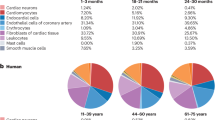
Abstract
Chronic inflammation has long been recognized as a major risk factor for and a causal contributor to cardiovascular disease (CVD). However, advances in omics technologies and deepening insights into CVD pathogenesis have expanded our understanding of the underlying mechanisms. Inflammation is now seen not as an isolated cause, but as one of several biological responses to cumulative tissue damage over time. In this Review, we propose that inflammation initially functions as a resilience mechanism, acting to resolve molecular and cellular damage driven by environmental stressors and intrinsic age-related entropy. With ageing, however, this protective response can become dysregulated and maladaptive, promoting collateral pathological changes. We illustrate this theory through two examples, atherosclerosis and age-related impairment of tissue perfusion, and support these conceptual models using proteomic data from large population studies with cardiovascular outcomes. Our findings reaffirm the central role of inflammation in CVD pathophysiology, but also indicate that the upstream biological driver of inflammation is molecular damage that is either not readily prevented or repaired by inadequate resilience mechanisms. Understanding the coordination of these responses offers new opportunities for targeted prevention and treatment of CVD.
Key points
-
Ageing, endothelial dysfunction and impaired perfusion lead to molecular damage and trigger resilience mechanisms to maintain tissue integrity.
-
Persistent, unrepaired molecular damage activates inflammatory responses that, over time, contribute to atherosclerosis.
-
In ageing, impaired vascular regulation and tissue fibrosis can cause recurrent hypoperfusion, leading to energy deficits and progressive molecular damage.
-
Chronic inflammation acts as a component of stress response pathways that, ultimately, lead to the onset and progression of cardiovascular disease.
-
Biomarkers of cardiovascular disease, such as fibroblast growth factor 23 and growth/differentiation factor 15, reflect not only inflammation, but also changes in the extracellular matrix, cellular senescence and proteostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Datasets and the custom R source code used in the proteomic meta-analysis are available on the Open Science Framework repository117.
References
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Ferrucci, L. et al. The origins of age-related proinflammatory state. Blood 105, 2294–2299 (2005).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A 69, S4–S9 (2014).
Ridker, P. M. Closing the loop on inflammation and atherothrombosis: why perform the CIRT and CANTOS trials? Trans. Am. Clin. Climatol. Assoc. 124, 174–190 (2013).
Dalton, J. E. et al. Failure of traditional risk factors to adequately predict cardiovascular events in older populations. J. Am. Geriatr. Soc. 68, 754–761 (2020).
Walker, K. A. et al. Connecting aging biology and inflammation in the omics era. J. Clin. Invest. 132, e158448 (2022).
Picard, M. M. & Murugan, N. J. The energy resistance principle. Cell Metab. 37, 2107–2127 (2025).
Patel, S. et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 29, 707–718.e8 (2019).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Bortz, W. M. 2nd. Aging as entropy. Exp. Gerontol. 21, 321–328 (1986).
Ferrucci, L. et al. Measuring biological aging in humans: a quest. Aging Cell 19, e13080 (2020).
McMillen, P. & Levin, M. Collective intelligence: a unifying concept for integrating biology across scales and substrates. Commun. Biol. 7, 378 (2024).
Schumacher, B. et al. The central role of DNA damage in the ageing process. Nature 592, 695–703 (2021).
Ren, P., Dong, X. & Vijg, J. Age-related somatic mutation burden in human tissues. Front. Aging 3, 1018119 (2022).
Vijg, J. & Dong, X. Pathogenic mechanisms of somatic mutation and genome mosaicism in aging. Cell 182, 12–23 (2020).
Shaulson, E. D., Cohen, A. A. & Picard, M. The brain–body energy conservation model of aging. Nat. Aging 4, 1354–1371 (2024).
Zhao, Y. et al. DNA damage and repair in age-related inflammation. Nat. Rev. Immunol. 23, 75–89 (2023).
Wang, B. et al. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 25, 958–978 (2024).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Picca, A. et al. Mitophagy in human health, ageing and disease. Nat. Metab. 5, 2047–2061 (2023).
Walker, E. M. et al. Retrograde mitochondrial signaling governs the identity and maturity of metabolic tissues. Science 388, eadf2034 (2025).
Minamino, T. & Komuro, I. Vascular cell senescence: contribution to atherosclerosis. Circ. Res. 100, 15–26 (2007).
Phua, T. J. Understanding human aging and the fundamental cell signaling link in age-related diseases: the middle-aging hypovascularity hypoxia hypothesis. Front. Aging 4, 1196648 (2023).
Steinberg, D. In celebration of the 100th anniversary of the lipid hypothesis of atherosclerosis. J. Lipid Res. 54, 2946–2949 (2013).
Ross, R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Warboys, C. M. et al. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 34, 985–995 (2014).
Jia, G. et al. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1802–1809 (2019).
Erusalimsky, J. D. Vascular endothelial senescence: from mechanisms to pathophysiology. J. Appl. Physiol. 106, 326–332 (2009).
Belcastro, E. et al. Fluorescent nanocarriers targeting VCAM-1 for early detection of senescent endothelial cells. Nanomedicine 34, 102379 (2021).
Minamino, T. et al. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544 (2002).
Chen, C. & Khismatullin, D. B. Oxidized low-density lipoprotein contributes to atherogenesis via co-activation of macrophages and mast cells. PLoS ONE 10, e0123088 (2015).
Chinetti-Gbaguidi, G., Colin, S. & Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 12, 10–17 (2015).
Bloom, S. I. et al. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 20, 38–51 (2023).
Hwang, H. J. et al. Endothelial cells under therapy-induced senescence secrete CXCL11, which increases aggressiveness of breast cancer cells. Cancer Lett. 490, 100–110 (2020).
Jia, G. et al. Vascular stiffness in insulin resistance and obesity. Front. Physiol. 6, 231 (2015).
Gardner, S. E. et al. Senescent vascular smooth muscle cells drive inflammation through an interleukin-1α-dependent senescence-associated secretory phenotype. Arterioscler. Thromb. Vasc. Biol. 35, 1963–1974 (2015).
Herman, A. B. et al. DPP4 inhibition impairs senohemostasis to improve plaque stability in atherosclerotic mice. J. Clin. Invest. 133, e165933 (2023).
Owens, G. K. Molecular control of vascular smooth muscle cell differentiation and phenotypic plasticity. Novartis Found. Symp. 283, 174–191 (2007).
Shankman, L. S. et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 21, 628–637 (2015).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016).
Kawai, K. et al. Differences in stable and unstable atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 44, 1474–1484 (2024).
Virmani, R. et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275 (2000).
Nakajima, A. et al. Biomarkers associated with coronary high-risk plaques. J. Thromb. Thrombolysis 54, 647–659 (2022).
Kigka, V. I. et al. Serum biomarkers in carotid artery disease. Diagnostics 11, 2143 (2012).
Lutsey, P. L. et al. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J. Am. Heart Assoc. 3, e000936 (2014).
Chen, M. C., Hsu, B. G., Lee, C. J. & Wang, J. H. High-serum angiopoietin-like protein 3 levels associated with cardiovascular outcome in patients with coronary artery disease. Int. J. Hypertens. 2020, 2980954 (2020).
Schickling, B. M. & Miller, F. J. Jr. Outside-in signaling by adventitial fibroblasts. Arterioscler. Thromb. Vasc. Biol. 41, 711–713 (2021).
Sartore, S. et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ. Res. 89, 1111–1121 (2001).
Sirois, M. G., Simons, M. & Edelman, E. R. Antisense oligonucleotide inhibition of PDGFR-β receptor subunit expression directs suppression of intimal thickening. Circulation 95, 669–676 (1997).
Herrmann, J. et al. Differential effect of experimental hypertension and hypercholesterolemia on adventitial remodeling. Arterioscler. Thromb. Vasc. Biol. 25, 447–453 (2005).
Stenmark, K. R. et al. The adventitia: essential regulator of vascular wall structure and function. Annu. Rev. Physiol. 75, 23–47 (2013).
Buono, M. F. et al. Human plaque myofibroblasts to study mechanisms of atherosclerosis. J. Am. Heart Assoc. 12, e030243 (2023).
Dabravolski, S. A. et al. Molecular mechanisms underlying pathological and therapeutic roles of pericytes in atherosclerosis. Int. J. Mol. Sci. 23, 11663 (2022).
Campagnolo, P. et al. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation 121, 1735–1745 (2010).
Wang, N. et al. Novel mechanism of the pericyte–myofibroblast transition in renal interstitial fibrosis: core fucosylation regulation. Sci. Rep. 7, 16914 (2017).
Schrimpf, C. et al. TIMP3 is regulated by pericytes upon shear stress detection leading to a modified endothelial cell response. Eur. J. Vasc. Endovasc. Surg. 54, 524–533 (2017).
de Vries, M. R. & Quax, P. H. Plaque angiogenesis and its relation to inflammation and atherosclerotic plaque destabilization. Curr. Opin. Lipidol. 27, 499–506 (2016).
Kong, P. et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 7, 131 (2022).
Matter, M. A. et al. Inflammation in acute myocardial infarction: the good, the bad and the ugly. Eur. Heart J. 45, 89–103 (2024).
Liu, C. et al. Insight into cerebral microvessel endothelial regulation of cognitive impairment: a systematic review of the causes and consequences. Exp. Neurol. 385, 115116 (2025).
Halvorson, B. D. et al. Scoping review: integration of the major mechanisms underlying the regulation of arteriolar tone. J. Vasc. Res. 61, 1–15 (2024).
Altura, B. M. Chemical and humoral regulation of blood flow through the precapillary sphincter. Microvasc. Res. 3, 361–384 (1971).
Zambach, S. A. et al. Precapillary sphincters and pericytes at first-order capillaries as key regulators for brain capillary perfusion. Proc. Natl Acad. Sci. USA 118, e2023749118 (2021).
Rhodin, J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J. Ultrastruct. Res. 18, 181–223 (1967).
Federspiel, W. J. & Popel, A. S. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc. Res. 32, 164–189 (1986).
Dore-Duffy, P. & Cleary, K. Morphology and properties of pericytes. Methods Mol. Biol. 686, 49–68 (2011).
Banks, W. A. et al. Healthy aging and the blood–brain barrier. Nat. Aging 1, 243–254 (2021).
Avolio, E. et al. The role of cardiac pericytes in health and disease: therapeutic targets for myocardial infarction. Nat. Rev. Cardiol. 21, 106–118 (2024).
Donato, A. J., Machin, D. R. & Lesniewski, L. A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 123, 825–848 (2018).
Mick, E. et al. Distinct mitochondrial defects trigger the integrated stress response depending on the metabolic state of the cell. eLife 9, e49178 (2020).
Stasch, J. P. et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J. Clin. Invest. 116, 2552–2561 (2006).
Landmesser, U. et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 111, 1201–1209 (2003).
AlGhatrif, M. et al. Longitudinal decline in peak Vo2 with aging in a healthy population is associated with a reduction in peripheral oxygen utilization but not in cardiac output. Am. J. Physiol. Heart Circ. Physiol 327, H509–H517 (2024).
Ungvari, Z. et al. Mechanisms of vascular aging. Circ. Res. 123, 849–867 (2018).
Hu, H. H. et al. Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res. Rev. 60, 101063 (2020).
Janotka, M. & Ostadal, P. Biochemical markers for clinical monitoring of tissue perfusion. Mol. Cell Biochem. 476, 1313–1326 (2021).
Coppel, J. et al. A comparison of the quality of image acquisition between two different sidestream dark field video-microscopes. J. Clin. Monit. Comput. 35, 577–583 (2021).
McDermott, M. M. et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J. Vasc. Surg. 32, 1164–1171 (2000).
Oberdier, M. T. et al. Ankle-brachial index and energy production in people without peripheral artery disease: the blsa. J. Am. Heart Assoc. 11, e019014 (2022).
Kim, J., Kim, H. S. & Chung, J. H. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS–STING pathway. Exp. Mol. Med. 55, 510–519 (2023).
Zampino, M. et al. Poor mitochondrial health and systemic inflammation? Test of a classic hypothesis in the Baltimore longitudinal study of aging. Geroscience 42, 1175–1182 (2020).
Morciano, G. et al. The mitochondrial permeability transition pore: an evolving concept critical for cell life and death. Biol. Rev. Camb. Philos. Soc. 96, 2489–2521 (2021).
Ershler, W. B. et al. Serum erythropoietin and aging: a longitudinal analysis. J. Am. Geriatr. Soc. 53, 1360–1365 (2005).
Ren, J. et al. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat. Rev. Cardiol. 18, 499–521 (2021).
Chipurupalli, S., Samavedam, U. & Robinson, N. Crosstalk between ER stress, autophagy and inflammation. Front. Med. 8, 758311 (2021).
Pluquet, O., Pourtier, A. & Abbadie, C. The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am. J. Physiol. Cell Physiol 308, C415–C425 (2015).
Costa-Mattioli, M. & Walter, P. The integrated stress response: from mechanism to disease. Science 368, eaat5314 (2020).
Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep. 17, 1374–1395 (2016).
Roy, R. et al. Epigenetic signature of human immune aging in the GESTALT study. eLife 12, e86136 (2023).
McGuire, D. K. et al. Oral semaglutide and cardiovascular outcomes in high-risk type 2 diabetes. N. Engl. J. Med. 392, 2001–2012 (2025).
Knauf, C. et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J. Clin. Invest. 115, 3554–3563 (2005).
Xu, X. Y. et al. GLP-1 in the hypothalamic paraventricular nucleus promotes sympathetic activation and hypertension. J. Neurosci. 44, e2032232024 (2024).
Aung, M. M. et al. Locally delivered GLP-1 analogues liraglutide and exenatide enhance microvascular perfusion in individuals with and without type 2 diabetes. Diabetologia 62, 1701–1711 (2019).
Wei, R. et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am. J. Physiol. Endocrinol. Metab. 310, E947–E957 (2016).
Smits, M. M. et al. GLP-1 receptor agonist exenatide increases capillary perfusion independent of nitric oxide in healthy overweight men. Arterioscler. Thromb. Vasc. Biol. 35, 1538–1543 (2015).
Ho, J. E. et al. Protein biomarkers of cardiovascular disease and mortality in the community. J. Am. Heart Assoc. 7, e008108 (2018).
Hoogeveen, R. M. et al. Improved cardiovascular risk prediction using targeted plasma proteomics in primary prevention. Eur. Heart J. 41, 3998–4007 (2020).
Nurmohamed, N. S. et al. Targeted proteomics improves cardiovascular risk prediction in secondary prevention. Eur. Heart J. 43, 1569–1577 (2022).
Williams, S. A. et al. A proteomic surrogate for cardiovascular outcomes that is sensitive to multiple mechanisms of change in risk. Sci. Transl. Med. 14, eabj9625 (2022).
Helgason, H. et al. Evaluation of large-scale proteomics for prediction of cardiovascular events. JAMA 330, 725–735 (2023).
Mazidi, M. et al. Plasma proteomics to identify drug targets for ischemic heart disease. J. Am. Coll. Cardiol. 82, 1906–1920 (2023).
Carrasco-Zanini, J. et al. Proteomic signatures improve risk prediction for common and rare diseases. Nat. Med. 30, 2489–2498 (2024).
Ho, F. K. et al. A proteomics-based approach for prediction of different cardiovascular diseases and dementia. Circulation 151, 277–287 (2025).
Kuku, K. O. et al. Development and validation of a protein risk score for mortality in heart failure: a community cohort study. Ann. Intern. Med. 177, 39–49 (2024).
Ramonfaur, D. et al. High throughput plasma proteomics and risk of heart failure and frailty in late life. JAMA Cardiol. 9, 649–658 (2024).
Schuermans, A. et al. Integrative proteomic analyses across common cardiac diseases yield mechanistic insights and enhanced prediction. Nat. Cardiovasc. Res. 3, 1516–1530 (2024).
Titova, O. E. et al. Plasma proteome and incident myocardial infarction: sex-specific differences. Eur. Heart J. 45, 4647–4657 (2024).
Coppe, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 28, 436–453 (2018).
Wiley, C. D. et al. SILAC analysis reveals increased secretion of hemostasis-related factors by senescent cells. Cell Rep. 28, 3329–3337 (2019).
Basisty, N. et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18, e3000599 (2020).
Casella, G. et al. Transcriptome signature of cellular senescence. Nucleic Acids Res. 47, 7294–7305 (2019).
Wang, Y. et al. PCSK9 affects vascular senescence through the SIRT1 pathway. Exp. Gerontol. 201, 112701 (2025).
GTEx Consortium. The genotype-tissue expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Oh, H. S. et al. Organ aging signatures in the plasma proteome track health and disease. Nature 624, 164–172 (2023).
Kivimaki, M. et al. Proteomic organ-specific ageing signatures and 20-year risk of age-related diseases: the Whitehall II observational cohort study. Lancet Digit. Health 7, e195–e204 (2025).
Candia, J. Aging-associated accumulation of molecular damage drives inflammation in cardiovascular disease. Open Science Framework https://osf.io/p4kbg/files/xvjcy (2025).
Kapil, V. et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65, 320–327 (2015).
Cosentino, F. et al. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart 94, 487–492 (2008).
Lewis, G. D. et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 116, 1555–1562 (2007).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).
Kooy, A. et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch. Intern. Med. 169, 616–625 (2009).
Mannick, J. B. et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 6, 268ra179 (2014).
Rossman, M. J. et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71, 1056–1063 (2018).
Martens, C. R. et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 9, 1286 (2018).
Mortensen, S. A. et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2, 641–649 (2014).
Daubert, M. A. et al. Novel mitochondria-targeting peptide in heart failure treatment: a randomized, placebo-controlled trial of elamipretide. Circ. Heart Fail. 10, e004389 (2017).
Andreux, P. A. et al. The mitophagy activator urolithin a is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 1, 595–603 (2019).
Singh, A. et al. Direct supplementation with urolithin a overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr. 76, 297–308 (2022).
Singh, A. et al. Urolithin a improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 3, 100633 (2022).
Acknowledgements
The authors acknowledge the support of the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Baltimore, MD, USA.
Author information
Authors and Affiliations
Contributions
All the authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Mika Kivimaki, Esther Lutgens, Andrew Steptoe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Biomarkers
-
Measurable indicators of biological and pathogenic processes or therapeutic efficacy.
- Cellular senescence
-
A state of stable cell cycle arrest caused by sublethal DNA damage and stress.
- Entropy
-
The measure of a system’s thermal energy per unit temperature that is unavailable for doing useful work.
- Immunosenescence
-
Innate and adaptive immune dysregulation that occurs with ageing.
- Integrated stress response
-
The signalling pathway activated in response to various forms of stress, including nutrient and oxygen deprivation, oxidative stress and viral infections.
- Macromolecular damage
-
The accumulation of structural and chemical alterations in DNA, proteins, lipids and polysaccharides within cells.
- Perfusion
-
The passing of a fluid through spaces.
- Proteostasis or proteostatic
-
Protein homeostasis or homeostatic state.
- Resilience response
-
The capacity of an organism to adapt or recover from stress. The response involves molecular pathways that detect stress, promote survival and enable repair.
- Senescence-associated secretory phenotype
-
The secretion of bioactive molecules associated with damage-induced cellular senescence.
- Stochastic
-
Occurring by chance or random variables.
- Thermodynamics
-
The relationships between heat, work, temperature and energy.
- Unfolded protein response
-
The signalling pathway responsible for the fidelity of protein folding in the lumen of the endoplasmic reticulum.
Rights and permissions
About this article
Cite this article
Herman, A.B., Candia, J., Wilson, D.M. et al. Molecular damage associated with ageing drives inflammation in cardiovascular disease. Nat Rev Cardiol (2026). https://doi.org/10.1038/s41569-026-01253-3
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41569-026-01253-3