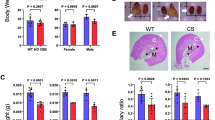
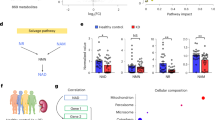
Abstract
Acute kidney injury (AKI) impairs the energy metabolism and antioxidant capacity of renal proximal tubular cells. Here we show that ultrasound-responsive liposomes integrating thylakoid fragments and encapsulating l-ascorbic acid can restore the energy supply and antioxidant capacity of the tubular cells as well as renal function in animal models of AKI. After intravenous injection, the liposomes preferentially accumulated in the injured kidneys and were internalized by proximal tubular cells. Quinolinate phosphoribosyltransferase expressed in thylakoid catalysed the biosynthesis of nicotinamide adenine dinucleotide (NAD+), prompting the recovery of damaged mitochondria. Local ultrasound stimulation activated electron transfer from ascorbic acid, which led to the cytoplasmic formation of NADH and to the restoration of adenosine triphosphate through the malate-aspartate shuttle. Concurrently, the enhanced pentose phosphate pathway facilitated NADPH biosynthesis and reduced the levels of reactive oxygen species. In mice and piglets with AKI, low doses of the liposomes prevented kidney damage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Ronco, C., Bellomo, R. & Kellum, J. A. Acute kidney injury. Lancet 394, 1949–1964 (2019).
Ralto, K. M., Rhee, E. P. & Parikh, S. M. NAD+ homeostasis in renal health and disease. Nat. Rev. Nephrol. 16, 99–111 (2020).
Bhargava, P. & Schnellmann, R. G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 13, 629–646 (2017).
van der Rijt, S., Leemans, J. C., Florquin, S., Houtkooper, R. H. & Tammaro, A. Immunometabolic rewiring of tubular epithelial cells in kidney disease. Nat. Rev. Nephrol. 18, 588–603 (2022).
Wen, L. et al. Glucose metabolism in acute kidney injury and kidney repair. Front. Med. 8, 744122 (2021).
Zheng, T. et al. Alternative polyadenylation trans-factor FIP1 exacerbates UUO/IRI-induced kidney injury and contributes to AKI-CKD transition via ROS-NLRP3 axis. Cell Death Dis. 12, 512 (2021).
Liu, D. et al. ROS-responsive chitosan-SS31 prodrug for AKI therapy via rapid distribution in the kidney and long-term retention in the renal tubule. Sci. Adv. 6, eabb7422 (2020).
Zhang, R. et al. Ultra-small natural product based coordination polymer nanodots for acute kidney injury relief. Mater. Horiz. 8, 1314–1322 (2021).
Liu, T. et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 11, 2788 (2020).
Ni, D. et al. Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 9, 5421 (2018).
Hershberger, K. A., Martin, A. S. & Hirschey, M. D. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat. Rev. Nephrol. 13, 213–225 (2017).
Poyan Mehr, A. et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat. Med. 24, 1351–1359 (2018).
Katsyuba, E. et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature 563, 354–359 (2018).
Xie, N. et al. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal. Transduct. Target. Ther. 5, 227 (2020).
Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 350, 1208–1213 (2015).
Faivre, A. et al. Differential role of nicotinamide adenine dinucleotide deficiency in acute and chronic kidney disease. Nephrol. Dial. Transplant. 36, 60–68 (2021).
Piedrafita, A. et al. The tryptophan pathway and nicotinamide supplementation in ischaemic acute kidney injury. Clin. Kidney J. 14, 2490–2496 (2021).
Li, X. et al. NAD+ anabolism disturbance causes glomerular mesangial cell injury in diabetic nephropathy. Int. J. Mol. Sci. 23, 3458 (2022).
Trammell, S. A. et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 7, 12948 (2016).
Jia, Y. et al. Nicotinamide mononucleotide attenuates renal interstitial fibrosis after AKI by suppressing tubular DNA damage and senescence. Front. Physiol. 12, 649547 (2021).
Guan, Y. et al. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-dependent manner. J. Am. Soc. Nephrol. 28, 2337–2352 (2017).
Bignon, Y. et al. Cell stress response impairs de novo NAD+ biosynthesis in the kidney. JCI Insight 7, e153019 (2022).
Xu, S. et al. Nuclear farnesoid X receptor attenuates acute kidney injury through fatty acid oxidation. Kidney Int. 101, 987–1002 (2022).
Katoh, A., Uenohara, K., Akita, M. & Hashimoto, T. Early steps in the biosynthesis of NAD in Arabidopsis start with aspartate and occur in the plastid. Plant Physiol. 141, 851–857 (2006).
Pétriacq, P. et al. Inducible NAD overproduction in Arabidopsis alters metabolic pools and gene expression correlated with increased salicylate content and resistance to Pst-AvrRpm1. Plant J. 70, 650–665 (2012).
Wang, Y., Li, S., Liu, L., Lv, F. & Wang, S. Conjugated polymer nanoparticles to augment photosynthesis of chloroplasts. Angew. Chem. Int. Ed. Engl. 56, 5308–5311 (2017).
Li, Y. et al. Supramolecular assembly of photosystem II and adenosine triphosphate synthase in artificially designed honeycomb multilayers for photophosphorylation. ACS Nano 12, 1455–1461 (2018).
Geo, H. N. et al. Renal nano-drug delivery for acute kidney injury: current status and future perspectives. J. Control. Release 343, 237–254 (2022).
Yu, H. et al. Size and temporal-dependent efficacy of oltipraz-loaded PLGA nanoparticles for treatment of acute kidney injury and fibrosis. Biomaterials 219, 119368 (2019).
Sercombe, L. et al. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 6, 286 (2015).
Molinaro, R. et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 15, 1037–1046 (2016).
Feng, X., Jia, Y., Cai, P., Fei, J. & Li, J. Coassembly of photosystem II and ATPase as artificial chloroplast for light-driven ATP synthesis. ACS Nano 10, 556–561 (2016).
Wang, W. et al. Bioactive metal-organic frameworks with specific metal-nitrogen (M-N) active sites for efficient sonodynamic tumor therapy. ACS Nano 15, 20003–20012 (2021).
Sahm, F. et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res. 73, 3225–3234 (2013).
Zhao, Y. et al. NAD+ improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion models through Sirt1/PGC-1α pathway. J. Neuroinflammation 18, 207 (2021).
Fanibunda, S. E. et al. Serotonin regulates mitochondrial biogenesis and function in rodent cortical neurons via the 5-HT(2A) receptor and SIRT1-PGC-1α axis. Proc. Natl Acad. Sci. USA 116, 11028–11037 (2019).
Zuo, L. et al. Smart tumor-cell-derived microparticles provide on-demand photosensitizer synthesis and hypoxia relief for photodynamic therapy. Angew. Chem. Int. Ed. Engl. 60, 25365–25371 (2021).
Galgamuwa, R. et al. Dichloroacetate prevents cisplatin-induced nephrotoxicity without compromising cisplatin anticancer properties. J. Am. Soc. Nephrol. 27, 3331–3344 (2016).
Josephine, A. et al. Beneficial effects of sulfated polysaccharides from Sargassum wightii against mitochondrial alterations induced by Cyclosporine A in rat kidney. Mol. Nutr. Food Res. 51, 1413–1422 (2007).
Agudelo, L. Z. et al. Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat. Commun. 10, 2767 (2019).
Zheng, D. W. et al. Photo-powered artificial organelles for ATP generation and life-sustainment. Adv. Mater. 30, e1805038 (2018).
Zhou, H. L. et al. Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury. Nature 565, 96–100 (2019).
Cisternas, P. et al. The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J. Neurochem. 129, 663–671 (2014).
Tang, T. T. et al. Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci. Adv. 6, eaaz0748 (2020).
Zheng, D. W. et al. An orally delivered microbial cocktail for the removal of nitrogenous metabolic waste in animal models of kidney failure. Nat. Biomed. Eng. 4, 853–862 (2020).
Zhang, C. et al. Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS Nano 14, 12133–12147 (2020).
Okubo, K. et al. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat. Med. 24, 232–238 (2018).
Rosenkrans, Z. T. et al. Selenium-doped carbon quantum dots act as broad-spectrum antioxidants for acute kidney injury management. Adv. Sci. 7, 2000420 (2020).
Romagnani, P. et al. Chronic kidney disease. Nat. Rev. Dis. Primers 3, 17088 (2017).
LeBleu, V. S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013).
Falke, L. L., Gholizadeh, S., Goldschmeding, R., Kok, R. J. & Nguyen, T. Q. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nat. Rev. Nephrol. 11, 233–244 (2015).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 10, 493–503 (2014).
Tanaka, T. & Nangaku, M. Angiogenesis and hypoxia in the kidney. Nat. Rev. Nephrol. 9, 211–222 (2013).
Su, H. et al. Subcellular trafficking of tubular MDM2 implicates in acute kidney injury to chronic kidney disease transition during multiple low-dose cisplatin exposure. FASEB J. 34, 1620–1636 (2020).
Guo, X. et al. Kidney-targeted renalase agonist prevents cisplatin-induced chronic kidney disease by inhibiting regulated necrosis and inflammation. J. Am. Soc. Nephrol. 33, 342–356 (2022).
Hukriede, N. A. et al. Experimental models of acute kidney injury for translational research. Nat. Rev. Nephrol. 18, 277–293 (2022).
Chawla, L. S., Eggers, P. W., Star, R. A. & Kimmel, P. L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 371, 58–66 (2014).
Jiang, D. et al. DNA origami nanostructures can exhibit preferential renal uptake and alleviate acute kidney injury. Nat. Biomed. Eng. 2, 865–877 (2018).
Weng, Q. et al. Catalytic activity tunable ceria nanoparticles prevent chemotherapy-induced acute kidney injury without interference with chemotherapeutics. Nat. Commun. 12, 1436 (2021).
Tran, M. T. et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531, 528–532 (2016).
Perico, L. et al. Human mesenchymal stromal cells transplanted into mice stimulate renal tubular cells and enhance mitochondrial function. Nat. Commun. 8, 983 (2017).
Miller, T. E. et al. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts. Science 368, 649–654 (2020).
Porra, R. J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 73, 149–156 (2002).
Ouyang, J. et al. Biomimetic nanothylakoids for efficient imaging-guided photodynamic therapy for cancer. Chem. Commun. 54, 3468–3471 (2018).
Huang, J. et al. A renal-clearable duplex optical reporter for real-time imaging of contrast-induced acute kidney injury. Angew. Chem. Int. Ed. Engl. 58, 17796–17804 (2019).
Acknowledgements
This work was supported by the National Science Fund for Distinguished Young Scholars (22025401) and the National Natural Science Foundation of China (22293034, 22293030, 32101140 and 22104005). We thank the Biological and Medical Engineering Core Facilities (Beijing Institute of Technology) for providing advanced equipment.
Author information
Authors and Affiliations
Contributions
H.-Y.X., Y.L. and D.-W.P. conceived the study, designed the experiments, performed the cellular and animal experiments and wrote the paper. Y.L., Y.W. and H.Z. performed the cellular and animal experiments and evaluated the anti-AKI activity in vivo. Y.L., W.-R.Z., W.N. and G.W. collected and analysed the data. H.-Y.X. provided financial support and directed the research. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Ana Sanz, Wei Tao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Fluorescence imaging and corresponding quantitative analysis of mitochondrial membrane potential changes.
Mitochondria in PTCs were labelled with the potential sensor JC-1. Scale bar, 50 μm. Replicates are biological (n = 3) and all values are shown as the mean ± s.d. Significance calculated using a two-tailed t-test.
Extended Data Fig. 2 Representative imaging analysis of KIM-1+ tubules and proximal tubuli in renal tissue.
(a) Representative immunofluorescence staining of KIM-1+ tubules in renal tissue sections after 24 h different treatments. Higher-magnification images of the KIM-1+ tubules in renal tissue sections are shown at the bottom, and renal tubules were stained with anti-KIM-1 antibody and FITC-conjugated secondary antibody (green: KIM-1; blue: DAPI; wireframe indicates the magnified image). Scale bars, 500 μ (top) and 50 μm (bottom). (b) Representative fluorescence imaging (green: wheat germ agglutinin (WGA) lectin; blue: DAPI) and scanning electron microscopy (SEM) micrographs of proximal tubuli in mice after 24 h different treatments. The renal tubuli were labelled with FITC-conjugated-WGA lectin. Scale bars, 10 μm (top) and 5 μm (bottom).
Extended Data Fig. 3 Therapeutic efficacy of LipTk-AA in sepsis-induced AKI mice.
(a) Schematic diagram of the treatment schedule. (b) Representative immunofluorescence staining of KIM-1+ tubules (green: KIM-1; blue: DAPI) in renal sections after different treatments. Renal tubules were stained with anti-KIM-1 antibody and FITC-conjugated secondary antibody (green: KIM-1; blue: DAPI). Scale bar, 100 μm. (c) Representative images of PAS staining of different groups and corresponding tubule injury scores. Scale bar, 20 μm. (d) Representative immunofluorescence images and corresponding quantitative analysis of tubules with Ly6G+ neutrophils or CD68+ macrophages in kidney sections. Renal cells were stained separately with FITC conjugated-anti-CD68 antibody, anti-Ly6G+ antibody and FITC-conjugated secondary antibody. Scale bars, 20 μm. (e-f) Quantification of IL-1β and IL-6 levels in the serum of mice given different treatments. (g-h) Quantification of BUN and CRE levels in the serum of mice after different treatments. Replicates are biological (n = 5) and all values are shown as the mean ± s.d. Significance calculated using a two-tailed t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, no significance.
Extended Data Fig. 4 Therapeutic efficacy of LipTk-AA in rhabdomyolysis (RM)-induced AKI mice.
(a) Schematic diagram of the treatment schedule. (b) Representative immunofluorescence staining of KIM-1+ tubules in renal tissue sections. Higher-magnification images of the KIM-1+ tubules in renal tissue sections are shown at the bottom, and renal tubules were stained with anti-KIM-1 antibody and FITC-conjugated secondary antibody (green: KIM-1; blue: DAPI; wireframe indicates the magnified image). Scale bars, 500 μm (top) and 50 μm (bottom). (c) Representative fluorescence imaging (green: wheat germ agglutinin (WGA) lectin; blue: DAPI) of renal proximal tubuli after 48 h different treatments. Scale bar, 10 μm. (d-e) Representative images of PAS staining of different groups and corresponding tubule injury scores. Scale bar, 40 μm. (f-g) Quantification of BUN and CRE levels in the serum of mice after different treatments for 24 h or 48 h. Replicates are biological (n = 5) and all values are shown as the mean ± s.d. Significance calculated using a two-tailed t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, no significance.
Extended Data Fig. 5 Effective inhibition of AKI-CKD transition in repeated low doses of cisplatin induced nephrotoxic models.
(a) Illustration of the treatment schedule. (b) Representative images of PAS staining of renal sections from different groups. Scale bar, 20 μm. (c) Representative images of Masson trichrome staining on sagittal sections from different groups. Scale bar, 50 μm. (d) Representative fluorescence imaging of collagen I (Col-I) and (α-SMA) in kidneys of mice given different treatments. Renal tubules were stained with anti-α-SMA, Col-I antibody, and PE or FITC-conjugated secondary antibody. Scale bars, 50 μm. (e) Tubular injury score of the kidneys after different treatments. (f) Quantitative analysis of renal fibrosis in different groups, and (g-h) the mean fluorescence intensity (MFI) of collagen I (Col-I) and (α-SMA) in kidneys of mice given different treatments. Replicates are biological (n = 5) and all values are shown as the mean ± s.d. Significance calculated using a two-tailed t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, no significance.
Extended Data Fig. 6 The assessment of system antigenicity in LipTk-AA&US-treated mice.
(a) Immunohistochemical analysis of CD3+ T cells and CD68+ macrophages in tissue sections. Scale bars, 20 μm. (b) Quantification of IL-6, IL-1β and TNF-α levels in the serum of mice after treated with LipTk-AA&US. Replicates are biological (n = 5) and all values are shown as the mean ± s.d.
Supplementary information
Supplementary Information
Supplementary figures and table.
Data
Source data for the Supplementary figures.
Source data
Source Data Figs. 2–7 and Extended Data Figs. 1–6
Source data and unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lei, Y., Wu, Y., Zhuang, WR. et al. NAD+ biosynthesis and mitochondrial repair in acute kidney injury via ultrasound-responsive thylakoid-integrating liposomes. Nat. Biomed. Eng 9, 1740–1757 (2025). https://doi.org/10.1038/s41551-025-01402-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01402-y
This article is cited by
-
Metabolic reprogramming with ultrasound-responsive nanovesicles
Nature Biomedical Engineering (2025)
-
Harnessing plant thylakoids to restore cellular function in AKI
Nature Reviews Nephrology (2025)