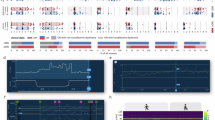
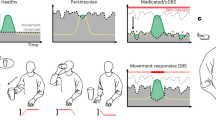
Abstract
Deep brain stimulation (DBS) substantially improves motor symptoms and quality of life in people with movement disorders such as Parkinson disease and dystonia, and it is also being explored as a treatment option for other brain disorders, including treatment-resistant obsessive–compulsive disorder, Alzheimer disease and depression. Two major developments are currently driving progress in DBS research: first, the framework of adaptive DBS, which senses brain activity to infer the momentary state of the symptoms of a patient and reacts by adapting stimulation settings, and second, the concept of connectomic DBS, which identifies brain circuits that should optimally be stimulated to reduce specific symptoms. In this Perspective, we propose a unified framework that combines these two concepts. Our approach, termed adaptive circuit targeting, decodes symptom severity from brain signals and adaptively activates the most relevant symptom-response circuits. We discuss the state of the art in the adaptive and connectomic DBS fields and the research gaps that need to be addressed to unify these concepts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Deuschl, G. et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 355, 896–908 (2006).
Kupsch, A. et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N. Engl. J. Med. 355, 1978–1990 (2006).
Nuttin, B., Cosyns, P., Demeulemeester, H., Gybels, J. & Meyerson, B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet 354, 1526 (1999).
Lozano, A. M. et al. A phase II study of fornix deep brain stimulation in mild Alzheimer’s disease. J. Alzheimers Dis. 54, 777–787 (2016).
Mayberg, H. S. et al. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 (2005).
Krauss, J. K. et al. Technology of deep brain stimulation: current status and future directions. Nat. Rev. Neurol. 17, 75–87 (2021).
Harmsen, I. E., Wolff Fernandes, F., Krauss, J. K. & Lozano, A. M. Where are we with deep brain stimulation? A review of scientific publications and ongoing research. Stereotact. Funct. Neurosurg. 100, 184–197 (2022).
Lozano, A. M. & Lipsman, N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 77, 406–424 (2013).
Neumann, W.-J., Horn, A. & Kühn, A. A. Insights and opportunities for deep brain stimulation as a brain circuit intervention. Trends Neurosci. 46, 472–487 (2023).
Vedam-Mai, V. et al. Proceedings of the Eighth Annual Deep Brain Stimulation Think Tank: advances in optogenetics, ethical issues affecting DBS research, neuromodulatory approaches for depression, adaptive neurostimulation, and emerging DBS technologies. Front. Hum. Neurosci. 15, 644593 (2021).
Little, S. et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 74, 449–457 (2013).
Arlotti, M. et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology 90, e971–e976 (2018).
Oehrn, C. R. et al. Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson’s disease: a blinded randomized feasibility trial. Nat. Med. 30, 3345–3356 (2024).
Neumann, W.-J., Gilron, R., Little, S. & Tinkhauser, G. Adaptive deep brain stimulation: from experimental evidence toward practical implementation. Mov. Disord. 38, 937–948 (2023).
Krook-Magnuson, E., Gelinas, J. N., Soltesz, I. & Buzsáki, G. Neuroelectronics and biooptics: closed-loop technologies in neurological disorders. JAMA Neurol. 72, 823–829 (2015).
Delgado, J. M., Johnston, V. S., Wallace, J. D. & Bradley, R. J. Operant conditioning of EEG in the unrestrained chimpanzee. Electroencephalogr. Clin. Neurophysiol. 27, 701–702 (1969).
Bechtereva, N. P., Bondartchuk, A. N., Smirnov, V. M., Meliutcheva, L. A. & Shandurina, A. N. Method of electrostimulation of the deep brain structures in treatment of some chronic diseases. Stereotact. Funct. Neurosurg. 37, 136–140 (1975).
Sem-Jacobsen, C. W. Depth-Electrographic Stimulation of the Human Brain and Behavior: From Fourteen Years of Studies and Treatment of Parkinson’s Disease and Mental Disorders with Implanted Electrodes (Thomas, 1968).
Heath, R. G. Physiological and biochemical studies in schizophrenia with particular emphasis on mind-brain relationships. Int. Rev. Neurobiol. 1, 299–331 (1959).
Blomstedt, P. & Hariz, M. I. Are complications less common in deep brain stimulation than in ablative procedures for movement disorders? Stereotact. Funct. Neurosurg. 84, 72–81 (2006).
Brice, J. & McLellan, L. Suppression of intention tremor by contingent deep-brain stimulation. Lancet 1, 1221–1222 (1980).
Rosin, B. et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72, 370–384 (2011).
He, S. et al. Beta-triggered adaptive deep brain stimulation during reaching movement in Parkinson’s disease. Brain 146, 5015–5030 (2023).
Piña-Fuentes, D. et al. Adaptive DBS in a Parkinson’s patient with chronically implanted DBS: a proof of principle. Mov. Disord. 32, 1253–1254 (2017).
Schmidt, S. L. et al. At home adaptive dual target deep brain stimulation in Parkinson’s disease with proportional control. Brain 147, 911–922 (2024).
Caffi, L. et al. Adaptive vs. conventional deep brain stimulation: one-year subthalamic recordings and clinical monitoring in a patient with Parkinson’s disease. Bioengineering 11, 990 (2024).
Busch, J. L. et al. Single threshold adaptive deep brain stimulation in Parkinson’s disease depends on parameter selection, movement state and controllability of subthalamic beta activity. Brain Stimul. 17, 125–133 (2024).
Neumann, W.-J. et al. Deep brain recordings using an implanted pulse generator in Parkinson’s disease. Neuromodulation 19, 20–24 (2016).
Bronte-Stewart, H. et al. Adaptive DBS algorithm for personalized therapy in Parkinson’s disease: ADAPT-PD clinical trial methodology and early data (P1-11.002). Neurology 100, 3204 (2023).
Johnson, V. et al. Embedded adaptive deep brain stimulation for cervical dystonia controlled by motor cortex theta oscillations. Exp. Neurol. 345, 113825 (2021).
Okun, M. S. et al. Responsive deep brain stimulation for the treatment of Tourette syndrome. Sci. Rep. 14, 6467 (2024).
Provenza, N. R. et al. Disruption of neural periodicity predicts clinical response after deep brain stimulation for obsessive-compulsive disorder. Nat. Med. 30, 3004–3014 (2024).
Lofredi, R. et al. Subthalamic beta bursts correlate with dopamine-dependent motor symptoms in 106 Parkinson’s patients. NPJ Parkinsons Dis. 9, 2 (2023).
Yin, Z. et al. Local field potentials in Parkinson’s disease: a frequency-based review. Neurobiol. Dis. 155, 105372 (2021).
Thenaisie, Y. et al. Principles of gait encoding in the subthalamic nucleus of people with Parkinson’s disease. Sci. Transl. Med. 14, eabo1800 (2022).
Yin, Z. et al. Cortical phase-amplitude coupling is key to the occurrence and treatment of freezing of gait. Brain 145, 2407–2421 (2022).
Yin, Z. et al. Pathological pallidal beta activity in Parkinson’s disease is sustained during sleep and associated with sleep disturbance. Nat. Commun. 14, 5434 (2023).
Gilron, R. et al. Sleep-aware adaptive deep brain stimulation control: chronic use at home with dual independent linear discriminate detectors. Front. Neurosci. 15, 732499 (2021).
Mizrahi-Kliger, A. D., Kaplan, A., Israel, Z., Deffains, M. & Bergman, H. Basal ganglia beta oscillations during sleep underlie Parkinsonian insomnia. Proc. Natl Acad. Sci. USA 117, 17359–17368 (2020).
Cole, S. R. et al. Nonsinusoidal beta oscillations reflect cortical pathophysiology in Parkinson’s disease. J. Neurosci. 37, 4830–4840 (2017).
Donoghue, T. et al. Evaluating and comparing measures of aperiodic neural activity. Preprint at bioRxiv https://doi.org/10.1101/2024.09.15.613114 (2024).
Gerster, M. et al. Beyond beta rhythms: aperiodic broadband power reflects Parkinson’s disease severity — a multicenter study. Preprint at bioRxiv https://doi.org/10.1101/2025.03.11.642600 (2025).
Neumann, W.-J. et al. Toward electrophysiology-based intelligent adaptive deep brain stimulation for movement disorders. Neurotherapeutics 16, 105–118 (2019).
Wolpaw, J. R. in Handbook of Clinical Neurology Vol. 110 (eds Barnes, M. P. & Good, D. C.) Ch. 6, 67–74 (Elsevier, 2013).
Miller, K. J., Hermes, D. & Staff, N. P. The current state of electrocorticography-based brain–computer interfaces. Neurosurg. Focus. 49, E2 (2020).
Herron, J. et al. The convergence of neuromodulation and brain–computer interfaces. Nat. Rev. Bioeng. 2, 628–630 (2024).
Köhler, R. M. et al. Dopamine and deep brain stimulation accelerate the neural dynamics of volitional action in Parkinson’s disease. Brain 147, 3358–3369 (2024).
Merk, T. et al. Electrocorticography is superior to subthalamic local field potentials for movement decoding in Parkinson’s disease. eLife 11, e75126 (2022).
Lauro, P. M. et al. Concurrent decoding of distinct neurophysiological fingerprints of tremor and bradykinesia in Parkinson’s disease. eLife 12, e84135 (2023).
Hirschmann, J., Schoffelen, J. M., Schnitzler, A. & Van Gerven, M. A. J. Parkinsonian rest tremor can be detected accurately based on neuronal oscillations recorded from the subthalamic nucleus. Clin. Neurophysiol. 128, 2029–2036 (2017).
Cagnan, H. et al. Stimulating at the right time: phase-specific deep brain stimulation. Brain 140, 132–145 (2017).
Cernera, S. et al. Wearable sensor-driven responsive deep brain stimulation for essential tremor. Brain Stimul. 14, 1434–1443 (2021).
Dixon, T. C. et al. Movement-responsive deep brain stimulation for Parkinson’s disease using a remotely optimized neural decoder. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-025-01438-0 (2025).
He, S. et al. Closed-loop deep brain stimulation for essential tremor based on thalamic local field potentials. Mov. Disord. 36, 863–873 (2021).
Merk, T. et al. Invasive neurophysiology and whole brain connectomics for neural decoding in patients with brain implants. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-025-01467-9 (in the press).
Opri, E. et al. Chronic embedded cortico-thalamic closed-loop deep brain stimulation for the treatment of essential tremor. Sci. Transl. Med. 12, eaay7680 (2020).
Rj, P. et al. Towards network-guided neuromodulation for epilepsy. Brain 145, 3347–3362 (2022).
Henderson, J. M. “Connectomic surgery”: diffusion tensor imaging (DTI) tractography as a targeting modality for surgical modulation of neural networks. Front. Integr. Neurosci. 6, 15 (2012).
Horn, A., Al-Fatly, B., Neumann, W.-J. & Neudorfer, C. in Connectomic Deep Brain Stimulation (ed. Horn, A.) Ch. 1, 3–23 (Academic, 2022).
Horn, A. & Fox, M. D. Opportunities of connectomic neuromodulation. Neuroimage 221, 117180 (2020).
Sporns, O., Tononi, G. & Kötter, R. The human connectome: a structural description of the human brain. PLoS Comp. Biol. 1, e42 (2005).
Cobb, M. The Idea of the Brain (Hachette UK, 2020).
Michaleas, S. N., Tsoucalas, G., Tzavellas, E., Stranjalis, G. & Karamanou, M. Gottlieb Burckhardt (1836-1907): 19th-century pioneer of psychosurgery. Surg. Innov. 28, 381–387 (2021).
Foerster, O. Zur analyse und pathophysiologie der striären bewegungsstörungen. Z. Gesamte Neurol. Psychiatr. 73, 1–169 (1921).
Vogt, C. & Vogt, O. Zur Lehre Der Erkrankungen Des Striären Systems (Barth, 1920).
Spiegel, E. A., Wycis, H. T., Marks, M. & Lee, A. J. Stereotaxic apparatus for operations on the human brain. Science 106, 349–350 (1947).
Spiegel, E. A. & Wycis, H. T. Ansotomy in paralysis agitans. AMA Arch. Neurol. Psychiatry 71, 598–614 (1954).
Hassler, R., Mundinger, F. & Riechert, T. Stereotaxis in Parkinson Syndrome (Springer, 1979).
Hassler, R., Riechert, T., Mundinger, F., Umbach, W. & Ganglberger, J. A. Physiological observations in stereotaxic operations in extrapyramidal motor disturbances. Brain 83, 337–350 (1960).
Hagmann, P. From Diffusion MRI to Brain Connectomics. PhD dissertation, Univ. Lausanne (2005).
Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267 (1994).
Mori, S., Crain, B. J., Chacko, V. P. & Van Zijl, P. C. M. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 45, 265–269 (1999).
Fox, M. D. M. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl Acad. Sci. USA 102, 9673–9678 (2005).
Biswal, B. B. et al. Toward discovery science of human brain function. Proc. Natl Acad. Sci. USA 107, 4734–4739 (2010).
Biswal, B., Zerrin Yetkin, F., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 (1995).
Butson, C. R., Cooper, S. E., Henderson, J. M. & McIntyre, C. C. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage 34, 661–670 (2007).
McIntyre, C. C. & Hahn, P. J. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol. Dis. 38, 329–337 (2010).
Bello, L., et al. Intraoperative use of diffusion tensor imaging fiber tractography and subcortical mapping for resection of gliomas: technical considerations. Neurosurg. Focus. 28, E6 (2010).
Leclercq, D. et al. Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations. J. Neurosurg. 112, 503–511 (2010).
Coenen, V. A., Mädler, B., Schiffbauer, H., Urbach, H. & Allert, N. Individual fiber anatomy of the subthalamic region revealed with diffusion tensor imaging: a concept to identify the deep brain stimulation target for tremor suppression. Neurosurgery 68, 1069–1075 (2011).
Coenen, V. A. et al. Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson’s disease. Neurosurgery 64, 1106–1114 (2009).
Choi, K. S., Riva-Posse, P., Gross, R. E. & Mayberg, H. S. Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 72, 1252–1260 (2015).
Riva-Posse, P. et al. Rapid antidepressant effects of deep brain stimulation and their relation to surgical protocol. Biol. Psychiatry 88, e37–e39 (2020).
Riva-Posse, P. et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol. Psychiatry 23, 843–849 (2018).
Horn, A. et al. Connectivity predicts deep brain stimulation outcome in Parkinson disease. Ann. Neurol. 82, 67–78 (2017).
Sobesky, L. et al. Subthalamic and pallidal deep brain stimulation: are we modulating the same network? Brain 145, 251–262 (2022).
Li, N. et al. A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nat. Commun. 11, 3364 (2020).
Baldermann, J. C. et al. Connectivity profile predictive of effective deep brain stimulation in obsessive-compulsive disorder. Biol. Psychiatry 85, 735–743 (2019).
Hollunder, B. et al. Mapping dysfunctional circuits in the frontal cortex using deep brain stimulation. Nat. Neurosci. 27, 573–586 (2024).
Smith, A. H. et al. Replicable effects of deep brain stimulation for obsessive-compulsive disorder. Brain Stimul. 14, 1–3 (2020).
Bouwens van der Vlis, T. A. M. et al. Ventral capsule/ventral striatum stimulation in obsessive-compulsive disorder: toward a unified connectomic target for deep brain stimulation? Neuromodulation 24, 316–323 (2020).
Mosley, P. E. et al. A randomised, double-blind, sham-controlled trial of deep brain stimulation of the bed nucleus of the stria terminalis for treatment-resistant obsessive-compulsive disorder. Transl. Psychiatry 11, 190 (2021).
Johnson, K. A. et al. Basal ganglia pathways associated with therapeutic pallidal deep brain stimulation for Tourette syndrome. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6, 961–972 (2021).
Gadot, R. et al. Tractography-based modeling explains treatment outcomes in patients undergoing deep brain stimulation for obsessive–compulsive disorder. Biol. Psychiatry 96, 95–100 (2024).
Li, N. et al. A unified functional network target for deep brain stimulation in obsessive-compulsive disorder. Biol. Psychiatry 90, 701–713 (2021).
Al-Fatly, B. et al. Connectivity profile of thalamic deep brain stimulation to effectively treat essential tremor. Brain 142, 3086–3098 (2019).
Neudorfer, C. et al. Personalizing deep brain stimulation using advanced imaging sequences. Ann. Neurol. 91, 613–628 (2022).
Horn, A. et al. Optimal deep brain stimulation sites and networks for cervical vs. generalized dystonia. Proc. Natl Acad. Sci. USA 119, e2114985119 (2022).
Schaper, F. L. W. V. J. et al. Mapping lesion-related epilepsy to a human brain network. JAMA Neurol. 80, 891–902 (2023).
Ganos, C. et al. A neural network for tics: insights from causal brain lesions and deep brain stimulation. Brain 145, 4385–4397 (2022).
Elias, G. J. B. et al. Local neuroanatomical and tract-based proxies of optimal subcallosal cingulate deep brain stimulation. Brain Stimul. 16, 1259–1272 (2023).
Elias, G. J. B. et al. Structuro-functional surrogates of response to subcallosal cingulate deep brain stimulation for depression. Brain 145, 362–377 (2022).
Ríos, A. S. et al. Optimal deep brain stimulation sites and networks for stimulation of the fornix in Alzheimer’s disease. Nat. Commun. 13, 7707 (2022).
Akram, H. et al. Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in Parkinson’s disease. Neuroimage 158, 332–345 (2017).
Rajamani, N. et al. Deep brain stimulation of symptom-specific networks in Parkinson’s disease. Nat. Commun. 15, 4662 (2024).
Neudorfer, C. et al. The role of the motor thalamus in deep brain stimulation for essential tremor. Neurotherapeutics 21, e00313 (2024).
Hollunder, B. et al. Toward personalized medicine in connectomic deep brain stimulation. Prog. Neurobiol. 210, 102211 (2022).
Yin, Z. et al. Generalized sleep decoding with basal ganglia signals in multiple movement disorders. npj Digit. Med. 7, 122 (2024).
Shute, J. B. et al. Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. Neuroimage Clin. 12, 165–172 (2016).
Mathis, M. W., Rotondo, A. P., Chang, E. F., Tolias, A. S. & Mathis, A. Decoding the brain: from neural representations to mechanistic models. Cell 187, 5814–5832 (2024).
Schneider, S., Lee, J. H. & Mathis, M. W. Learnable latent embeddings for joint behavioural and neural analysis. Nature 617, 360–368 (2023).
Lofredi, R. et al. Subthalamic stimulation impairs stopping of ongoing movements. Brain 144, 44–52 (2021).
Goede, L. L. et al. Convergent mapping of a tremor treatment network. Nat. Commun. 16, 4772 (2025).
Irmen, F. et al. Left prefrontal connectivity links subthalamic stimulation with depressive symptoms. Ann. Neurol. 87, 962–975 (2020).
Meyer, G. M. et al. Subthalamic deep brain stimulation: mapping non-motor outcomes to structural connections. Hum. Brain Mapp. 46, e70207 (2025).
Irmen, F. et al. Sensorimotor subthalamic stimulation restores risk-reward trade-off in Parkinson’s disease. Mov. Disord. 34, 366–376 (2018).
Chua, M. M. J. et al. Optimal focused ultrasound lesion location in essential tremor. Sci. Adv. 11, eadp0532 (2025).
Hollunder, B. & Horn, A. Mapping the dysfunctome provides an avenue for targeted brain circuit therapy. Nat. Neurosci. 27, 401–402 (2024).
Petersen, M. V. et al. Holographic reconstruction of axonal pathways in the human brain. Neuron 104, 1056–1064.e3 (2019).
Alho, E. J. L. et al. The ansa subthalamica: a neglected fiber tract. Mov. Disord. 35, 75–80 (2020).
Alho, E. J. L., Fonoff, E. T., Di Lorenzo Alho, A. T., Nagy, J. & Heinsen, H. Use of computational fluid dynamics for 3D fiber tract visualization on human high-thickness histological slices: histological mesh tractography. Brain Struct. Funct. 226, 323–333 (2021).
Castaño-Candamil, S. et al. A pilot study on data-driven adaptive deep brain stimulation in chronically implanted essential tremor patients. Front. Hum. Neurosci. 14, 541625 (2020).
Ferleger, B. I. et al. Fully implanted adaptive deep brain stimulation in freely moving essential tremor patients. J. Neural Eng. 17, 056026 (2020).
Benabid, A. L. et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337, 403–406 (1991).
Soh, D., Lozano, A. M. & Fasano, A. Hybrid deep brain stimulation system to manage stimulation-induced side effects in essential tremor patients. Parkinsonism Relat. Disord. 58, 85–86 (2019).
Alagapan, S. et al. Cingulate dynamics track depression recovery with deep brain stimulation. Nature 622, 130–138 (2023).
Gilron, R. et al. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson’s disease. Nat. Biotechnol. 39, 1078–1085 (2021).
Vissani, M. et al. Toward closed-loop intracranial neurostimulation in obsessive-compulsive disorder. Biol. Psychiatry 93, e43–e46 (2023).
Neudorfer, C. et al. Lead-DBS v3.0: mapping deep brain stimulation effects to local anatomy and global networks. Neuroimage 268, 119862 (2023).
Butenko, K. et al. Engaging dystonia networks with subthalamic stimulation. Proc. Natl Acad. Sci. USA 122, e2417617122 (2025).
Metzger, S. L. et al. A high-performance neuroprosthesis for speech decoding and avatar control. Nature 620, 1037–1046 (2023).
Rodriguez-Rojas, R. et al. Functional anatomy of the subthalamic nucleus and the pathophysiology of cardinal features of Parkinson’s disease unraveled by focused ultrasound ablation. Sci. Adv. 10, eadr9891 (2024).
Neumann, W. et al. A localized pallidal physiomarker in cervical dystonia. Ann. Neurol. 82, 912–924 (2017).
Meyer, G. M. et al. Deep brain stimulation for obsessive-compulsive disorder: optimal stimulation sites. Biol. Psychiatry 96, 101–113 (2024).
Hollunder, B. Neuromodulation-informed connectomics as a roadmap towards personalized brain circuit therapy in obsessive-compulsive disorder. Brain Stimul. 18, 243–244 (2025).
Horn, A. et al. Deep brain stimulation response circuits in obsessive–compulsive disorder. Biol. Psychiatry https://doi.org/10.1016/j.biopsych.2025.03.008 (2025).
Siddiqi, S. H. et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am. J. Psychiatry 177, 435–446 (2020).
Ji, G.-J. et al. A generalized epilepsy network derived from brain abnormalities and deep brain stimulation. Nat. Commun. 16, 2783 (2025).
Sheth, S. A. & Mayberg, H. S. Deep brain stimulation for obsessive-compulsive disorder and depression. Annu. Rev. Neurosci. 46, 341–358 (2023).
Merk, T. et al. Machine learning based brain signal decoding for intelligent adaptive deep brain stimulation. Exp. Neurol. 351, 113993 (2022).
Treu, S. et al. Deep brain stimulation: imaging on a group level. Neuroimage 219, 117018 (2020).
Ehlen, F. et al. Thalamic deep brain stimulation decelerates automatic lexical activation. Brain Cogn. 111, 34–43 (2017).
Neumann, W.-J. & Rodriguez-Oroz, M. C. Machine learning will extend the clinical utility of adaptive deep brain stimulation. Mov. Disord. 36, 796–799 (2021).
Mosley, P. E., et al. The structural connectivity of subthalamic deep brain stimulation correlates with impulsivity in Parkinson’s disease. Brain 143, 2235–2254 (2020).
Acknowledgements
The authors acknowledge the pioneering work by N. Rajamani and T. Merk in the development of this concept. A.H. was supported by the Schilling Foundation. W.-J.N. was funded by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) — Project-ID 424778381 — TRR 295 and the European Union (ERC, ReinforceBG, 101077060). Views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the European Union or the European Research Council. Neither the European Union nor the granting authority can be held responsible for them.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
A.H. reports lecture fees from Boston Scientific, is a consultant for Modulight.bio, was a consultant for FxNeuromodulation and Abbott in recent years, and serves as a co-inventor on a patent granted to Charité University Medicine Berlin that covers multisymptom deep brain stimulation (DBS) fibre filtering and an automated DBS parameter suggestion algorithm (patent #LU103178). W.J.N. received honoraria for consulting from INBRAIN Neuroelectronics, which is a neurotechnology company, and honoraria for talks from Medtronic, which is a manufacturer of DBS devices unrelated to this manuscript.
Peer review
Peer review information
Nature Reviews Neurology thanks P. Starr and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Horn, A., Neumann, WJ. From adaptive deep brain stimulation to adaptive circuit targeting. Nat Rev Neurol 21, 556–566 (2025). https://doi.org/10.1038/s41582-025-01131-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-025-01131-5