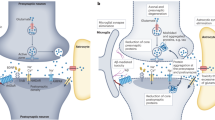
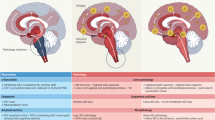
Abstract
Preservation of synapses is crucial for healthy cognitive ageing, and synapse loss is one of the closest anatomical correlates of cognitive decline in Alzheimer disease, dementia with Lewy bodies and frontotemporal dementia. In these conditions, some synapses seem particularly vulnerable to degeneration whereas others are resilient and remain preserved. Evidence has highlighted that vulnerability and resilience are intrinsically distinct phenomena linked to specific brain structural and/or functional signatures, yet the key features of vulnerable and resilient synapses in the dementias remain incompletely understood. Defining the characteristics of vulnerable and resilient synapses in each form of dementia could offer novel insight into the mechanisms of synapse preservation and of synapse loss that underlies cognitive decline, thereby facilitating the discovery of targeted biomarkers and disease-modifying therapies. In this Review, we consider the concepts of synapse vulnerability and resilience, and provide an overview of our current understanding of the associations between synaptic protein changes, neuropathology and cognitive decline. We also consider how understanding of the underlying mechanisms could identify novel strategies to mitigate the cognitive dysfunction associated with dementias.
Key points
-
Synaptic pathology is a robust hallmark of dementia, and selective synapse degeneration could be the anatomical basis of varying clinical presentations and disease severities of dementia disorders.
-
Vulnerable synapses are preferentially affected in dementia, whereas resilient synapses are comparatively spared; understanding the mechanisms of selective synapse degeneration could provide clinically more relevant outcomes than current neuropathology-centred approaches.
-
Studies of temporal synapse changes are now possible with biofluid analysis and brain imaging; disease- and stage-specific synaptic signatures could reveal novel biomarkers for dementia, and their study in brain tissue may propose tailored synapse-protective targets for timely interventions.
-
Neuroinflammation, which involves central (glial cells) and peripheral (innate immunity, adaptive immunity) elements, has a key role in synapse pathology across dementias, and its modulation with central and/or peripheral treatment approaches holds promise for mitigating dementia symptoms.
-
Convergence of multiple oligomeric proteins and neuroinflammatory elements at synapses is a shared feature of dementias; dissecting their intricate crosstalk and combined cognitive effects could unravel critical therapeutic targets to block potentially deleterious interactions at synapses.
-
Synapse pathology in rapidly progressive dementias remains under-studied but could reveal novel mechanisms of fulminant synapse degeneration and/or synapse recovery that could be exploited therapeutically in all dementias.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Abraham, W. C., Jones, O. D. & Glanzman, D. L. Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci. Learn. 4, 9 (2019).
von Bartheld, C. S. Myths and truths about the cellular composition of the human brain: a review of influential concepts. J. Chem. Neuroanat. 93, 2–15 (2018).
Liu, Y. et al. Interactions of glial cells with neuronal synapses, from astrocytes to microglia and oligodendrocyte lineage cells. Glia 71, 1383–1401 (2023).
Perrone-Capano, C., Volpicelli, F., Penna, E., Chun, J. T. & Crispino, M. Presynaptic protein synthesis and brain plasticity: from physiology to neuropathology. Prog. Neurobiol. 202, 102051 (2021).
Chater, T. E., Eggl, M. F., Goda, Y. & Tchumatchenko, T. Competitive processes shape multi-synapse plasticity along dendritic segments. Nat. Commun. 15, 7572 (2024).
De Francesco, S. et al. Differential diagnosis of neurodegenerative dementias with the explainable MRI based machine learning algorithm MUQUBIA. Sci. Rep. 13, 17355 (2023).
Henstridge, C. M., Pickett, E. & Spires-Jones, T. L. Synaptic pathology: a shared mechanism in neurological disease. Ageing Res. Rev. 28, 72–84 (2016).
DeKosky, S. T. & Scheff, S. W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 27, 457–464 (1990).
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
Griffiths, J. & Grant, S. G. N. Synapse pathology in Alzheimer’s disease. Semin. Cell Dev. Biol. 139, 13–23 (2023).
Frigerio, I. et al. Regional differences in synaptic degeneration are linked to alpha-synuclein burden and axonal damage in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 12, 4 (2024).
Malpetti, M. et al. Synaptic loss in frontotemporal dementia revealed by [11C]UCB-J positron emission tomography. Ann. Neurol. 93, 142–154 (2023).
Hermann, P. & Zerr, I. Rapidly progressive dementias — aetiologies, diagnosis and management. Nat. Rev. Neurol. 18, 363–376 (2022).
Colom-Cadena, M. et al. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain 140, 3204–3214 (2017).
Clayton, E. L., Huggon, L., Cousin, M. A. & Mizielinska, S. Synaptopathy: presynaptic convergence in frontotemporal dementia and amyotrophic lateral sclerosis. Brain 147, 2289–2307 (2024).
Anschuetz, A., Schwab, K., Harrington, C. R., Wischik, C. M. & Riedel, G. A meta-analysis on presynaptic changes in Alzheimer’s disease. J. Alzheimers Dis. 97, 145–162 (2024).
Graff-Radford, J. et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 20, 222–234 (2021).
Oh, H. S.-H. et al. A cerebrospinal fluid synaptic protein biomarker for prediction of cognitive resilience versus decline in Alzheimer’s disease. Nat. Med. https://doi.org/10.1038/s41591-025-03565-2 (2025).
Sogorb-Esteve, A. et al. Differential impairment of cerebrospinal fluid synaptic biomarkers in the genetic forms of frontotemporal dementia. Alzheimers Res. Ther. 14, 118 (2022).
Borczyk, M., Radwanska, K. & Giese, K. P. The importance of ultrastructural analysis of memory. Brain Res. Bull. 173, 28–36 (2021).
Taoufik, E., Kouroupi, G., Zygogianni, O. & Matsas, R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: an overview of induced pluripotent stem-cell-based disease models. Open. Biol. 8, 180138 (2018).
Nilsson, J. et al. Cerebrospinal fluid biomarker panel for synaptic dysfunction in a broad spectrum of neurodegenerative diseases. Brain 147, 2414–2427 (2024).
Meftah, S. & Gan, J. Alzheimer’s disease as a synaptopathy: evidence for dysfunction of synapses during disease progression. Front. Synaptic Neurosci. 15, 1129036 (2023).
Minoshima, S., Cross, D., Thientunyakit, T., Foster, N. L. & Drzezga, A. 18F-FDG PET imaging in neurodegenerative dementing disorders: insights into subtype classification, emerging disease categories, and mixed dementia with copathologies. J. Nucl. Med. 63, 2S–12S (2022).
Martínez-Serra, R., Alonso-Nanclares, L., Cho, K. & Giese, K. P. Emerging insights into synapse dysregulation in Alzheimer’s disease. Brain Commun. 4, fcac083 (2022).
Goossens, J. et al. Evaluation of cerebrospinal fluid levels of synaptic vesicle protein, VAMP-2, across the sporadic Alzheimer’s disease continuum. Alzheimers Res. Ther. 15, 186 (2023).
Martín-de-Saavedra, M. D., Santos, M. D. & Penzes, P. Intercellular signaling by ectodomain shedding at the synapse. Trends Neurosci. 45, 483–498 (2022).
Cheng, Q. et al. Biomarkers of synaptic degeneration in Alzheimer’s disease. Ageing Res. Rev. https://doi.org/10.1016/j.arr.2024.102642 (2024).
Iascone, D. M. et al. Whole-neuron synaptic mapping reveals spatially precise excitatory/inhibitory balance limiting dendritic and somatic spiking. Neuron 106, 566–578.e8 (2020).
Lauterborn, J. C. et al. Increased excitatory to inhibitory synaptic ratio in parietal cortex samples from individuals with Alzheimer’s disease. Nat. Commun. 12, 2603 (2021).
Tok, S., Ahnaou, A. & Drinkenburg, W. Functional neurophysiological biomarkers of early-stage Alzheimer’s disease: a perspective of network hyperexcitability in disease progression. J. Alzheimers Dis. 88, 809–836 (2022).
Targa Dias Anastacio, H., Matosin, N. & Ooi, L. Neuronal hyperexcitability in Alzheimer’s disease: what are the drivers behind this aberrant phenotype? Transl. Psychiatry 12, 257 (2022).
Kurucu, H. et al. Inhibitory synapse loss and accumulation of amyloid beta in inhibitory presynaptic terminals in Alzheimer’s disease. Eur. J. Neurol. 29, 1311–1323 (2022).
Lorenc, F., Dupuis, L. & Cassel, R. Impairments of inhibitory neurons in amyotrophic lateral sclerosis and frontotemporal dementia. Neurobiol. Dis. 203, 106748 (2024).
Smeralda, C. L. et al. The role of parvalbumin interneuron dysfunction across neurodegenerative dementias. Ageing Res. Rev. 101, 102509 (2024).
Wesenhagen, K. E. J. et al. Synaptic protein CSF levels relate to memory scores in individuals without dementia. Alzheimers Res. Ther. 17, 56 (2025).
Scaduto, P. et al. Functional excitatory to inhibitory synaptic imbalance and loss of cognitive performance in people with Alzheimer’s disease neuropathologic change. Acta Neuropathol. 145, 303–324 (2023).
Kumar, P. et al. Native-state proteomics of parvalbumin interneurons identifies unique molecular signatures and vulnerabilities to early Alzheimer’s pathology. Nat. Commun. 15, 2823 (2024).
Pascual-Leone, A. & Bartres-Faz, D. Human brain resilience: a call to action. Ann. Neurol. 90, 336–349 (2021).
Neuner, S. M. et al. Translational approaches to understanding resilience to Alzheimer’s disease. Trends Neurosci. 45, 369–383 (2022).
Boyle, R. et al. Identifying longitudinal cognitive resilience from cross-sectional amyloid, tau, and neurodegeneration. Alzheimers Res. Ther. 16, 148 (2024).
Latimer, C. S. et al. Resistance and resilience to Alzheimer’s disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort. Acta Neuropathol. Commun. 7, 91 (2019).
Gómez-Isla, T. & Frosch, M. P. Lesions without symptoms: understanding resilience to Alzheimer disease neuropathological changes. Nat. Rev. Neurol. 18, 323–332 (2022).
Ahangari, N., Fischer, C. E., Schweizer, T. A. & Munoz, D. G. Cognitive resilience and severe Alzheimer’s disease neuropathology. Aging Brain 3, 100065 (2023).
de Vries, L. E., Huitinga, I., Kessels, H. W., Swaab, D. F. & Verhaagen, J. The concept of resilience to Alzheimer’s disease: current definitions and cellular and molecular mechanisms. Mol. Neurodegener. 19, 33 (2024).
Nascimento, C. et al. Prevalence of transactive response DNA-binding protein 43 (TDP-43) proteinopathy in cognitively normal older adults: systematic review and meta-analysis. Neuropathol. Appl. Neurobiol. 44, 286–297 (2018).
Parkkinen, L., Soininen, H. & Alafuzoff, I. Regional distribution of α-synuclein pathology in unimpaired aging and Alzheimer disease. J. Neuropathol. Exp. Neurol. 62, 363–367 (2003).
Melikyan, Z. A. et al. Cognitive resilience to three dementia-related neuropathologies in an oldest-old man: a case report from the 90+ study. Neurobiol. Aging 116, 12–15 (2022).
Zhang, J. et al. Uncovering the system vulnerability and criticality of human brain under dynamical neuropathological events in Alzheimer’s disease. J. Alzheimers Dis. 95, 1201–1219 (2023).
Siebert, M., Carello-Collar, G., Bastiani, M. A. D. & Zimmer, E. R. Transcriptomic similarities in vulnerable and resilient brain regions of Parkinson’s disease and Alzheimer’s disease. Alzheimers Dement. 19, e080097 (2023).
Anand, C., Torok, J., Abdelnour, F., Maia, P. D. & Raj, A. Selective vulnerability and resilience to Alzheimer’s disease tauopathy as a function of genes and the connectome. Preprint at bioRxiv https://doi.org/10.1101/2024.03.04.583403 (2024).
Serrano, G. E. et al. Correlation of presynaptic and postsynaptic proteins with pathology in Alzheimer’s disease. Int. J. Mol. Sci. 25, 3130 (2024).
Askenazi, M. et al. Compilation of reported protein changes in the brain in Alzheimer’s disease. Nat. Commun. 14, 4466 (2023).
Huang, Z. et al. Brain proteomic analysis implicates actin filament processes and injury response in resilience to Alzheimer’s disease. Nat. Commun. 14, 2747 (2023).
Camporesi, E. et al. Neuroligin-1 in brain and CSF of neurodegenerative disorders: investigation for synaptic biomarkers. Acta Neuropathol. Commun. 9, 19 (2021).
Mrdjen, D. et al. The basis of cellular and regional vulnerability in Alzheimer’s disease. Acta Neuropathol. 138, 729–749 (2019).
Gabitto, M. I. et al. Integrated multimodal cell atlas of Alzheimer’s disease. Nat. Neurosci. 27, 2366–2383 (2024).
Pham, A. Q. & Dore, K. Novel approaches to increase synaptic resilience as potential treatments for Alzheimer’s disease. Semin. Cell Dev. Biol. 139, 84–92 (2023).
Castanho, I. et al. Molecular hallmarks of excitatory and inhibitory neuronal resilience and resistance to Alzheimer’s disease. Preprint at bioRxiv https://doi.org/10.1101/2025.01.13.632801 (2025).
Higginbotham, L. et al. Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer’s disease. Sci. Adv. 6, eaaz9360 (2020).
[No authors listed] 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 20, 3708–3821 (2024).
Tzioras, M., McGeachan, R. I., Durrant, C. S. & Spires-Jones, T. L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 19, 19–38 (2023).
Kang, X. et al. Convergent neuroimaging and molecular signatures in mild cognitive impairment and Alzheimer’s disease: a data-driven meta-analysis with N = 3,118. Neurosci. Bull. 40, 1274–1286 (2024).
Lleó, A. et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s disease cerebrospinal fluid. Mol. Cell. Proteom. 18, 546–560 (2019).
de Wilde, M. C., Overk, C. R., Sijben, J. W. & Masliah, E. Meta-analysis of synaptic pathology in Alzheimer’s disease reveals selective molecular vesicular machinery vulnerability. Alzheimers Dement. 12, 633–644 (2016).
Mathys, H. et al. Single-cell multiregion dissection of Alzheimer’s disease. Nature 632, 858–868 (2024).
John, A. & Reddy, P. H. Synaptic basis of Alzheimer’s disease: focus on synaptic amyloid beta, p-tau and mitochondria. Ageing Res. Rev. 65, 101208 (2021).
Blennow, K., Bogdanovic, N., Alafuzoff, I., Ekman, R. & Davidsson, P. Synaptic pathology in Alzheimer’s disease: relation to severity of dementia, but not to senile plaques, neurofibrillary tangles, or the APOE4 allele. J. Neural Transm. 103, 603–618 (1996).
Domínguez-Álvaro, M. et al. Three-dimensional analysis of synapses in the transentorhinal cortex of Alzheimer’s disease patients. Acta Neuropathol. Commun. 6, 20 (2018).
Scheff, S. W., Price, D. A., Schmitt, F. A. & Mufson, E. J. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 27, 1372–1384 (2006).
Mecca, A. P. et al. Synaptic density and cognitive performance in Alzheimer’s disease: A PET imaging study with [11C]UCB-J. Alzheimers Dement. 18, 2527–2536 (2022).
Vanderlinden, G. et al. Longitudinal synaptic loss versus tau Braak staging in amnestic mild cognitive impairment. Alzheimers Dement. 21, e14412 (2025).
Kumar, A., Scarpa, M. & Nordberg, A. Tracing synaptic loss in Alzheimer’s brain with SV2A PET-tracer UCB-J. Alzheimers Dement. 20, 2589–2605 (2024).
Holland, N. et al. Synaptic loss in primary tauopathies revealed by [11C]UCB-J positron emission tomography. Mov. Disord. 35, 1834–1842 (2020).
Papanikolaou, A. et al. Selectively vulnerable deep cortical layer 5/6 fast-spiking interneurons in Alzheimer’s disease models in vivo. Neuron https://doi.org/10.1016/j.neuron.2025.04.010 (2025).
Escamilla, S. et al. Synaptic vs extrasynaptic NMDA receptors distribution in Alzheimer’s human brain. Alzheimers Dement. 19, e076281 (2023).
Higginbotham, L. et al. Unbiased classification of the elderly human brain proteome resolves distinct clinical and pathophysiological subtypes of cognitive impairment. Neurobiol. Dis. 186, 106286 (2023).
Pelucchi, S., Stringhi, R. & Marcello, E. Dendritic spines in Alzheimer’s disease: how the actin cytoskeleton contributes to synaptic failure. Int. J. Mol. Sci. 21, 908 (2020).
Dorostkar, M. M., Zou, C., Blazquez-Llorca, L. & Herms, J. Analyzing dendritic spine pathology in Alzheimer’s disease: problems and opportunities. Acta Neuropathol. 130, 1–19 (2015).
Counts, S. E. et al. Hippocampal drebrin loss in mild cognitive impairment. Neurodegenerative Dis. 10, 216–219 (2012).
Landry, O. et al. Postsynaptic protein Shank3a deficiency synergizes with Alzheimer’s disease neuropathology to impair cognitive performance in the 3xTg-AD murine model. J. Neurosci. 43, 4941–4954 (2023).
Pchitskaya, E. & Bezprozvanny, I. Dendritic spines shape analysis – classification or clusterization? Perspective. Front. Synaptic Neurosci. 12, 31 (2020).
Boros, B. D. et al. Dendritic spines provide cognitive resilience against Alzheimer’s disease. Ann. Neurol. 82, 602–614 (2017).
Walker, C. K. & Herskowitz, J. H. Dendritic spines: mediators of cognitive resilience in aging and Alzheimer’s disease. Neuroscientist 27, 487–505 (2021).
Dalal, S., Ramirez-Gomez, J., Sharma, B., Devara, D. & Kumar, S. MicroRNAs and synapse turnover in Alzheimer’s disease. Ageing Res. Rev. 99, 102377 (2024).
Zhang, W., Xiao, D., Mao, Q. & Xia, H. Role of neuroinflammation in neurodegeneration development. Signal. Transduct. Target. Ther. 8, 267 (2023).
Taddei, R. N. et al. Tau oligomer-containing synapse elimination by microglia and astrocytes in Alzheimer disease. JAMA Neurol. 80, 1209–1221 (2023).
Tzioras, M. et al. Human astrocytes and microglia show augmented ingestion of synapses in Alzheimer’s disease via MFG-E8. Cell Rep. Med. 4, 101175 (2023).
Shen, F.-S. et al. Emerging evidence of context-dependent synapse elimination by phagocytes in the CNS. J. Leukoc. Biol. 116, 511–522 (2024).
Dejanovic, B. et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2, 837–850 (2022).
De Schepper, S. et al. Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer’s disease. Nat. Neurosci. 26, 406–415 (2023).
Guo, Y. et al. Multiplex cerebrospinal fluid proteomics identifies biomarkers for diagnosis and prediction of Alzheimer’s disease. Nat. Hum. Behav. 8, 2047–2066 (2024).
Hurst, C. et al. Integrated proteomics to understand the role of neuritin (NRN1) as a mediator of cognitive resilience to Alzheimer’s disease. Mol. Cell. Proteom. 22, 100542 (2023).
Hesse, R. et al. Comparative profiling of the synaptic proteome from Alzheimer’s disease patients with focus on the APOE genotype. Acta Neuropathol. Commun. 7, 214 (2019).
Paasila, P. J., Aramideh, J. A., Sutherland, G. T. & Graeber, M. B. Synapses, microglia, and lipids in Alzheimer’s disease. Front. Neurosci. 15, 778822 (2022).
Fu, W.-Y. & Ip, N. Y. The role of genetic risk factors of Alzheimer’s disease in synaptic dysfunction. Semin. Cell Dev. Biol. 139, 3–12 (2023).
Zhao, J. et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat. Commun. 11, 5540 (2020).
Williams, J. B., Cao, Q. & Yan, Z. Transcriptomic analysis of human brains with Alzheimer’s disease reveals the altered expression of synaptic genes linked to cognitive deficits. Brain Commun. 3, fcab123 (2021).
Milà-Alomà, M. et al. CSF synaptic biomarkers in the preclinical stage of Alzheimer disease and their association with MRI and PET. Neurology 97, e2065 (2021).
Oeckl, P. et al. Higher plasma β-synuclein indicates early synaptic degeneration in Alzheimer’s disease. Alzheimers Dement. 19, 5095–5102 (2023).
van der Ende, E. L. et al. CSF proteomics in autosomal dominant Alzheimer’s disease highlights parallels with sporadic disease. Brain 146, 4495–4507 (2023).
Johnson, E. C. B. et al. Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 26, 769–780 (2020).
Zhang, H., Lyu, D. & Jia, J. The trajectory of cerebrospinal fluid growth-associated protein 43 in the Alzheimer’s disease continuum: a longitudinal study. J. Alzheimers Dis. 85, 1441–1452 (2022).
Utz, J. et al. Cerebrospinal fluid of patients with Alzheimer’s disease contains increased percentages of synaptophysin-bearing microvesicles. Front. Aging Neurosci. 13, 682115 (2021).
Haytural, H. et al. Insights into the changes in the proteome of Alzheimer disease elucidated by a meta-analysis. Sci. Data 8, 312 (2021).
Vrillon, A. et al. Plasma neuregulin 1 as a synaptic biomarker in Alzheimer’s disease: a discovery cohort study. Alzheimers Res. Ther. 14, 71 (2022).
Galasko, D. et al. Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer’s disease. Alzheimers Dement. 5, 871–882 (2019).
Libiger, O. et al. Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer’s disease. Alzheimers Dement. 17, 1976–1987 (2021).
Steffen, Halbgebauer. et al. CSF levels of SNAP-25 are increased early in Creutzfeldt-Jakob and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 93, 1059–1065 (2022).
Dulewicz, M., Kulczyńska-Przybik, A. & Mroczko, B. Neurogranin and VILIP-1 as molecular indicators of neurodegeneration in Alzheimer’s disease: a systematic review and meta-analysis. Int. J. Mol. Sci. 21, 8335 (2020).
Halbgebauer, S. et al. Visinin-like protein 1 levels in blood and CSF as emerging markers for Alzheimer’s and other neurodegenerative diseases. Alzheimers Res. Ther. 14, 175 (2022).
Das, S. et al. The use of synaptic biomarkers in cerebrospinal fluid to differentiate behavioral variant of frontotemporal dementia from primary psychiatric disorders and Alzheimer’s disease. Alzheimers Res. Ther. 16, 34 (2024).
Das, S. et al. Synaptic biomarkers in the cerebrospinal fluid associate differentially with classical neuronal biomarkers in patients with Alzheimer’s disease and frontotemporal dementia. Alzheimers Res. Ther. 15, 62 (2023).
Saunders, T. S. et al. Associations between cerebrospinal fluid markers and cognition in ageing and dementia: a systematic review. Eur. J. Neurosci. 56, 5650–5713 (2022).
Kivisäkk, P. et al. Increased levels of the synaptic proteins PSD-95, SNAP-25, and neurogranin in the cerebrospinal fluid of patients with Alzheimer’s disease. Alzheimers Res. Ther. 14, 58 (2022).
Lista, S. et al. Monitoring synaptic pathology in Alzheimer’s disease through fluid and PET imaging biomarkers: a comprehensive review and future perspectives. Mol. Psychiatry 29, 847–857 (2024).
Thal, D. R., Rüb, U., Orantes, M. & Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800 (2002).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Willumsen, N. et al. Variability in the type and layer distribution of cortical Aβ pathology in familial Alzheimer’s disease. Brain Pathol. 32, e13009 (2022).
Carroll, T., Guha, S., Nehrke, K. & Johnson, G. V. W. Tau post-translational modifications: potentiators of selective vulnerability in sporadic Alzheimer’s disease. Biology 10, 1047 (2021).
DeTure, M. A. & Dickson, D. W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 14, 32 (2019).
Spires-Jones, T. L., Kopeikina, K. J., Koffie, R. M., de Calignon, A. & Hyman, B. T. Are tangles as toxic as they look? J. Mol. Neurosci. 45, 438–444 (2011).
Goure, W. F., Krafft, G. A., Jerecic, J. & Hefti, F. Targeting the proper amyloid-beta neuronal toxins: a path forward for Alzheimer’s disease immunotherapeutics. Alzheimers Res. Ther. 6, 42 (2014).
Robinson, J. L. et al. Pathological combinations in neurodegenerative disease are heterogeneous and disease-associated. Brain 146, 2557–2569 (2023).
Nelson, R. S. et al. Neurodegenerative pathologies associated with behavioral and psychological symptoms of dementia in a community-based autopsy cohort. Acta Neuropathol. Commun. 11, 89 (2023).
Colom-Cadena, M. et al. Synaptic oligomeric tau in Alzheimer’s disease – a potential culprit in the spread of tau pathology through the brain. Neuron 111, 2170–2183.e6 (2023).
Spires-Jones, T. L. & Hyman, B. T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 82, 756–771 (2014).
Gkanatsiou, E. et al. Amyloid pathology and synaptic loss in pathological aging. J. Neurochem. 159, 258–272 (2021).
O’Dell, R. S. et al. Association of Aβ deposition and regional synaptic density in early Alzheimer’s disease: a PET imaging study with [11C]UCB-J. Alzheimers Res. Ther. 13, 11 (2021).
Puliatti, G. et al. Intracellular accumulation of tau oligomers in astrocytes and their synaptotoxic action rely on amyloid precursor protein intracellular domain-dependent expression of glypican-4. Prog. Neurobiol. 227, 102482 (2023).
Kadamangudi, S. et al. Amyloid-β oligomers increase the binding and internalization of tau oligomers in human synapses. Acta Neuropathol. 149, 2 (2024).
Sengupta, U. & Kayed, R. Amyloid β, tau, and α-synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 214, 102270 (2022).
English, L. A., Boeken, D., Cheetham, M. R., Danial, J. S. & Klenerman, D. Brains in focus: uncovering protein aggregate changes across Alzheimer’s disease with single-molecule imaging. Alzheimers Dement. 19, e078222 (2023).
Almeida, Z. L., Vaz, D. C. & Brito, R. M. M. Morphological and molecular profiling of amyloid-β species in Alzheimer’s pathogenesis. Mol. Neurobiol. 62, 4391–4419 (2025).
Pereira, J. B. et al. Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer’s disease. Brain 144, 310–324 (2021).
Walker, J. M. et al. Spatial proteomics of hippocampal subfield-specific pathology in Alzheimer’s disease and primary age-related tauopathy. Alzheimers Dement. 20, 783–797 (2024).
Lo Cascio, F. et al. Brain-derived tau oligomer polymorphs: distinct aggregations, stability profiles, and biological activities. Commun. Biol. 8, 53 (2025).
Dunot, J., Ribera, A., Pousinha, P. A. & Marie, H. Spatiotemporal insights of APP function. Curr. Opin. Neurobiol. 82, 102754 (2023).
Bold, C. S. et al. APPsα rescues tau-induced synaptic pathology. J. Neurosci. 42, 5782 (2022).
Hur, J.-Y. γ-Secretase in Alzheimer’s disease. Exp. Mol. Med. 54, 433–446 (2022).
Sirisi, S., Sánchez-Aced, É., Belbin, O. & Lleó, A. APP dyshomeostasis in the pathogenesis of Alzheimer’s disease: implications for current drug targets. Alzheimers Res. Ther. 16, 144 (2024).
Musardo, S. et al. The development of ADAM10 endocytosis inhibitors for the treatment of Alzheimer’s disease. Mol. Ther. 30, 2474–2490 (2022).
Jones, A. A. et al. A multilayer network analysis of Alzheimer’s disease pathogenesis: roles for p-tau, synaptic peptides, and physical activity. Alzheimers Dement. 20, 8012–8027 (2024).
Colom-Cadena, M. et al. Transmembrane protein 97 is a potential synaptic amyloid beta receptor in human Alzheimer’s disease. Acta Neuropathol. 147, 32 (2024).
Scaduto, P., Marcatti, M., Bhatt, N., Kayed, R. & Taglialatela, G. Calcineurin inhibition prevents synaptic plasticity deficit induced by brain-derived tau oligomers. Brain Commun. 6, fcae277 (2024).
Morgan, G. R. & Carlyle, B. C. Interrogation of the human cortical peptidome uncovers cell-type specific signatures of cognitive resilience against Alzheimer’s disease. Sci. Rep. 14, 7161 (2024).
Barroeta-Espar, I. et al. Distinct cytokine profiles in human brains resilient to Alzheimer’s pathology. Neurobiol. Dis. 121, 327–337 (2019).
Johnson, E. C. B. et al. Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nat. Neurosci. 25, 213–225 (2022).
Yu, L. et al. Cortical proteins associated with cognitive resilience in community-dwelling older persons. JAMA Psychiat. 77, 1172–1180 (2020).
Ramos-Miguel, A. et al. Proteomic identification of select protein variants of the SNARE interactome associated with cognitive reserve in a large community sample. Acta Neuropathol. 141, 755–770 (2021).
Mathys, H. et al. Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer’s disease pathology. Cell 186, 4365–4385.e27 (2023).
O’Neill, N. et al. Cognitive resilience to Alzheimer’s disease characterized by cell-type abundance. Alzheimers Dement. 20, 6910–6921 (2024).
Guptarak, J. et al. Cognitive integrity in non-demented individuals with Alzheimer’s neuropathology is associated with preservation and remodeling of dendritic spines. Alzheimers Dement. 20, 4677–4691 (2024).
Aguado, C. et al. Resilience to structural and molecular changes in excitatory synapses in the hippocampus contributes to cognitive function recovery in Tg2576 mice. Neural Regen. Res. 19, 2068–2074 (2024).
Perez-Nievas, B. G. et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain 136, 2510–2526 (2013).
Taddei, R. N. et al. Changes in glial cell phenotypes precede overt neurofibrillary tangle formation, correlate with markers of cortical cell damage, and predict cognitive status of individuals at Braak III-IV stages. Acta Neuropathol. Commun. 10, 72 (2022).
Zolochevska, O. & Taglialatela, G. Selected microRNAs increase synaptic resilience to the damaging binding of the Alzheimer’s disease amyloid beta oligomers. Mol. Neurobiol. 57, 2232–2243 (2020).
Singh, A. et al. Functional integrity of synapses in the central nervous system of cognitively intact individuals with high Alzheimer’s disease neuropathology is associated with absence of synaptic tau oligomers. J. Alzheimers Dis. 78, 1661–1678 (2020).
Gutierrez, D. A. et al. c-Abl deficiency provides synaptic resiliency against Aβ-oligomers. Front. Cell. Neurosci. 13, 526 (2019).
King, D. et al. Synaptic resilience is associated with maintained cognition during ageing. Alzheimers Dement. 19, 2560–2574 (2023).
Arboleda-Velasquez, J. F. et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat. Med. 25, 1680–1683 (2019).
Lopera, F. et al. Resilience to autosomal dominant Alzheimer’s disease in a Reelin-COLBOS heterozygous man. Nat. Med. 29, 1243–1252 (2023).
Villalba-Moreno, N. D. et al. Differential spatial transcriptome profile of the hippocampal formation of protected PSEN1 E280A familial Alzheimer’s disease cases. Alzheimers Dement. 19, e083204 (2023).
Kauwe, G. et al. KIBRA repairs synaptic plasticity and promotes resilience to tauopathy-related memory loss. J. Clin. Invest. 134, e169064 (2024).
Bhattarai, P. et al. Rare genetic variation in fibronectin 1 (FN1) protects against APOEε4 in Alzheimer’s disease. Acta Neuropathol. 147, 70 (2024).
Seto, M., Weiner, R. L., Dumitrescu, L. & Hohman, T. J. Protective genes and pathways in Alzheimer’s disease: moving towards precision interventions. Mol. Neurodegener. 16, 29 (2021).
Camporesi, E. et al. Quantification of the trans-synaptic partners neurexin-neuroligin in CSF of neurodegenerative diseases by parallel reaction monitoring mass spectrometry. eBioMedicine 75, 103793 (2022).
Krafft, G. A., Jerecic, J., Siemers, E. & Cline, E. N. ACU193: an Immunotherapeutic poised to test the amyloid β oligomer hypothesis of Alzheimer’s disease. Front. Neurosci. 16, 848215 (2022).
van Dyck, C. H. et al. A pilot study to evaluate the effect of CT1812 treatment on synaptic density and other biomarkers in Alzheimer’s disease. Alzheimers Res. Ther. 16, 20 (2024).
Kiss, E. et al. Artesunate restores the levels of inhibitory synapse proteins and reduces amyloid-β and C-terminal fragments (CTFs) of the amyloid precursor protein in an AD-mouse model. Mol. Cell. Neurosci. 113, 103624 (2021).
Peng, L., Bestard-Lorigados, I. & Song, W. The synapse as a treatment avenue for Alzheimer’s disease. Mol. Psychiatry 27, 2940–2949 (2022).
Piscopo, P. et al. A systematic review on drugs for synaptic plasticity in the treatment of dementia. Ageing Res. Rev. 81, 101726 (2022).
Cummings, J. et al. Alzheimer’s disease drug development pipeline: 2024. Alzheimers Dement. 10, e12465 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06427668 (2024).
Fei, M. et al. Characteristics of initial symptoms in patients with dementia with Lewy body disease. Front. Neurol. 13, 1024995 (2022).
Armstrong, M. J. & Barnes, L. L. Under-diagnosis of dementia with Lewy bodies in individuals racialized as black: hypotheses regarding potential contributors. J. Alzheimers Dis. 97, 1571–1580 (2024).
Alzheimer’s Society. Dementia with Lewy bodies (DLB): what is it and what causes it? http://www.alzheimers.org.uk/about-dementia/types-dementia/dementia-with-lewy-bodies (2021).
Orme, T., Guerreiro, R. & Bras, J. The genetics of dementia with Lewy bodies: current understanding and future directions. Curr. Neurol. Neurosci. Rep. 18, 67 (2018).
Perovnik, M. et al. Metabolic brain pattern in dementia with Lewy bodies: relationship to Alzheimer’s disease topography. Neuromage Clin. 35, 103080 (2022).
Gomperts, S. N. Lewy body dementias: dementia with Lewy bodies and Parkinson disease dementia. Continuum 22, 435–463 (2016).
Macoir, J. The cognitive and language profile of dementia with Lewy bodies. Am. J. Alzheimers Dis. Other Dement. 37, 15333175221106901 (2022).
Caminiti, S. P. et al. Brain glucose metabolism in Lewy body dementia: implications for diagnostic criteria. Alzheimers Res. Ther. 11, 20 (2019).
Nicastro, N. et al. 11C-UCB-J synaptic PET and multimodal imaging in dementia with Lewy bodies. Eur. J. Hybrid. Imaging 4, 25 (2020).
Menšíková, K. et al. Lewy body disease or diseases with Lewy bodies? NPJ Parkinsons Dis. 8, 3 (2022).
Shantaraman, A. et al. Network proteomics of the Lewy body dementia brain reveals presynaptic signatures distinct from Alzheimer’s disease. Mol. Neurodegener. 19, 60 (2024).
Canal-Garcia, A. et al. Proteomic signatures of Alzheimer’s disease and Lewy body dementias: a comparative analysis. Alzheimers Dement. 21, e14375 (2024).
Andersen, K. B. et al. Reduced synaptic density in patients with Lewy body dementia: an [11C]UCB-J PET imaging study. Mov. Disord. 36, 2057–2065 (2021).
Vanderlinden, G., Carron, C., Vandenberghe, R., Vandenbulcke, M. & Van Laere, K. In vivo PET of synaptic density as potential diagnostic marker for cognitive disorders: prospective comparison with current imaging markers for neuronal dysfunction and relation to symptomatology – study protocol. BMC Med. Imaging 24, 41 (2024).
Pérez-Acuña, D., Shin, S. J., Rhee, K. H., Kim, S. J. & Lee, S.-J. α-Synuclein propagation leads to synaptic abnormalities in the cortex through microglial synapse phagocytosis. Mol. Brain 16, 72 (2023).
Gao, C., Jiang, J., Tan, Y. & Chen, S. Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal. Transduct. Target. Ther. 8, 359 (2023).
Shen, J., Bian, N., Zhao, L. & Wei, J. The role of T-lymphocytes in central nervous system diseases. Brain Res. Bull. 209, 110904 (2024).
Gate, D. et al. CD4+ T cells contribute to neurodegeneration in Lewy body dementia. Science 374, 868–874 (2021).
van Steenoven, I. et al. Identification of novel cerebrospinal fluid biomarker candidates for dementia with Lewy bodies: a proteomic approach. Mol. Neurodegener. 15, 36 (2020).
Xiang, Y., Xin, J., Le, W. & Yang, Y. Neurogranin: a potential biomarker of neurological and mental diseases. Front. Aging Neurosci. 12, 584743 (2020).
Willemse, E. A. J. et al. Neurogranin as biomarker in CSF is non-specific to Alzheimer’s disease dementia. Neurobiol. Aging 108, 99–109 (2021).
Nilsson, J. et al. Cerebrospinal fluid biomarker panel of synaptic dysfunction in Alzheimer’s disease and other neurodegenerative disorders. Alzheimers Dement. 19, 1775–1784 (2023).
Barba, L. et al. CSF synaptic biomarkers in AT(N)-based subgroups of Lewy body disease. Neurology 101, e50–e62 (2023).
Mohaupt, P. et al. β-Synuclein as a candidate blood biomarker for synaptic degeneration in Alzheimer’s disease. Alzheimers Res. Ther. 14, 179 (2022).
Bousiges, O. & Blanc, F. Biomarkers of dementia with Lewy bodies: differential diagnostic with Alzheimer’s disease. Int. J. Mol. Sci. 23, 6371 (2022).
Kannarkat, G. et al. Alpha-synuclein strains in plasma distinguish Parkinson’s disease from dementia with Lewy bodies (S26.006). Neurology 102, 6274 (2024).
Bartl, M. et al. Lysosomal and synaptic dysfunction markers in longitudinal cerebrospinal fluid of de novo Parkinson’s disease. NPJ Parkinsons Dis. 10, 102 (2024).
Attems, J. et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathologica 141, 159–172 (2021).
Markesbery, W. R., Jicha, G. A., Liu, H. & Schmitt, F. A. Lewy body pathology in normal elderly subjects. J. Neuropathol. Exp. Neurol. 68, 816–822 (2009).
Borghammer, P. et al. Neuropathological evidence of body-first vs. brain-first Lewy body disease. Neurobiol. Dis. 161, 105557 (2021).
Geertsma, H. M. et al. A topographical atlas of α-synuclein dosage and cell type-specific expression in adult mouse brain and peripheral organs. NPJ Parkinsons Dis. 10, 65 (2024).
Goralski, T. M. et al. Spatial transcriptomics reveals molecular dysfunction associated with cortical Lewy pathology. Nat. Commun. 15, 2642 (2024).
Simon, C., Soga, T., Okano, H. J. & Parhar, I. α-Synuclein-mediated neurodegeneration in dementia with Lewy bodies: the pathobiology of a paradox. Cell Biosci. 11, 196 (2021).
de Boni, L. et al. Brain region-specific susceptibility of Lewy body pathology in synucleinopathies is governed by α-synuclein conformations. Acta Neuropathol. 143, 453–469 (2022).
Gao, V., Briano, J. A., Komer, L. E. & Burré, J. Functional and pathological effects of α-synuclein on synaptic SNARE complexes. J. Mol. Biol. 435, 167714 (2023).
Nordengen, K. & Morland, C. From synaptic physiology to synaptic pathology: the enigma of α-synuclein. Int. J. Mol. Sci. 25, 986 (2024).
Krawczuk, D., Groblewska, M., Mroczko, J., Winkel, I. & Mroczko, B. The role of α-synuclein in etiology of neurodegenerative diseases. Int. J. Mol. Sci. 25, 9197 (2024).
Roodveldt, C. et al. The immune system in Parkinson’s disease: what we know so far. Brain 147, 3306–3324 (2024).
Lilamand, M. et al. Cerebrospinal fluid alpha-synuclein improves the differentiation between dementia with Lewy bodies and Alzheimer’s disease in clinical practice. Int. J. Mol. Sci. 23, 13488 (2022).
Bousiges, O. et al. Differential diagnostic value of total alpha-synuclein assay in the cerebrospinal fluid between Alzheimer’s disease and dementia with Lewy bodies from the prodromal stage. Alzheimers Res. Ther. 12, 120 (2020).
Coughlin, D. G. et al. Association of CSF α-synuclein seeding amplification assay results with clinical features of possible and probable dementia with Lewy bodies. Neurology 103, e209656 (2024).
Ardah, M. T. et al. Inhibition of alpha-synuclein seeded fibril formation and toxicity by herbal medicinal extracts. BMC Complement. Med. Ther. 20, 73 (2020).
Hyman, B. T. et al. National Institute on Aging – Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 8, 1–13 (2012).
Hepp, D. H. et al. Distribution and load of amyloid-β pathology in Parkinson disease and dementia with Lewy bodies. J. Neuropathol. Exp. Neurol. 75, 936–945 (2016).
Toledo, J. B. et al. Dementia with Lewy bodies: impact of co-pathologies and implications for clinical trial design. Alzheimers Dement. 19, 318–332 (2023).
Gifford, A., Praschan, N., Newhouse, A. & Chemali, Z. Biomarkers in frontotemporal dementia: current landscape and future directions. Biomark. Neuropsychiatry 8, 100065 (2023).
Mayo Clinic. Frontotemporal dementia. http://www.mayoclinic.org/diseases-conditions/frontotemporal-dementia/symptoms-causes/syc-20354737 (2023).
Chung, D. C., Roemer, S., Petrucelli, L. & Dickson, D. W. Cellular and pathological heterogeneity of primary tauopathies. Mol. Neurodegener. 16, 57 (2021).
Mehta, P. R., Brown, A.-L., Ward, M. E. & Fratta, P. The era of cryptic exons: implications for ALS-FTD. Mol. Neurodegener. 18, 16 (2023).
Giannini, L. A. A. et al. Isoform-specific patterns of tau burden and neuronal degeneration in MAPT-associated frontotemporal lobar degeneration. Acta Neuropathol. 144, 1065–1084 (2022).
Restrepo-Martínez, M. et al. Defining repetitive behaviours in frontotemporal dementia. Brain 147, 1149–1165 (2024).
Mirbod, M. et al. FDG-PET in the diagnosis of primary progressive aphasia: a systematic review. Ann. Nucl. Med. 38, 673–687 (2024).
Whiteside, D. J. et al. Synaptic density affects clinical severity via network dysfunction in syndromes associated with frontotemporal lobar degeneration. Nat. Commun. 14, 8458 (2023).
Dando, O. et al. Synaptic gene expression changes in frontotemporal dementia due to the MAPT 10+16 mutation. Neuropathol. Appl. Neurobiol. 50, e13006 (2024).
Nana, A. L. et al. Neurons selectively targeted in frontotemporal dementia reveal early stage TDP-43 pathobiology. Acta Neuropathol. 137, 27–46 (2019).
Ayala, I. et al. Loss and microglia phagocytosis of synaptic proteins in frontotemporal lobar degeneration with TDP-43 proteinopathy. Neurochem. Int. 175, 105719 (2024).
Italia, M. et al. Anti-GluA3 autoantibodies define a new sub-population of frontotemporal lobar degeneration patients with distinct neuropathological features. Brain, Behav., Immun. 118, 380–397 (2024).
Chu, M. et al. Peripheral inflammation in behavioural variant frontotemporal dementia: associations with central degeneration and clinical measures. J. Neuroinflammation 20, 65 (2023).
Spalloni, A. et al. Cerebrospinal fluid from frontotemporal dementia patients is toxic to neurons. Biochim. Biophysica Acta Mol. Basis Dis. 1867, 166122 (2021).
Laszlo, Z. I. et al. Synaptic proteomics reveal distinct molecular signatures of cognitive change and C9ORF72 repeat expansion in the human ALS cortex. Acta Neuropathol. Commun. 10, 156 (2022).
Bridel, C. et al. Clusters of co-abundant proteins in the brain cortex associated with fronto-temporal lobar degeneration. Alzheimers Res. Ther. 15, 59 (2023).
Cervantes González, A. et al. Multimarker synaptic protein cerebrospinal fluid panels reflect TDP-43 pathology and cognitive performance in a pathological cohort of frontotemporal lobar degeneration. Mol. Neurodegener. 17, 29 (2022).
Bauer, C. S. et al. An interaction between synapsin and C9orf72 regulates excitatory synapses and is impaired in ALS/FTD. Acta Neuropathol. 144, 437–464 (2022).
Camporesi, E. et al. Fluid biomarkers for synaptic dysfunction and loss. Biomark. Insights 15, 1177271920950319 (2020).
Lin, P.-Y. et al. Neurexin-2: an inhibitory neurexin that restricts excitatory synapse formation in the hippocampus. Sci. Adv. 9, eadd8856 (2023).
Clarke, M. T. M. et al. CSF synaptic protein concentrations are raised in those with atypical Alzheimer’s disease but not frontotemporal dementia. Alzheimers Res. Ther. 11, 105 (2019).
Bavato, F. et al. Introducing neurofilament light chain measure in psychiatry: current evidence, opportunities, and pitfalls. Mol. Psychiatry 29, 2543–2559 (2024).
Meneses, A. et al. TDP-43 pathology in Alzheimer’s disease. Mol. Neurodegener. 16, 84 (2021).
Keating, S. S., San Gil, R., Swanson, M. E. V., Scotter, E. L. & Walker, A. K. TDP-43 pathology: from noxious assembly to therapeutic removal. Prog. Neurobiol. 211, 102229 (2022).
Young, A. L. et al. Data-driven neuropathological staging and subtyping of TDP-43 proteinopathies. Brain 146, 2975–2988 (2023).
Kawakami, I., Arai, T. & Hasegawa, M. The basis of clinicopathological heterogeneity in TDP-43 proteinopathy. Acta Neuropathol. 138, 751–770 (2019).
Brettschneider, J. et al. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD). Acta Neuropathol. 127, 423–439 (2014).
Brettschneider, J. et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 74, 20–38 (2013).
Keszycki, R. et al. Propagation of TDP-43 proteinopathy in neurodegenerative disorders. Neural Regen. Res. 17, 1498–1500 (2022).
Versluys, L. et al. Expanding the TDP-43 proteinopathy pathway from neurons to muscle: physiological and pathophysiological functions. Front. Neurosci. 16, 815765 (2022).
Jo, M. et al. The role of TDP-43 propagation in neurodegenerative diseases: integrating insights from clinical and experimental studies. Exp. Mol. Med. 52, 1652–1662 (2020).
Yang, L., Jasiqi, Y. & Lashuel, H. Recombinant full-length TDP-43 oligomers retain their ability to bind RNAs, are not toxic, and do not seed TDP-43 aggregation in vitro. ACS Chem. Neurosci. 15, 193–204 (2024).
Forrest, S. L. et al. Coexisting Lewy body disease and clinical parkinsonism in frontotemporal lobar degeneration. Neurology 92, e2472–e2482 (2019).
Woodworth, D. C. et al. Comprehensive assessment of TDP-43 neuropathology data in the National Alzheimer’s Coordinating Center database. Acta Neuropathol. 147, 103 (2024).
Yuan, L. et al. Typical metabolic pattern of 18F-FDG PET in Anti-NMDAR encephalitis in the acute and subacute phases and its correlation with T2 FLAIR-MRI features. BMC Neurosci. 24, 51 (2023).
Kvam, K. A. et al. Outcome and sequelae of aueoimmune encephalitis. J. Clin. Neurol. 20, 3–22 (2024).
Gong, X. et al. Long-term functional outcomes and relapse of anti-NMDA receptor encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 8, e958 (2021).
Zhong, R., Chen, Q., Zhang, X., Zhang, H. & Lin, W. Risk factors for mortality in anti-NMDAR, anti-LGI1, and anti-GABABR encephalitis. Front. Immunol. 13, 845365 (2022).
Qiao, S. et al. Characteristics and prognosis of autoimmune encephalitis in the east of China: a multi-center study. Front. Neurol. 12, 642078 (2021).
Gibson, L. L., McKeever, A., Coutinho, E., Finke, C. & Pollak, T. A. Cognitive impact of neuronal antibodies: encephalitis and beyond. Transl. Psychiatry 10, 304 (2020).
Tirado-García, L.-A., Piña-Ballantyne, S.-A., Cienfuegos-Meza, J. & Tena-Suck, M.-L. Anti-N-methyl-D-aspartate receptor encephalitis with diffuse demyelinating plaques: a case report of an atypical presentation. Cureus 15, e41595 (2023).
Huang, Y. Q. & Xiong, H. Anti-NMDA receptor encephalitis: a review of mechanistic studies. Int. J. Physiol. Pathophysiol. Pharmacol. 13, 1–11 (2021).
Dalmau, J. et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 18, 1045–1057 (2019).
Shim, Y.-M. et al. An autopsy-proven case-based review of autoimmune encephalitis. Exp. Neurobiol. 33, 1–17 (2024).
Zhang, C. et al. Hypometabolism of the left middle/medial frontal lobe on FDG-PET in anti-NMDA receptor encephalitis: comparison with MRI and EEG findings. CNS Neurosci. Therapeutics 29, 1624–1635 (2023).
Wei, Y.-C. et al. Different FDG-PET metabolic patterns of anti-AMPAR and anti-NMDAR encephalitis: case report and literature review. Brain Behav. 10, e01540 (2020).
Ahmed, H. et al. Evaluation of (rac)-, (R)-, and (S)-18F-OF-NB1 for imaging GluN2B subunit-containing N-methyl-d-aspartate receptors in nonhuman primates. J. Nucl. Med. 63, 1912 (2022).
Ramirez-Bermudez, J., Restrepo-Martinez, M., Diaz-Victoria, A. R. & Espinola-Nadurille, M. Memantine as adjuntive therapy in a patient with anti-N-methyl-d-aspartate receptor encephalitis. J. Clin. Psychopharmacol. 40, 92–93 (2020).
Kortazar-Zubizarreta, I. et al. Sporadic Creutzfeldt–Jakob disease with extremely long 14-year survival period. Eur. J. Neurol. 28, 2901–2906 (2021).
Jurcau, M. C., Jurcau, A., Diaconu, R. G., Hogea, V. O. & Nunkoo, V. S. A systematic review of sporadic Creutzfeldt–Jakob disease: pathogenesis, diagnosis, and therapeutic attempts. Neurol. Int. 16, 1039–1065 (2024).
Sigurdson, C. J., Bartz, J. C. & Glatzel, M. Cellular and molecular mechanisms of prion disease. Annu. Rev. Pathol.: Mechanisms Dis. 14, 497–516 (2019).
Chu, M. et al. A longitudinal 18F-FDG PET/MRI study in asymptomatic stage of genetic Creutzfeldt–Jakob disease linked to G114V mutation. J. Neurol. 269, 6094–6103 (2022).
Foliaki, S. T. et al. Limbic system synaptic dysfunctions associated with prion disease onset. Acta Neuropathol. Commun. 12, 192 (2024).
Matsuo, K. et al. An autopsy case of MV2K-type sporadic Creutzfeldt–Jakob disease presenting with characteristic clinical, radiological, and neuropathological findings. Neuropathology 42, 245–253 (2022).
Jankovska, N. et al. Biomarkers analysis and clinical manifestations in comorbid Creutzfeldt–Jakob disease: a retrospective study in 215 autopsy cases. Biomedicines 10, 680 (2022).
Ferrer, I., Puig, B., Blanco, R. & Martí, E. Prion protein deposition and abnormal synaptic protein expression in the cerebellum in Creutzfeldt–Jakob disease. Neuroscience 97, 715–726 (2000).
Fyfe, I. Neurogranin in CSF identifies Creutzfeldt–Jakob disease. Nat. Rev. Neurol. 15, 434–435 (2019).
Antonell, A. et al. Synaptic, axonal damage and inflammatory cerebrospinal fluid biomarkers in neurodegenerative dementias. Alzheimers Dement. 16, 262–272 (2020).
Bentivenga, G. M. et al. Diagnostic and prognostic value of cerebrospinal fluid SNAP-25 and neurogranin in Creutzfeldt–Jakob disease in a clinical setting cohort of rapidly progressive dementias. Alzheimers Res. Ther. 15, 150 (2023).
Hermann, P. et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 20, 235–246 (2021).
Le, N. T. T., Wu, B. & Harris, D. A. Prion neurotoxicity. Brain Pathol. 29, 263–277 (2019).
Wang, Y. et al. Loss of homeostatic microglia signature in prion diseases. Cells 11, 2948 (2022).
de Vries, L. E., Bahnerth, A., Swaab, D. F., Verhaagen, J. & Carulli, D. Resilience to Alzheimer’s disease associates with alterations in perineuronal nets. Alzheimers Dement. 21, e14504 (2024).
Herden, J. M. et al. Comparative evaluation of clinical and cerebrospinal fluid biomarker characteristics in rapidly and non-rapidly progressive Alzheimer’s disease. Alzheimers Res. Ther. 15, 106 (2023).
Remnestål, J. et al. Altered levels of CSF proteins in patients with FTD, presymptomatic mutation carriers and non-carriers. Transl. Neurodegener. 9, 27 (2020).
Huber, N. et al. Deficient neurotransmitter systems and synaptic function in frontotemporal lobar degeneration – insights into disease mechanisms and current therapeutic approaches. Mol. Psychiatry 27, 1300–1309 (2022).
Fratiglioni, L., Marseglia, A. & Dekhtyar, S. Ageing without dementia: can stimulating psychosocial and lifestyle experiences make a difference? Lancet Neurol. 19, 533–543 (2020).
Kramer, M., Cutty, M., Knox, S., Alekseyenko, A. V. & Mollalo, A. Rural–urban disparities of Alzheimer’s disease and related dementias: a scoping review. Alzheimers Dement (N Y) 11, e70047 (2025).
Bocancea, D. I. et al. Measuring resilience and resistance in aging and Alzheimer disease using residual methods. Neurology 97, 474–488 (2021).
Sepulveda-Falla, D. Resistant and resilient mutations in protection against familial Alzheimer’s disease: learning from nature. Mol. Neurodegen. 18, 36 (2023).
Author information
Authors and Affiliations
Contributions
R.N.T. researched data for the article and wrote the article. Both authors made substantial contributions to discussion of the content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks M. di Luca, T. Spires-Jones and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
We searched PubMed and the Cochrane Library for studies published in English up to August 2024, using the search terms “Alzheimer disease”, “AD”, “dementia with Lewy bodies”, ”DLB”, ”frontotemporal dementia”, “FTD”, “cognitive decline”, “mild cognitive impairment”, “dementia severity”, “autoimmune encephalitis”, “prion disease”, “NMDA-R”, “CJD”, “synapse dysfunction”, “synapse loss”, “synapse biomarkers”, “plasma biomarkers”, “proteomic studies”, “resilience”, “resistance”, “vulnerability” and “locoregional”. Meta-analyses, systematic reviews and observational studies were selected from the results. In addition, we manually searched the references of selected meta-analyses, systematic reviews, observational studies, internet articles from international institutions and hospitals, and practice guidelines. Emphasis was given to meta-analyses and observational studies published in the past 5 years. The studies included focused on human-derived data obtained from biofluids and/or brain tissue samples unless otherwise indicated. The articles selected were agreed upon by the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taddei, R.N., Duff, K.E. Synapse vulnerability and resilience across the clinical spectrum of dementias. Nat Rev Neurol 21, 353–369 (2025). https://doi.org/10.1038/s41582-025-01094-7
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-025-01094-7
This article is cited by
-
SerpinA3N-APOE interaction in astrocytes exacerbates Alzheimer’s disease progression through NFκB activation
Journal of Neuroinflammation (2025)
-
Primary age-related tauopathy
Acta Neuropathologica (2025)