Abstract
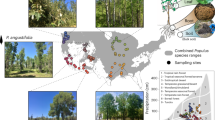
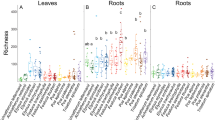
Understanding how species interactions impact population dynamics and long-term persistence over broad temporal and spatial scales is crucial for predicting species distributions and responses to global change. Here we investigate how microbial mutualisms can promote long-term and range-wide population persistence of plants, particularly by ameliorating drought stress. We integrate range-wide field surveys of ~90 grass host populations spanning 13 years with demographic modelling based on 6-year common garden experiments conducted across the host range. We found that mutualistic fungal endophytes (genus Epichloë) promote population-level persistence and growth of their native host grass (Bromus laevipes) across its distribution, with non-mutualistic populations four times more likely to go locally extinct. However, endophyte prevalence declined eightfold more in historically mutualistic populations that experienced high climate variability. This demonstrates that mutualisms can underpin population persistence and buffer hosts against environmental stress but may themselves be vulnerable to global change, with concerning implications for long-term population viability and, ultimately, species distributions under an increasingly uncertain climate.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All datasets involved are available via Zenodo at https://doi.org/10.5281/zenodo.17379577 (ref. 83). Raw climate data are available from the PRISM Group (https://prism.oregonstate.edu/).
Code availability
Code to replicate our analyses and associated datasets are available via Zenodo at https://doi.org/10.5281/zenodo.17379577 (ref. 83).
References
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003).
Wiens, J. J. Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol. 14, e2001104 (2016).
Román-Palacios, C. & Wiens, J. J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl Acad. Sci. USA 117, 4211–4217 (2020).
Bruno, J. F., Stachowicz, J. J. & Bertness, M. D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18, 119–125 (2003).
Porter, S. S. et al. Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct. Ecol. 34, 2075–2086 (2020).
Benning, J. W. & Moeller, D. A. Microbes, mutualism, and range margins: testing the fitness consequences of soil microbial communities across and beyond a native plant’s range. New Phytol. 229, 2886–2900 (2021).
Redman, R. S., Sheehan, K. B., Stout, R. G., Rodriguez, R. J. & Henson, J. M. Thermotolerance generated by plant/fungal symbiosis. Science 298, 1581 (2002).
Donald, M. L. et al. Context-dependent variability in the population prevalence and individual fitness effects of plant–fungal symbiosis. J. Ecol. 109, 847–859 (2021).
Biesmeijer, J. C. et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 313, 351–354 (2006).
Oliver, T. H. et al. Interacting effects of climate change and habitat fragmentation on drought-sensitive butterflies. Nat. Clim. Change 5, 941–945 (2015).
David, A. S. et al. Soil microbiomes underlie population persistence of an endangered plant species. Am. Nat. 194, 488–494 (2019).
Angelini, C. et al. A keystone mutualism underpins resilience of a coastal ecosystem to drought. Nat. Commun. 7, 12473 (2016).
Afkhami, M. E., McIntyre, P. J. & Strauss, S. Y. Mutualist-mediated effects on species’ range limits across large geographic scales. Ecol. Lett. 17, 1265–1273 (2014).
Kivlin, S. N., Emery, S. M. & Rudgers, J. A. Fungal symbionts alter plant responses to global change. Am. J. Bot. 100, 1445–1457 (2013).
Baynes, M., Newcombe, G., Dixon, L., Castlebury, L. & O’Donnell, K. A novel plant–fungal mutualism associated with fire. Fungal Biol. 116, 133–144 (2012).
Fowler, J. C., Ziegler, S., Whitney, K. D., Rudgers, J. A. & Miller, T. E. X. Microbial symbionts buffer hosts from the demographic costs of environmental stochasticity. Ecol. Lett. 27, e14438 (2024).
Vázquez, D. P., Morris, W. F. & Jordano, P. Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol. Lett. 8, 1088–1094 (2005).
Moeller, D. A., Geber, M. A., Eckhart, V. M. & Tiffin, P. Reduced pollinator service and elevated pollen limitation at the geographic range limit of an annual plant. Ecology 93, 1036–1048 (2012).
Harrower, J. & Gilbert, G. S. Context-dependent mutualisms in the Joshua tree–yucca moth system shift along a climate gradient. Ecosphere 9, e02439 (2018).
Lopez, Z. C., Friesen, M. L., Von Wettberg, E., New, L. & Porter, S. Microbial mutualist distribution limits spread of the invasive legume Medicago polymorpha. Biol. Invasions 23, 843–856 (2021).
Leal, L. C. & Peixoto, P. E. C. Decreasing water availability across the globe improves the effectiveness of protective ant–plant mutualisms: a meta-analysis. Biol. Rev. 92, 1785–1794 (2017).
Iannone, L. J., Irisarri, J. G. N., Mc Cargo, P. D., Pérez, L. I. & Gundel, P. E. Occurrence of Epichloë fungal endophytes in the sheep-preferred grass Hordeum comosum from Patagonia. J. Arid. Environ. 115, 19–26 (2015).
Clay, K., Holah, J. & Rudgers, J. A. Herbivores cause a rapid increase in hereditary symbiosis and alter plant community composition. Proc. Natl Acad. Sci. USA 102, 12465–12470 (2005).
Panaccione, D. G., Beaulieu, W. T. & Cook, D. Bioactive alkaloids in vertically transmitted fungal endophytes. Funct. Ecol. 28, 299–314 (2014).
Shen, Y. & Duan, T. The interaction between arbuscular mycorrhizal fungi (AMF) and grass endophyte (Epichloë) on host plants: a review. J. Fungi 10, 174 (2024).
Decunta, F. A., Pérez, L. I., Malinowski, D. P., Molina-Montenegro, M. A. & Gundel, P. E. A systematic review on the effects of Epichloë fungal endophytes on drought tolerance in cool-season grasses. Front. Plant Sci. 12, 644731 (2021).
Kiers, T. E., Palmer, T. M., Ives, A. R., Bruno, J. F. & Bronstein, J. L. Mutualisms in a changing world: an evolutionary perspective. Ecol. Lett. 13, 1459–1474 (2010).
Fowler, J. C., Moutouama, J. & Miller, T. E. X. Increasing prevalence of plant-fungal symbiosis across two centuries of environmental change. Glob. Change Biol. 31, e70577 (2025).
Rudgers, J. A. et al. Climate disruption of plant-microbe interactions. Annu. Rev. Ecol. Evol. Syst. 51, 561–586 (2020).
Rafferty, N. E., CaraDonna, P. J. & Bronstein, J. L. Phenological shifts and the fate of mutualisms. Oikos 124, 14–21 (2015).
Charlton, N. D. et al. Interspecific hybridization and bioactive alkaloid variation increases diversity in endophytic Epichloë species of Bromus laevipes. FEMS Microbiol. Ecol. 90, 276–289 (2014).
Rodriguez, R. J., White, J. F. Jr, Arnold, A. E. & Redman, R. S. Fungal endophytes: diversity and functional roles. New Phytol. 182, 314–330 (2009).
Card, S. D. et al. Mutualistic fungal endophytes in the Triticeae – Survey and description. FEMS Microbiol. Ecol. 88, 94–106 (2014).
Cabral, D., Iannone, L. J., Stewart, A. & Novas, M. V. The distribution and incidence of Neotyphodium endophytes in native grasses from Argentina and its association with environmental factors. NZGA Res. Pract. Ser. 13, 79–82 (2007).
Iannone, L. J., White, J. F., Giussani, L. M., Cabral, D. & Victoria Novas, M. Diversity and distribution of Neotyphodium-infected grasses in Argentina. Mycol. Prog. 10, 9–19 (2011).
Afkhami, M. E. Fungal endophyte–grass symbioses are rare in the California floristic province and other regions with Mediterranean-influenced climates. Fungal Ecol. 5, 345–352 (2012).
IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability (eds Pörtner, H.-O. et al.) (Cambridge Univ. Press, 2023).
Vicente-Serrano, S. M., Beguería, S. & López-Moreno, J. I. A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J. Clim. 23, 1696–1718 (2010).
Yule, K. M., Miller, T. E. X. & Rudgers, J. A. Costs, benefits, and loss of vertically transmitted symbionts affect host population dynamics. Oikos 122, 1512–1520 (2013).
Chung, Y. A., Miller, T. E. X. & Rudgers, J. A. Fungal symbionts maintain a rare plant population but demographic advantage drives the dominance of a common host. J. Ecol. 103, 967–977 (2015).
Gibert, A., Magda, D. & Hazard, L. Interplay between endophyte prevalence, effects and transmission: insights from a natural grass population. PLoS ONE 10, e0139919 (2015).
Rudgers, J. A., Miller, T. E. X., Ziegler, S. M. & Craven, K. D. There are many ways to be a mutualist: endophytic fungus reduces plant survival but increases population growth. Ecology 93, 565–574 (2012).
Gibert, A., Magda, D. & Hazard, L. Endophytic fungus fine-tunes the persistence strategy of its alpine host grass in response to soil resource levels. Oikos 122, 367–376 (2013).
Fowler, J. C., Donald, M. L., Bronstein, J. L. & Miller, T. E. X. The geographic footprint of mutualism: how mutualists influence species’ range limits. Ecol. Monogr. 93, e1558 (2023).
Stanton-Geddes, J. & Anderson, C. G. Does a facultative mutualism limit species range expansion?. Oecologia 167, 149–155 (2011).
Benning, J. W. & Moeller, D. A. Maladaptation beyond a geographic range limit driven by antagonistic and mutualistic biotic interactions across an abiotic gradient. Evolution 73, 2044–2059 (2019).
Nuñez, M. A., Horton, T. R. & Simberloff, D. Lack of belowground mutualisms hinders Pinaceae invasions. Ecology 90, 2352–2359 (2009).
Pierce, D. W., Kalansky, J. F. & Cayan, D. R. Climate, drought, and sea level rise scenarios for the fourth California climate assessment (California Energy Commission, 2018).
Afkhami, M. E. & Rudgers, J. A. Symbiosis lost: imperfect vertical transmission of fungal endophytes in grasses. Am. Nat. 172, 405–416 (2008).
Martínez-García, L. B., de Dios Miranda, J. & Pugnaire, F. I. Impacts of changing rainfall patterns on mycorrhizal status of a shrub from arid environments. Eur. J. Soil Biol. 50, 64–67 (2012).
Chamberlain, S. A., Bronstein, J. L. & Rudgers, J. A. How context dependent are species interactions?. Ecol. Lett. 17, 881–890 (2014).
Bruijning, M., Henry, L. P., Forsberg, S. K. G., Metcalf, C. J. E. & Ayroles, J. F. Natural selection for imprecise vertical transmission in host–microbiota systems. Nat. Ecol. Evol. 6, 77–87 (2022).
Sneck, M. E., Rudgers, J. A., Young, C. A. & Miller, T. E. X. Variation in the prevalence and transmission of heritable symbionts across host populations in heterogeneous environments. Microb. Ecol. 74, 640–653 (2017).
Rodriguez, R. & Redman, R. More than 400 million years of evolution and some plants still can’t make it on their own: plant stress tolerance via fungal symbiosis. J. Exp. Bot. 59, 1109–1114 (2008).
do Valle Ribeiro, M. A. M. Transmission and survival of Acremonium and the implications for grass breeding. Agric. Ecosyst. Environ. 44, 195–213 (1993).
Freitas, P. P. et al. A tale of two grass species: temperature affects the symbiosis of a mutualistic Epichloë endophyte in both tall fescue and perennial ryegrass. Front. Plant Sci. 11, 530 (2020).
Corbin, C., Heyworth, E. R., Ferrari, J. & Hurst, G. D. D. Heritable symbionts in a world of varying temperature. Heredity 118, 10–20 (2017).
Saikkonen, K., Faeth, S. H., Helander, M. & Sullivan, T. J. Fungal endophytes: a continuum of interactions with host plants. Annu. Rev. Ecol. Evol. Syst. 29, 319–343 (1998).
Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Evol. Syst. 31, 343–366 (2000).
Miller, T. E. X. & Rudgers, J. A. Niche differentiation in the dynamics of host–symbiont interactions: symbiont prevalence as a coexistence problem. Am. Nat. 183, 506–518 (2014).
Aslan, C. E., Zavaleta, E. S., Tershy, B. & Croll, D. Mutualism disruption threatens global plant biodiversity: a systematic review. PLoS ONE 8, e66993 (2013).
Hale, K. R. S., Valdovinos, F. S. & Martinez, N. D. Mutualism increases diversity, stability, and function of multiplex networks that integrate pollinators into food webs. Nat. Commun. 11, 2182 (2020).
Taylor, B. N., Simms, E. L. & Komatsu, K. J. More than a functional group: diversity within the legume–rhizobia mutualism and its relationship with ecosystem function. Diversity 12, 50 (2020).
Wei, W. et al. Long-term fertilization coupled with rhizobium inoculation promotes soybean yield and alters soil bacterial community composition. Front. Microbiol. 14, 1161983 (2023).
Thom, E. R., Popay, A. J., Hume, D. E. & Fletcher, L. R. Evaluating the performance of endophytes in farm systems to improve farmer outcomes – A review. Crop Pasture Sci. 63, 927–943 (2012).
Csorba, A. B. et al. Aphid adaptation in a changing environment through their bacterial endosymbionts: an overview, including a new major cereal pest (Rhopalosiphum maidis (Fitch) scenario. Symbiosis 93, 139–152 (2024).
Apprill, A. The role of symbioses in the adaptation and stress responses of marine organisms. Annu. Rev. Mar. Sci. 12, 291–314 (2020).
Remke, M. J., Johnson, N. C., Wright, J., Williamson, M. & Bowker, M. A. Sympatric pairings of dryland grass populations, mycorrhizal fungi and associated soil biota enhance mutualism and ameliorate drought stress. J. Ecol. 109, 1210–1223 (2021).
Rodriguez, R. J. et al. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2, 404–416 (2008).
Leuchtmann, A. Systematics, distribution, and host specificity of grass endophytes. Nat. Toxins 1, 150–162 (1993).
Xia, C. et al. Role of Epichloë endophytes in defense responses of cool-season grasses to pathogens: a review. Plant Dis. 102, 2061–2073 (2018).
Ahlholm, J. U., Helander, M., Lehtimäki, S., Wäli, P. & Saikkonen, K. Vertically transmitted fungal endophytes: different responses of host-parasite systems to environmental conditions. Oikos 99, 173–183 (2002).
Hickman, J. C. The Jepson Manual: Higher Plants of California (Univ. California Press, 1993).
Bacon, C. W. & White, J. F. in Biotechnology of Endophytic Fungi of Grasses (eds Bacon, C. W. & White, J. F.) 47–56 (CRC, 1994).
Dombrowski, J. E., Baldwin, J. C., Azevedo, M. D. & Banowetz, G. M. A sensitive PCR-based assay to detect Neotyphodium fungi in seed and plant tissue of tall fescue and ryegrass species. Crop Sci. 46, 1064–1070 (2006).
Beguería, S. & Vicente-Serrano, S. M. SPEI: Calculation of the Standardized Precipitation-evapotranspiration Index. R version 1.8.1 https://cran.r-project.org/web/packages/SPEI (2023).
Bartoń, K. MuMIn: Multi-model Inference. R version 1.48.4 https://cran.r-project.org/web/packages/MuMIn (2024).
Zeileis, A. et al. betareg: Beta Regression. R version 3.2-3 https://cran.r-project.org/web/packages/betareg (2025).
Paradis, E. et al. ape: Analyses of Phylogenetics and Evolution. R version 5.7-1 https://cran.r-project.org/web/packages/ape (2023).
Afkhami, M. E. & Strauss, S. Y. Native fungal endophytes suppress an exotic dominant and increase plant diversity over small and large spatial scales. Ecology 97, 1159–1169 (2016).
Kohl, M. MKinfer: Inferential Statistics. R version 1.2 https://cran.r-project.org/web/packages/MKinfer (2024).
Caswell, H. Matrix Population Models: Construction, Analysis, and Interpretation (Sinauer Associates, 2001).
Li, V. et al. Climate variability disrupts microbial mutualism-driven population persistence. Zenodo https://doi.org/10.5281/zenodo.17379578 (2025).
Moon, K.-W. ggiraphExtra: Make Interactive ‘ggplot2’. Extension to ‘ggplot2’ and ‘ggiraph’. R version 0.3.0 https://cran.r-project.org/web/packages/ggiraphExtra (2020).
Acknowledgements
We thank the University of California Natural Reserve System, particularly the McLaughlin, Quail Ridge, Hastings and Angelo Reserves, for providing protected natural habitats in which to conduct our experiments; the US Forest Service for their support of this project; and UC Reserve and Forest Service staff: J. Huhndorf, P. Aigner, C. Koehler, M. Power, J. Hunter, P. Steel, A. Spyres, R. Brennan, V. Boucher, J. Clary, L. Johnson and M. Stromberg. We also thank the Afkhami and Searcy laboratories, especially D. Hernandez, K. Kiesewetter, A. O’Brien and R. Rumelt, as well as W. Browne and D. DeAngelis for helpful feedback on analyses and the manuscript. This research was supported by the National Science Foundation (grant nos. NSF DEB-2030060 and NSF DEB-1922521 to M.E.A. and C.A.S.).
Author information
Authors and Affiliations
Contributions
V.W.L. collected field survey data, performed data analysis and demographic modelling and wrote the manuscript. J.C.F. contributed to demographic model construction and manuscript revisions. A.S.D. contributed to building and writing methods for the demographic model and manuscript revisions. S.Y.S. contributed to the conceptualization and design of the common garden experiments as well as the manuscript revisions and student supervision. C.A.S. contributed to survey data collection, manuscript revisions and feedback on modelling and statistical analyses. M.E.A. led overall project conception and contributed to data collection for field surveys and common gardens, common garden experimental design and establishment, feedback on analyses, manuscript revisions and student supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Discussion, Figs 1–17, Tables 1–13 and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, V.W., Fowler, J.C., David, A.S. et al. Climate variability disrupts microbial mutualism-driven population persistence. Nat Ecol Evol 10, 221–231 (2026). https://doi.org/10.1038/s41559-025-02943-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41559-025-02943-w