glycosylated mammalian proteins.
Mouse monoclonal antibodies prepared with immunogens containing limited GAC
These antibodies were prepared by immunizing mice with GAS membranes and boosted with either GAS membranes, lysin solubilized cell walls or a pepsin-digested M protein fragment with or without oil-based adjuvants (e.g. IFA) (see Box 4 and Fig. 2). They were generated by selecting hybridomas secreting mouse mAbs reacting with GAS by testing hybridoma supernatants by ELISA on plates coated with group A serotype M5 streptococci pelleted onto ELISA plates before fixing with glutaraldehyde55 and on ELISA plates coated with an extract of human heart55. Fourteen unique mouse mAbs were identified (Fig. 2)56 all of which were IgMκ. (Fifteen were identified, but two mAbs 6.5.1 and 113.2.1 have identical antibody gene sequences and specificities, were from the same mouse and are presumed identical.)
Vaccination strategies using antigens with no significant GAC or M protein have a white background; strategies using antigens containing GAC have a light green background; and strategies using antigens with no significant GAC but with M protein have a light blue background. Highlighting has also been used to emphasize mAbs that recognise GlcNAc-BSA (mid green background) and other antigens (mid blue background) as well as the level of cytotoxicity where tested (red background) and the number of mutations in the genes encoding the mAbs (brown). n.d. not determined.
As shown in Fig. 2, these 14 mouse antistreptococcal mAbs bind a wide selection of human proteins. Three patterns of specificity were assigned based on binding to DNA and GlcNAc56. There was no significant association between three types of vaccination (Fig. 2) and binding to GlcNAc-BSA while binding to GAC was not reported. Five of the mouse mAbs bound GlcNAc-BSA conjugate and for the four of these tested, they required high concentrations (>100 mM) of soluble GlcNAc to inhibit this binding: a detailed titration curve for one (101.4.1) has been published with a 50% inhibition of binding at ~400 mM GlcNAc42. These concentrations are much higher than the 0.1 to 10 mM dissociation constant (KD) measured for the binding to GlcNAc of mAbs that bind GAC29,57, or the 0.7 mM and 30 mM, respectively, required to give 50% inhibition of binding of HGAC58 and HGAC78 to GlcNAc-BSA42.
The VH and VL nucleotide sequences of these 14 mAbs were determined56. As originally reported, these antibody V gene sequences had either germline or minimally mutated sequences. Reanalysis for this review, using IgBLAST41, further strengthened these original conclusions: now 11/14 hybridomas have BALB/c unmutated germline VH and VL sequences, and for the remaining three, only a single substitution was observed.
Three mAbs (36.2.2, 54.2.8 and 49.8.9) were cytotoxic in the presence of complement on cultured cells58 with 36.2.2 showing the strongest response and the only mouse mAb binding to laminin. In addition to their binding to human proteins, mAbs 36.2.2 and 54.2.6 neutralize polio virus, and 48.8.9 neutralized coxsackieviruses B3 and B458. Of these three, only 49.8.9 bound GlcNAc-BSA and all three mAbs came from mice immunized only with GAS membranes (Box 4), i.e. a preparation that contained no significant quantities of GAC.
In conclusion, highly cross-reactive mAbs that recognize human, streptococcal, and viral proteins, can neutralize viruses in vitro and in vivo, and can be prepared from mice vaccinated with GAS membranes followed by boosting with GAS membranes, M protein fragment or solubilized cells. Some of these mAbs have cytotoxic activity against human or rat cells but it is unlikely that these mAbs resulted from vaccination with GAC.
Human mAbs prepared from healthy subjects as well as GAS, ARF and RHD patients
The fusions leading to these human mAbs falls into four groups (see also Box 4):
Set 1: The PB and T series mAbs derived from peripheral blood lymphocytes (PBL) from a cellulitis patient (PB series) or tonsillar lymphocytes from a patient with recurrent GAS pharyngitis (T2 mAbs) or from normal individuals with low ASO titres (T1, T6 and T7 series)59. Lymphocytes were stimulated in vitro with pokeweed mitogen or a fragment of the type 5 M protein released by pepsin digestion of whole GAS cells (Box 4). Binding to GlcNAc-BSA or GAC were not reported: all bound to human heart extract, rabbit skeletal myosin and GAS membranes. Individual mAbs bound to one or more of a panel of autoantigens (e.g. actin, calf thymus DNA), or GAS M protein.
Set 2: Hybridomas were generated from PBLs from an individual with chronic GAS carriage (9.B12 and 2.H11)60 or ARF/rheumatic heart disease (RHD) patients (1.C3, 1.C6, 1.C8, 1.H9, 3.B6, 4.F2, 5.G3, 5.G7)61,62. Lymphocytes were stimulated in vitro with a GAS whole cell digest likely to have contained GAC and peptidoglycan (Box 4), prepared by dissolving GAS cell walls with mutanolysin before affinity chromatography on Wheat germ agglutinin. Cells were then simulated with pokeweed mitogen and selected for binding to GlcNAc-BSA and for lack of binding to BSA. Not surprisingly all mAbs bound GlcNAc-BSA but also bound human skin keratin. Some also bound cytokeratin 8 and 18 (1.C8, 2.H11), human cardiac myosin (1.C8, 1.H9), rabbit skeletal myosin (4.F2, 5.G7), vimentin (1.C8), laminin (1.C3), and heat aggregated immunoglobulin (5.G7).
Set 3: Two closely related mAbs (10.2.3 and 10.2.5) were produced from tonsillar lymphocytes from a patient with recent GAS pharyngitis, stimulated in vitro with GAS membranes (Box 4) then pokeweed mitogen63 and selected for binding to GAS. These two mAbs have identical VH chains, differing by one amino acid in the VL chain, and have very similar binding profiles61.
Set 4: Three mAbs derived from PBL from a patient with Sydenham’s chorea (24.3.1, 31.1.1, 37.2.1). Lymphocytes were stimulated in vitro with streptococcal membranes, but not with pokeweed mitogen64 and selected for binding to GAS. All mAbs bound GlcNAc-BSA and lysoganglioside GM1 but had no detectible binding to double-stranded DNA, collagen, actin, human cardiac myosin, skeletal myosin and laminin. By immunohistochemistry, these mAbs bound to the surface of the human neuroblastoma SK-N-SH cell line.
Additionally, one of these mAbs (24.3.1) also bound tubulin65 and dopamine receptor D266. Cross-inhibition of binding showed the antibody combining site was highly likely to be genuinely cross-reactive: lysoganglioside GM1 inhibited binding of 24.3.1 to tubulin. Functionally, 24.3.1 binds to cultured neuroblastoma cells64, and with the appropriate cell targets, activates CAM kinase II64 and elicits dopamine release67, which can be inhibited by GlcNAc-BSA. Targeting of dopaminergic neurons by this mAb and its mouse IgG equivalents in transgenic mice may provide a mechanistic insight into the neurological symptoms observed in Sydenham’s chorea66,68.
In summary, sets 1–3 but not Set 4 came from cells simulated in vitro with pokeweed mitogen. Sets 1, 3 and 4 were from cells that had not been stimulated in vitro with GAC or other GlcNAc containing antigens. However, all mAbs tested (i.e. Set 1 not tested), bound GlcNAc-BSA. This is not unexpected since the initial screening of most of the hybridomas included binding of their mAb to GlcNAc-BSA.
Three mAbs from Set 1 were IgG but all of the remainder were IgM. The VL and VH sequences of the some of the mAbs in Set 261 and all the mAbs in Sets 361 and 465 have been reported, showing minimal changes from the closest germline sequences. With updated databases, reanalysis for this review shows the homology to germline sequences is even closer. For example, the VH sequence of mAb 37.2.1 (accession number DQ779566) now has a 100% match with the germline sequence IGHV3-64 * 02 (V) and IGHJ2 * 01 or IGHJ3 * 01 or IGHJ3 * 02 (J).
All human mAbs were compared to the IgG specificity of the sera from the human patient and found to have similar reactivity as the IgG responses found in the sera of these patients from which the human mAbs were derived. Studies have been published on both the heart and the brain cross-reactive autoantibodies which demonstrate their IgG responses with human tissues and group A streptococcal antigens64,66,69.
Implications for GAC containing vaccines
The mouse mAbs raised by vaccinating mice with GAS membranes and the human mAbs generated by in vitro stimulation of lymphocytes with GAS membranes and exposure to pokeweed mitogen provide a helpful model of the autoimmunity associated with ARF and other post-streptococcal diseases10. However, these monoclonal antibodies differ in multiple critical ways from the antibody responses generated by vaccinating with GAC containing preparations.
The “anti-GAC mAbs”:
-
1.
Have been generated by immunization with GAC preparations, primarily protease treated GAS cells;
-
2.
Bind GAC and, where tested, bind GlcNAc with lower affinity than GAC or GAC oligosaccharides, but still at substantially higher affinity than the “cross-reacting mAbs”;
-
3.
Where sequenced, exclusively, use IGHV6-3 or closely related heavy chains, as also observed in most individually sequenced GAC binding antibodies from V genes from mouse B cells54;
-
4.
Showed no binding to the surface of normal tissues. Some bind cytoplasmic components consistent with binding to O-GlcNAc, but there is no evidence of extensive binding to non-glycosylated proteins or to human N-linked oligosaccharides.
By contrast “cross-reacting, anti-GAS mAbs”:
-
1.
Are all generated from mice immunized in vivo or human cells in vitro from ARF/RHD patients exposed to immunogens mostly lacking significant GAC content and/or other GAS antigens and potent B cell mitogens;
-
2.
Gave highly cross-reactive binding to a range of human proteins by ELISA and by immunofluorescence to multiple human tissues;
-
3.
Use a wide array of very low or unmutated germline VH and VL sequences with none of the mouse anti-GAS mAbs using IGHV6-3 or related VH genes;
-
4.
Have either a lower affinity for GlcNAc, or no detectible binding to GlcNAc;
-
5.
In the case of the mouse mAbs, they show a degree of cross-reactivity and cytotoxicity that does not correlate with ability to bind GlcNAc (Fig. 2). The most cytotoxic mAb, 32.6.2 does not bind GlcNAc. Of the two mAbs with lower cytotoxicity, 54.2.8 and 49.8.9, one binds GlcNAc-BSA and the other does not.
The degree of cross-reactivity of the mAbs was apparently unrelated to vaccination with GAC containing immunogens: the median number of antigens in the cross-reactivity panel recognized by mAbs that did not bind GlcNAc was five compared to six for those that did bind GlcNAc (Fig. 2). This difference is not significant (p = 0.30, Mann-Whitney U test) implying that binding to GlcNAc-BSA is not required for cross-reactivity. Additionally binding to GlcNAc appeared related to binding to amino acid sequences which contained more amphipathic and aromatic amino acid residues potentially reflecting homology to alpha helical proteins43.
Additionally, the older data from rabbit and mouse anti-GAC (i.e. animals vaccinated with pepsin treated GAS or GAC conjugates) mirrored these findings: by immunofluorescence they showed no binding to surface antigens and no detectible binding to heart or brain sections.
Conclusions
While concerns about autoimmunity from vaccination with GAS proteins may remain, a wealth of studies with both anti-GAC monoclonal and polyclonal antibodies do not support the hypothesis that antibodies to GAC play a causal role in ARF. Moreover, most of these studies were done by vaccinating with protease treated GAS cells while human vaccines in development use GAC conjugates (e.g. GAC conjugated to CRM197). Conjugates are expected to generate antibodies with higher avidity and a more restricted specificity than the T-independent B-1 type response generated by protease treated GAS cells70, further reducing the risk of cross-reactivity. The antibodies to GAC are important for their broad reactivity to all GAS and their protective ability. GlcNAc does not represent the intact GAC molecule as described herein, and GAC should be strongly considered an important potential vaccine for use in humans.
We propose an alternative explanation for the extensive cross-reactivity of anti-GAS mAbs: that these cross-reactive mAbs were generated by polyclonal activation of B cells exposed to GAS membrane fractions (mouse and human) containing undetectable GAC, combined with pokeweed mitogen in human mAbs. In this scenario, the high frequency of recognition of GlcNAc-BSA reflects the propensity at which germline antibodies recognize GlcNAc and the ready availability of GlcNAc-BSA as a reagent for assays71. Plausibly, a similar mechanism may contribute to ARF pathogenesis and may be partly reflected in several more recent observations regarding ARF pathogenesis including the role of germline antibody gene variation72, the striking elevation of the IgG3 antibodies73 and the heterogeneous nature of the autoantibody repertoire74.
Finally, rather than causing harm, immunization with a safe, effective GAC-containing vaccine that reduces the frequency and duration of exposure to GAS could reduce the risk and the devastating consequences of ARF/RHD and its sequelae. It is expected that the first human vaccine trials of a combination vaccine containing a GAC conjugate will start in the near future75, providing initial human safety and immunogenicity data as an important step in developing a broad based, safe and effective vaccine to protect children and adults from the devastating consequences of invasive GAS disease as well as ARF, RHD and other GAS autoimmune sequelae.
Data availability
No datasets were generated or analysed during the current study.
Change history
24 November 2025
In this article the word count was published at the end of the abstract which needs to be removed. The original article has been corrected.
References
Gregory, C. J. et al. Invasive group A streptococcal infections in US States. JAMA 333, 1498−1507 (2025).
Karthikeyan, G. et al. Mortality and morbidity in adults with rheumatic heart disease. JAMA 332, 133–140 (2024).
Wirth, S. et al. Acute rheumatic fever and rheumatic heart disease. In Streptococcus Pyogenes: Basic Biology to Clinical Manifestations (eds. Ferretti, J. J., Stevens, D. L. & Fischetti, V. A.) (University of Oklahoma Health Sciences Center, 2022).
Sika-Paotonu, D., Beaton, A., Raghu, A., Steer, A. & Carapetis, J. Acute rheumatic fever and rheumatic heart disease. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations (eds. Ferretti, J. J., Stevens, D. L. & Fischetti, V. A.) (University of Oklahoma Health Sciences Center © The University of Oklahoma Health Sciences Center., 2016).
Cunningham, M. W. Molecular mimicry, autoimmunity, and infection: the cross-reactive antigens of group a streptococci and their sequelae. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.gpp3-0045-2018 7(2019).
Carapetis, J. R., Currie, B. J. & Mathews, J. D. Cumulative incidence of rheumatic fever in an endemic region: a guide to the susceptibility of the population?Epidemiol. Infect. 124, 239–244 (2000).
Muhamed, B., Parks, T. & Sliwa, K. Genetics of rheumatic fever and rheumatic heart disease. Nat. Rev. Cardiol. 17, 145–154 (2020).
Reynolds, S. et al. Streptococcus pyogenes vaccine candidates do not induce autoimmune responses in a rheumatic heart disease model. NPJ Vaccines 8, 9 (2023).
Cunningham, M. W. Pathogenesis of group A streptococcal infections. Clin. Microbiol Rev. 13, 470–511 (2000).
Cunningham, M. W. & Kirvan, C. A. Post-streptococcal autoimmune sequelae, rheumatic fever and beyond: a new perspective. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations (eds. Ferretti, J. J., Stevens, D. L. & Fischetti, V. A.) (University of Oklahoma Health Sciences Center © The University of Oklahoma Health Sciences Center., 2022).
Lancefield, R. C. The antigenic complex of Streptococcus haemolyticus: I. Demonstration of a type-specific substance in extracts of Streptococcus haemolyticus. J. Exp. Med. 47, 91–103 (1928).
Edgar, R. J. et al. Discovery of glycerol phosphate modification on streptococcal rhamnose polysaccharides. Nat. Chem. Biol. 15, 463–471 (2019).
Dudding, B. A. & Ayoub, E. M. Persistence of streptococcal group A antibody in patients with rheumatic valvular disease. J. Exp. Med 128, 1081–1098 (1968).
Whitcombe, A. L. et al. Increased breadth of group A streptococcus antibody responses in children with acute rheumatic fever compared to precursor pharyngitis and skin infections. J. Infect. Dis. 226, 167–176 (2022).
Burns, K., Dorfmueller, H. C., Wren, B. W., Mawas, F. & Shaw, H. A. Progress towards a glycoconjugate vaccine against group A streptococcus. NPJ Vaccines 8, 48 (2023).
McCarty, M. Missing links in the Streptococcal chain leading to rheumatic fever. Circulation 29, 488–493 (1964).
Oyelaran, O., McShane, L. M., Dodd, L. & Gildersleeve, J. C. Profiling human serum antibodies with a carbohydrate antigen microarray. J. Proteome Res. 8, 4301–4310 (2009).
Pasquali, J. L. & Martin, T. Control of B cells expressing naturally occurring autoantibodies. Adv. Exp. Med. Biol. 750, 145–156 (2012).
Mattos, M. S., Vandendriessche, S., Waisman, A. & Marques, P. E. The immunology of B-1 cells: from development to aging. Immun. Ageing 21, 54 (2024).
New, J. S. et al. Neonatal exposure to commensal-bacteria-derived antigens directs polysaccharide-specific B-1 B cell repertoire development. Immunity 53, 172–186.e176 (2020).
Harbison-Price, N. et al. Current approaches to vaccine development of streptococcus pyogenes. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations (eds. Ferretti, J. J., Stevens, D. L. & Fischetti, V. A.) (University of Oklahoma Health Sciences Center, The University of Oklahoma Health Sciences Center., Oklahoma City (OK), 2022).
Barnett, L. A. & Cunningham, M. W. Evidence for actinlike proteins in an M protein-negative strain of Streptococcus pyogenes. Infect. Immun. 60, 3932–3936 (1992).
Kaplan, M. H. & Meyeserian, M. An immunological cross-reaction between group-A streptococcal cells and human heart tissue. Lancet 1, 706–710 (1962).
Ellis, N. M., Li, Y., Hildebrand, W., Fischetti, V. A. & Cunningham, M. W. T cell mimicry and epitope specificity of cross-reactive T cell clones from rheumatic heart disease. J. Immunol. 175, 5448–5456 (2005).
Faé, K. C. et al. Mimicry in recognition of cardiac myosin peptides by heart-intralesional T cell clones from rheumatic heart disease. J. Immunol. 176, 5662–5670 (2006).
Goldstein, I., Halpern, B. & Robert, L. Immunological relationship between streptococcus A polysaccharide and the structural glycoproteins of heart valve. Nature 213, 44–47 (1967).
Goldstein, I., Rebeyrotte, P., Parlebas, J. & Halpern, B. Isolation from heart valves of glycopeptides which share immunological properties with Streptococcus haemolyticus group A polysaccharides. Nature 219, 866–868 (1968).
Kasp-Grochowska, E., Kingston, D. & Glynn, L. E. Immunology of bovine heart valves. I. Cross-reaction with the C-polysaccharide of Streptococcus pyogenes. Ann. Rheum. Dis. 31, 282–289 (1972).
Herbst, H., Lavanchy, D. & Braun, D. G. Grouping of haemolytic streptococci by monoclonal antibodies: determinant specificity, cross-reactivity and affinity. Ann. Immunol. (Paris) 134d, 349–371 (1983).
McCarty, M. & Lancefield, R. C. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J. Exp. Med 102, 11–28 (1955).
McCarty, M. Variation in the group-specific carbohydrate of group A streptococci. Ii. Stud. Chem. Basis Serological Specif. Carbohydr. J. Exp. Med. 104, 629–643 (1956).
Briles, D. E. & Davie, J. M. Clonal dominance. I. Restricted nature of the IgM antibody response to group A streptococcal carbohydrate in mice. J. Exp. Med. 141, 1291–1307 (1975).
Briles, D. E. & Davie, J. M. Clonal nature of the immune response. II. The effect of immunization on clonal commitment. J. Exp. Med. 152, 151–160 (1980).
Lutz, C. T. et al. Molecular dissection of the murine antibody response to streptococcal group A carbohydrate. J. Exp. Med. 165, 531–545 (1987).
Pitirollo, O. et al. Elucidating the role of N-acetylglucosamine in group A carbohydrate for the development of an effective glycoconjugate vaccine against group A Streptococcus. Carbohydr. Polym. 311, 120736 (2023).
Lyampert, I. M. et al. A cross-reactive antigen of thymus and skin epithelial cells common with the polysaccharide of group A streptococci. Immunology 31, 47–55 (1976).
Lyampert, I. M., Vvedenskaya, O. I. & Danilova, T. A. Study on streptococcus group A antigens common with heart tissue elements. Immunology 11, 313–320 (1966).
Ryzhikova, E. V. et al. Antibodies reacting with thymus and skin epithelium and antibodies to cell nuclei during immunization with group A streptococcal polysaccharide conjugated with synthetic polyelectrolytes. Bull. Exp. Biol. Med. 103, 805–808 (1987).
Nahm, M. H., Clevinger, B. L. & Davie, J. M. Monoclonal antibodies to streptococcal group A carbohydrate. I. A dominant idiotypic determinant is located on Vk. J. Immunol. 129, 1513–1518 (1982).
Perlmutter, R. M. et al. Multiple VH gene segments encode murine antistreptococcal antibodies. J. Exp. Med. 159, 179–192 (1984).
Ye, J., Ma, N., Madden, T. L. & Ostell, J. M. IgBLAST: an immunoglobulin variable domain sequence analysis tool. Nucleic Acids Res. 41, W34–W40 (2013).
Shikhman, A. R., Greenspan, N. S. & Cunningham, M. W. A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N-acetyl-beta-D-glucosamine. J. Immunol. 151, 3902–3913 (1993).
Shikhman, A. R., Greenspan, N. S. & Cunningham, M. W. Cytokeratin peptide SFGSGFGGGY mimics N-acetyl-beta-D-glucosamine in reaction with antibodies and lectins, and induces in vivo anti-carbohydrate antibody response. J. Immunol. 153, 5593–5606 (1994).
Turner, J. R., Tartakoff, A. M. & Greenspan, N. S. Cytologic assessment of nuclear and cytoplasmic O-linked N-acetylglucosamine distribution by using anti-streptococcal monoclonal antibodies. Proc. Natl. Acad. Sci. USA 87, 5608–5612 (1990).
Rook, G. A., Steele, J. & Rademacher, T. A monoclonal antibody raised by immunising mice with group A streptococci binds to agalactosyl IgG from rheumatoid arthritis. Ann. Rheum. Dis. 47, 247–250 (1988).
Sharif, M., Rook, G., Wilkinson, L. S., Worrall, J. G. & Edwards, J. C. Terminal N-acetylglucosamine in chronic synovitis. Br. J. Rheumatol. 29, 25–31 (1990).
Bazanova, E. A. et al. Preparation and characterization of monoclonal antibodies to group-specific antigenic determinant of group a streptococcus polysaccharide. Bull. Exp. Biol. Med. 128, 1032–1034 (1999).
Danilova, T. A., Asoskova, T. K., Borodiyuk, N. A., Beletskaya, L. V. & Nesterenko, V. G. Specificity of monoclonal antibodies obtained by immunization of mice with trypsin-treated group a streptococcus culture. Bull. Exp. Biol. Med. 118, 1189–1192 (1994).
Drobyshevskaya, E. I., Abyzov, V. N., Lyampert, I. M., Spitsyn, S. V. & Beletskaya, L. V. Monoclonal autoantibodies to epithelial structures of the thymus obtained by immunization with group a streptococcal antigens. Bull. Exp. Biol. Med. 107, 89–92 (1989).
Drobyshevskaya, E. I., Borodiyuk, N. A., Kolesnikova, V. Y. & Lyampert, I. M. Precipitating monoclonal antibodies to an antigenic determinant of group a streptococcal polysaccharide. Bull. Exp. Biol. Med. 101, 351–354 (1986).
Drobyshevskaya, E. I. et al. Monoclonal antibodies to group a streptococal polysaccharide, cross-reacting with mammalian connective tissue. Bull. Exp. Biol. Med. 105, 850–853 (1988).
Beletskaya, L. V. et al. Monoclonal antibodies to group a streptococcal polysaccharide, reacting with antigen of basal cell tumors histogenetically related to epidermal tissues. Bull. Exp. Biol. Med. 103, 491–493 (1987).
Cunningham, M. W. & Swerlick, R. A. Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J. Exp. Med. 164, 998–1012 (1986).
New, J. S., Dizon, B. L. P., King, R. G., Greenspan, N. S. & Kearney, J. F. B-1 B Cell-derived natural antibodies against N-acetyl-d-glucosamine suppress autoimmune diabetes pathogenesis. J. Immunol. 211, 1320–1331 (2023).
Cunningham, M. W. & Russell, S. M. Study of heart-reactive antibody in antisera and hybridoma culture fluids against group A Streptococci. Infect. Immun. 42, 531–538 (1983).
Mertens, N. M., Galvin, J. E., Adderson, E. E. & Cunningham, M. W. Molecular analysis of cross-reactive anti-myosin/anti-streptococcal mouse monoclonal antibodies. Mol. Immunol. 37, 901–913 (2000).
Pitner, J. B. et al. Bivalency and epitope specificity of a high-affinity IgG3 monoclonal antibody to the Streptococcus group A carbohydrate antigen. Molecular modeling of a Fv fragment. Carbohydr. Res. 324, 17–29 (2000).
Cunningham, M. W. et al. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc. Natl. Acad. Sci. USA 89, 1320–1324 (1992).
Cunningham, M. W. et al. Human monoclonal antibodies reactive with antigens of the group A Streptococcus and human heart. J. Immunol. 141, 2760–2766 (1988).
Shikhman, A. R. & Cunningham, M. W. Immunological mimicry between N-acetyl-beta-D-glucosamine and cytokeratin peptides. Evidence for a microbially driven anti-keratin antibody response. J. Immunol. 152, 4375–4387 (1994).
Adderson, E. E., Shikhman, A. R., Ward, K. E. & Cunningham, M. W. Molecular analysis of polyreactive monoclonal antibodies from rheumatic carditis: human anti-N-acetylglucosamine/anti-myosin antibody V region genes. J. Immunol. 161, 2020–2031 (1998).
Galvin, J. E., Hemric, M. E., Ward, K. & Cunningham, M. W. Cytotoxic monoclonal antibody from rheumatic carditis reacts with human endothelium: implications in rheumatic heart disease. J. Clin. Investig. 106, 217–224 (2000).
Cunningham, M. W. et al. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J. Immunol. 143, 2677–2683 (1989).
Kirvan, C. A., Swedo, S. E., Heuser, J. S. & Cunningham, M. W. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat. Med. 9, 914–920 (2003).
Kirvan, C. A., Cox, C. J., Swedo, S. E. & Cunningham, M. W. Tubulin is a neuronal target of autoantibodies in Sydenham’s chorea. J. Immunol. 178, 7412–7421 (2007).
Cox, C. J. et al. Brain human monoclonal autoantibody from sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: implications in human disease. J. Immunol. 191, 5524–5541 (2013).
Kirvan, C. A., Swedo, S. E., Kurahara, D. & Cunningham, M. W. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham’s chorea. Autoimmunity 39, 21–29 (2006).
Cox, C. J. et al. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder. J. Child Adolesc. Psychopharmacol. 25, 76–85 (2015).
Galvin, J. E., Hemric, M. E., Ward, K. & Cunningham, M. W. Cytotoxic mAb from rheumatic carditis recognizes heart valves and laminin. J. Clin. Invest. 106, 217–224 (2000).
Rappuoli, R. Glycoconjugate vaccines: principles and mechanisms. Sci. Transl. Med. 10, eaat4615 (2018).
Kirvan, C. A. et al. IgG2 rules: N-acetyl-beta-D-glucosamine-specific IgG2 and Th17/Th1 cooperation may promote the pathogenesis of acute rheumatic heart disease and be a biomarker of the autoimmune sequelae of Streptococcus pyogenes. Front. Cardiovasc. Med. 9, 919700 (2022).
Parks, T. et al. Association between a common immunoglobulin heavy chain allele and rheumatic heart disease risk in Oceania. Nat. Commun. 8, 14946 (2017).
Lorenz, N. et al. An acute rheumatic fever immune signature comprising inflammatory markers, IgG3, and Streptococcus pyogenes-specific antibodies. iScience 27, 110558 (2024).
McGregor, R. et al. Mapping autoantibodies in children with acute rheumatic fever. Front. Immunol. 12, 702877 (2021).
Walkinshaw, D. R. et al. The Streptococcus pyogenes vaccine landscape. NPJ Vaccines 8, 16 (2023).
Group, W. Glycam Web Polybuilder.https://glycam.org/ (2024).
Gao, N. J., Lima, E. R. & Nizet, V. Immunobiology of the classical lancefield group a streptococcal carbohydrate antigen. Infect. Immun. 89, e0029221 (2021).
NIH. Ig BLAST Tool.https://www.ncbi.nlm.nih.gov/igblast/ (2025).
Drobyshevskaya, E. I. et al. Production of monoclonal antibodies to mammalian cell nuclear DNA by immunization with streptococcal group a polysaccharide conjugated with synthetic polyelectrolytes. Bull. Exp. Biol. Med. 104, 1123–1126 (1987).
Sabharwal, H. et al. Group A streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J. Infect. Dis. 193, 129–135 (2006).
van Sorge, N. M. et al. The classical lancefield antigen of group a Streptococcus is a virulence determinant with implications for vaccine design. Cell Host Microbe 15, 729–740 (2014).
Kaplan, M. H. & Svec, K. H. Immunologic relation of Streptococcal and tissue antigens. Iii. Presence in human sera of streptococcal antibody cross-reactive with heart tissue. Association with Streptococcal infection, rheumatic fever, and glomerulonephritis. J. Exp. Med. 119, 651–666 (1964).
Cunningham, M. W. Autoimmunity and molecular mimicry in the pathogenesis of post-streptococcal heart disease. Front Biosci. 8, s533–s543 (2003).
Carapetis, J. R. et al. Acute rheumatic fever and rheumatic heart disease. Nat. Rev. Dis. Prim. 2, 15084 (2016).
Kasp-Grochowska, E., Kingston, D. & Glynn, L. E. Immunology of bovine heart valves. II. Reaction with connective tissue components. Ann. Rheum. Dis. 31, 290–297 (1972).
Cunningham, M. W., Krisher, K. & Graves, D. C. Murine monoclonal antibodies reactive with human heart and group A streptococcal membrane antigens. Infect. Immun. 46, 34–41 (1984).
Freimer, E. H. Studies on L forms and protoplasts of group A streptococci. Ii. Chem. Immunol. Prop. cell Membr. J. Exp. Med. 117, 377–399 (1963).
Gross, W. L. & Schlaak, M. Modulation of human lymphocyte functions by group A streptococci. Clin. Immunol. Immunopathol. 32, 234–247 (1984).
Fenderson, P. G., Fischetti, V. A. & Cunningham, M. W. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J. Immunol. 142, 2475–2481 (1989).
Morisaki, I. et al. Cell wall preparation consisting of group A carbohydrate and peptidoglycan moieties from Streptococcus pyogenes activates murine B lymphocytes. Immunobiology 170, 293–304 (1985).
Acknowledgements
The authors acknowledge valuable comments from the following group A streptococcal and immunology experts who reviewed the draft manuscript: Michael Good, Danilo Gomes Moriel, Jonathan Carapetis, Andrew Steer, Shiranee Sriskandan, John Kearney, Rodney King, David Briles and Alexander Shikhman. We acknowledge financial support to meet publication costs from the Strep A Vaccine Global Consortium (SAVAC 2.0) and the Australian Strep A Vaccine Initiative (ASAVI). TP acknowledges funding from the Wellcome Trust [222098/Z/20/Z]. The authors received no other specific funding for the work.
Author information
Authors and Affiliations
Contributions
T.P.: Interpreting literature, drafting and revising manuscript, tables and references. M.W.C.: Interpreting literature, contributed information and references to the manuscript, revising manuscript, tables, and references. A.S.: Concept, searching and interpreting literature, drafting and revising the manuscript, tables and references.
Corresponding authors
Ethics declarations
Competing interests
T.P. has no financial or other conflicts of interests. M.C. has a financial interest in and is Co-founder and Chief Scientific Officer of Moleculera Biosciences, a CLIA and COLA certified laboratory in Oklahoma City, OK, at the University of Oklahoma Research Park where the company offers diagnostic testing of blood samples for anti-neuronal Abs in postinfectious neuropsychiatric sequelae and movement disorders. Moleculera Biosciences owns the license for an autoimmune heart autoantibody panel for future diagnostic testing in autoimmune and inflammatory diseases of the heart. A.S. has received consulting payments, honoraria and travel funds from the Leducq Foundation, ASAVI, SAVAC and the NZ GAS vaccine programme (Rapua te mea ngaro ka tau) for advice on GAS vaccine development. A.S. was the director of the GSK Vaccines Institute for Global Health (GVGH), Siena from 2015 to November 2019. GVGH is developing a GAS vaccine containing a GAC conjugate. However, A.S. has no shares or other financial interest in GVGH, GSK or any other pharmaceutical company. A.S. is an inventor on two families of patents derived from WO2013038375A2 (family contains awarded patents) and WO2022101434A1 (all pending) describing methods for conjugation of polysaccharides to carriers, which may be relevant to production of a GAC conjugate vaccine the assignee of all these patents is GlaxoSmithKline Biologicals SA, GSK Vaccines Institute for Global Health S.R.L. or GSK Vaccines S.R.L. A.S. has no financial interest in these patents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This research was funded in part by the Wellcome Trust [222098/Z/20/Z]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parks, T., Cunningham, M.W. & Saul, A. No compelling evidence that vaccination with streptococcus pyogenes group A carbohydrate elicits cross-reactive rheumatic fever autoantibodies. npj Vaccines 10, 224 (2025). https://doi.org/10.1038/s41541-025-01272-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-025-01272-0