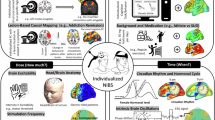
Abstract
Device-based non-invasive brain stimulation (NIBS) techniques show promise for the treatment of neurological and psychiatric disorders, although inconsistencies in protocol designs and study findings can make the field difficult to navigate. In this Review, we discuss applications of NIBS for enhancing cognitive and motor function in people with various neurological diseases that are characterized by disruption of large-scale brain networks, including neurodegenerative diseases and brain lesion disorders such as stroke and traumatic brain injury. In particular, we focus on repetitive transcranial magnetic stimulation and transcranial electrical stimulation, as these techniques have been widely used in clinical settings and randomized controlled trials. We summarize and synthesize current knowledge, and highlight gaps and shortcomings in the existing research that make it difficult to draw firm conclusions, including small sample sizes, heterogeneous patient populations and variations in stimulation protocols. We believe that a rapid evolution of NIBS techniques from state-dependent, network-informed, multifocal and subcortical paradigms to individualized electric field modelling and accelerated NIBS protocols will improve the management of neurological disorders. However, realizing this potential will require us to address crucial challenges and acquire deeper mechanistic insights, with the aim of developing adaptive, biomarker-driven protocols to optimize target engagement, dosing and timing for each patient.
Key points
-
Repetitive transcranial magnetic stimulation (rTMS) and transcranial electrical stimulation (tES), including transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS), are the most commonly used non-invasive brain stimulation (NIBS) techniques to enhance motor and cognitive functions, facilitate adaptation to deficits and promote recovery in people with neurological disorders. New technological developments are allowing non-invasive stimulation of deep brain structures.
-
In Alzheimer disease, rTMS and tES typically target either the dorsolateral prefrontal cortex or hubs of the default-mode network (for example, the precuneus). Accelerated rTMS protocols are feasible and well tolerated, producing clinically meaningful cognitive benefits.
-
In Parkinson disease, evidence is limited by the narrow range of stimulation targets, small sample sizes, heterogeneity of patient cohorts and effects of dopaminergic medication. Proof-of-concept work highlights the auditory feedback area as a target for home-based treatment for hypokinetic dysarthria.
-
In stroke, neuromodulation approaches such as TMS and tES target various cortical hubs of the motor network, including the primary and secondary motor cortices. The effects are heterogeneous, and targeting of multiple cortical or core deep structures of the motor circuitries might achieve larger, more homogeneous treatment effects.
-
In traumatic brain injury, the left dorsolateral prefrontal cortex is a key target for tDCS or rTMS to enhance cognitive function. However, evidence is limited by study heterogeneity and small sample sizes, and large-scale controlled trials are warranted to establish efficacy.
-
The rapid evolution of NIBS techniques from state-dependent, network-informed, multifocal and subcortical paradigms to individualized electric field modelling and accelerated NIBS protocols holds great promise to improve the management of neurological disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Boon, P. et al. A strategic neurological research agenda for Europe: towards clinically relevant and patient-centred neurological research priorities. Eur. J. Neurol. 31, e16171 (2024).
Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L. & Greicius, M. D. Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52 (2009).
Stam, C. J. Hub overload and failure as a final common pathway in neurological brain network disorders. Netw. Neurosci. 8, 1–23 (2024).
Murphy, K. R. et al. A practical guide to transcranial ultrasonic stimulation from the IFCN-endorsed ITRUSST consortium. Clin. Neurophysiol. 171, 192–226 (2025).
Violante, I. R. et al. Non-invasive temporal interference electrical stimulation of the human hippocampus. Nat. Neurosci. 26, 1994–2004 (2023).
Wessel, M. J. et al. Noninvasive theta-burst stimulation of the human striatum enhances striatal activity and motor skill learning. Nat. Neurosci. 26, 2005–2016 (2023).
Vassiliadis, P. et al. Non-invasive stimulation of the human striatum disrupts reinforcement learning of motor skills. Nat. Hum. Behav. 8, 1581–1598 (2024).
Beanato, E. et al. Noninvasive modulation of the hippocampal-entorhinal complex during spatial navigation in humans. Sci. Adv. 10, eado4103 (2024).
Yang, C. et al. Transcranial temporal interference stimulation of the right globus pallidus in Parkinson’s disease. Mov. Disord. 40, 1061–1069 (2025).
Piao, Y. et al. Safety evaluation of employing temporal interference transcranial alternating current stimulation in human studies. Brain Sci. 12, 1194 (2022).
Vassiliadis, P. et al. Safety, tolerability and blinding efficiency of non-invasive deep transcranial temporal interference stimulation: first experience from more than 250 sessions. J. Neural Eng. 21, 024001 (2024).
Bergmann, T. O., Karabanov, A., Hartwigsen, G., Thielscher, A. & Siebner, H. R. Combining non-invasive transcranial brain stimulation with neuroimaging and electrophysiology: current approaches and future perspectives. Neuroimage 140, 4–19 (2016).
Bradley, C., Nydam, A. S., Dux, P. E. & Mattingley, J. B. State-dependent effects of neural stimulation on brain function and cognition. Nat. Rev. Neurosci. 23, 459–475 (2022).
Hartz, S. M. et al. Assessing the clinical meaningfulness of slowing CDR-SB progression with disease-modifying therapies for Alzheimer’s disease. Alzheimers Dement. Transl. Res. Clin. Interv. 11, e70033 (2025).
Wei, N. et al. Repetitive transcranial magnetic stimulation may be superior to drug therapy in the treatment of Alzheimer’s disease: a systematic review and Bayesian network meta-analysis. CNS Neurosci. Ther. 29, 2912–2924 (2023).
Koch, G. et al. The emerging field of non-invasive brain stimulation in Alzheimer’s disease. Brain 147, 4003–4016 (2024).
Terao, I. & Kodama, W. Comparative efficacy, tolerability and acceptability of donanemab, lecanemab, aducanumab and lithium on cognitive function in mild cognitive impairment and Alzheimer’s disease: a systematic review and network meta-analysis. Ageing Res. Rev. 94, 102203 (2024).
Šimko, P., Kent, J. A. & Rektorova, I. Is non-invasive brain stimulation effective for cognitive enhancement in Alzheimer’s disease? An updated meta-analysis. Clin. Neurophysiol. 144, 23–40 (2022).
Yang, T. et al. The cognitive effect of non-invasive brain stimulation combined with cognitive training in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Alzheimers Res. Ther. 16, 140 (2024).
Rektorová, I. Non-invasive stimulation for treating cognitive impairment in Alzheimer disease. Nat. Rev. Neurol. 20, 445–446 (2024).
Moussavi, Z. et al. Repetitive transcranial magnetic stimulation as a treatment for Alzheimer’s disease: a randomized placebo-controlled double-blind clinical trial. Neurotherapeutics 21, e00331 (2024).
Lin, H. et al. Effects of accelerated intermittent theta-burst stimulation in modulating brain of Alzheimer’s disease. Cereb. Cortex 34, bhae106 (2024).
Wu, X. et al. Accelerated intermittent theta-burst stimulation broadly ameliorates symptoms and cognition in Alzheimer’s disease: a randomized controlled trial. Brain Stimul. 15, 35–45 (2022).
Tang, N., Shu, W. & Wang, H.-N. Accelerated transcranial magnetic stimulation for major depressive disorder: a quick path to relief? Wiley Interdiscip. Rev. Cogn. Sci. 15, e1666 (2024).
Wu, X. et al. Effects of a periodic intermittent theta burst stimulation in Alzheimer’s disease. Gen. Psychiatr. 37, e101106 (2024).
Jones, D. T. et al. Cascading network failure across the Alzheimer’s disease spectrum. Brain 139, 547–562 (2016).
Giorgio, J., Adams, J. N., Maass, A., Jagust, W. J. & Breakspear, M. Amyloid induced hyperexcitability in default mode network drives medial temporal hyperactivity and early tau accumulation. Neuron 112, 676–686.e4 (2024).
Krajcovicova, L., Marecek, R., Mikl, M. & Rektorova, I. Disruption of resting functional connectivity in Alzheimer’s patients and at-risk Subjects. Curr. Neurol. Neurosci. Rep. 14, 491 (2014).
Koch, G. et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage 169, 302–311 (2018).
Koch, G. et al. Precuneus magnetic stimulation for Alzheimer’s disease: a randomized, sham-controlled trial. Brain 145, 3776–3786 (2022).
Yao, Q. et al. Effect of cerebellum stimulation on cognitive recovery in patients with Alzheimer disease: a randomized clinical trial. Brain Stimul. 15, 910–920 (2022).
Chen, Y. et al. Integrated cerebellar radiomic-network model for predicting mild cognitive impairment in Alzheimer’s disease. Alzheimers Dement. 21, e14361 (2025).
Majdi, A., van Boekholdt, L., Sadigh-Eteghad, S. & Mc Laughlin, M. A systematic review and meta-analysis of transcranial direct-current stimulation effects on cognitive function in patients with Alzheimer’s disease. Mol. Psychiatry 27, 2000–2009 (2022).
Huo, L. et al. Effects of transcranial direct current stimulation on episodic memory in older adults: a meta-analysis. J. Gerontol. B 76, 692–702 (2021).
Rezakhani, S., Amiri, M., Hassani, A., Esmaeilpour, K. & Sheibani, V. Anodal HD-tDCS on the dominant anterior temporal lobe and dorsolateral prefrontal cortex: clinical results in patients with mild cognitive impairment. Alzheimers Res. Ther. 16, 27 (2024).
Šimko, P. et al. Exploring the impact of intensified multiple session tDCS over the left DLPFC on brain function in MCI: a randomized control trial. Sci. Rep. 14, 1512 (2024).
Im, J. J. et al. Effects of 6-month at-home transcranial direct current stimulation on cognition and cerebral glucose metabolism in Alzheimer’s disease. Brain Stimul. 12, 1222–1228 (2019).
Agboada, D., Mosayebi-Samani, M., Kuo, M. F. & Nitsche, M. A. Induction of long-term potentiation-like plasticity in the primary motor cortex with repeated anodal transcranial direct current stimulation — better effects with intensified protocols? Brain Stimul. 13, 987–997 (2020).
Nissim, N. R. et al. Efficacy of transcranial alternating current stimulation in the enhancement of working memory performance in healthy adults: a systematic meta-analysis. Neuromodulation 26, 728–737 (2023).
Manippa, V. et al. Cognitive and neuropathophysiological outcomes of gamma-tACS in dementia: a systematic review. Neuropsychol. Rev. 34, 338–361 (2023).
Jafari, Z., Kolb, B. E. & Mohajerani, M. H. Neural oscillations and brain stimulation in Alzheimer’s disease. Prog. Neurobiol. 194, 101878 (2020).
Gillespie, A. K. et al. Apolipoprotein E4 causes age-dependent disruption of slow gamma oscillations during hippocampal sharp-wave ripples. Neuron 90, 740–751 (2016).
Babiloni, C. et al. Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int. J. Psychophysiol. 103, 88–102 (2016).
Benussi, A. et al. Exposure to gamma tACS in Alzheimer’s disease: a randomized, double-blind, sham-controlled, crossover, pilot study. Brain Stimul. 14, 531–540 (2021).
Benussi, A. et al. Increasing brain gamma activity improves episodic memory and restores cholinergic dysfunction in Alzheimer’s disease. Ann. Neurol. 92, 322–334 (2022).
Chan, D. et al. Gamma frequency sensory stimulation in mild probable Alzheimer’s dementia patients: results of feasibility and pilot studies. PLoS ONE 17, e0278412 (2022).
Jung, Y. H. et al. Effectiveness of personalized hippocampal network–targeted stimulation in Alzheimer disease. JAMA Netw. Open 7, e249220 (2024).
Pupíková, M. et al. Physiology-inspired bifocal fronto-parietal tACS for working memory enhancement. Heliyon 10, e37427 (2024).
Anderkova, L., Eliasova, I., Marecek, R., Janousova, E. & Rektorova, I. Distinct pattern of gray matter atrophy in mild Alzheimer’s disease impacts on cognitive outcomes of noninvasive brain stimulation. J. Alzheimers Dis. 48, 251–260 (2015).
Altomare, D. et al. Home-based transcranial alternating current stimulation (tACS) in Alzheimer’s disease: rationale and study design. Alzheimers Res. Ther. 15, 155 (2023).
Benussi, A. et al. Alpha tACS improves cognition and modulates neurotransmission in dementia with Lewy bodies. Mov. Disord. 39, 1993–2003 (2024).
Grossman, M. et al. Frontotemporal lobar degeneration. Nat. Rev. Dis. Primers 9, 40 (2023).
Roheger, M. et al. Non-pharmacological interventions for improving language and communication in people with primary progressive aphasia. Cochrane Database Syst. Rev. 5, CD015067 (2024).
Lamoš, M., Morávková, I., Ondráček, D., Bočková, M. & Rektorová, I. Altered spatiotemporal dynamics of the resting brain in mild cognitive impairment with Lewy bodies. Mov. Disord. 36, 2435–2440 (2021).
Bonakdarpour, B. et al. Perturbations of language network connectivity in primary progressive aphasia. Cortex 121, 468–480 (2019).
Zhou, J. et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133, 1352–1367 (2010).
Höglinger, G. U. et al. A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria. Lancet Neurol. 23, 191–204 (2024).
Simuni, T. et al. A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research. Lancet Neurol. 23, 178–190 (2024).
Rektorova, I. Current treatment of behavioral and cognitive symptoms of Parkinson’s disease. Parkinsonism Relat. Disord. 59, 65–73 (2019).
Brabenec, L., Mekyska, J., Galaz, Z. & Rektorova, I. Speech disorders in Parkinson’s disease: early diagnostics and effects of medication and brain stimulation. J. Neural Transm. 124, 303–334 (2017).
Rektorova, I., Barrett, J., Mikl, M., Rektor, I. & Paus, T. Functional abnormalities in the primary orofacial sensorimotor cortex during speech in Parkinson’s disease. Mov. Disord. 22, 2043–2051 (2007).
Brabenec, L., Simko, P., Sejnoha Minsterova, A., Kostalova, M. & Rektorova, I. Repetitive transcranial magnetic stimulation for hypokinetic dysarthria in Parkinson’s disease enhances white matter integrity of the auditory–motor loop. Eur. J. Neurol. 30, 881–886 (2023).
Klobusiakova, P. et al. Articulatory network reorganization in Parkinson’s disease as assessed by multimodal MRI and acoustic measures. Parkinsonism Relat. Disord. 84, 122–128 (2021).
Brabenec, L. et al. Non-invasive stimulation of the auditory feedback area for improved articulation in Parkinson’s disease. Parkinsonism Relat. Disord. 61, 187–192 (2019).
Brabenec, L. et al. Non-invasive brain stimulation for speech in Parkinson’s disease: a randomized controlled trial. Brain Stimul. 14, 571–578 (2021).
Brabenec, L. et al. Short-term effects of transcranial direct current stimulation on motor speech in Parkinson’s disease: a pilot study. J. Neural Transm. 131, 791–797 (2024).
Aarsland, D., Brønnick, K., Larsen, J. P., Tysnes, O. B. & Alves, G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 72, 1121–1126 (2009).
Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement disorder society task force guidelines. Mov. Disord. 27, 349–356 (2012).
Giustiniani, A., Maistrello, L., Mologni, V., Danesin, L. & Burgio, F. TMS and tDCS as potential tools for the treatment of cognitive deficits in Parkinson’s disease: a meta-analysis. Neurol. Sci. 46, 579–592 (2025).
Pupíková, M. & Rektorová, I. Non-pharmacological management of cognitive impairment in Parkinson’s disease. J. Neural Transm. 127, 799–820 (2020).
Lee, H., Choi, B. J. & Kang, N. Non-invasive brain stimulation enhances motor and cognitive performances during dual tasks in patients with Parkinson’s disease: a systematic review and meta-analysis. J. Neuroeng. Rehabil. 21, 205 (2024).
Duan, Z. & Zhang, C. Transcranial direct current stimulation for Parkinson’s disease: systematic review and meta-analysis of motor and cognitive effects. npj Parkinsons Dis. 10, 214 (2024).
He, W., Wang, J.-C. & Tsai, P.-Y. Theta burst magnetic stimulation improves Parkinson’s-related cognitive impairment: a randomised controlled study. Neurorehabil. Neural Repair 35, 986–995 (2021).
Buard, I. et al. Transcranial magnetic stimulation does not improve mild cognitive impairment in Parkinson’s disease. Mov. Disord. 33, 489–491 (2018).
Del Felice, A. et al. Personalized transcranial alternating current stimulation (tACS) and physical therapy to treat motor and cognitive symptoms in Parkinson’s disease: a randomized cross-over trial. Neuroimage Clin. 22, 101768 (2019).
Wei, W. et al. Acute improvement in the attention network with repetitive transcranial magnetic stimulation in Parkinson’s disease. Disabil. Rehabil. 44, 7958–7966 (2022).
Pal, E., Nagy, F., Aschermann, Z., Balazs, E. & Kovacs, N. The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson’s disease: a randomized, double-blind, placebo-controlled study. Mov. Disord. 25, 2311–2317 (2010).
Aksu, S. et al. Does transcranial direct current stimulation enhance cognitive performance in Parkinson’s disease mild cognitive impairment? An event-related potentials and neuropsychological assessment study. Neurol. Sci. 43, 4029–4044 (2022).
Chung, C. L., Mak, M. K. & Hallett, M. Transcranial magnetic stimulation promotes gait training in Parkinson disease. Ann. Neurol. 88, 933–945 (2020).
Wong, P.-L., Yang, Y.-R., Huang, S.-F. & Wang, R.-Y. Effects of DLPFC tDCS followed by treadmill training on dual-task gait and cortical excitability in Parkinson’s disease: a randomized controlled trial. Neurorehabil. Neural Repair 38, 680–692 (2024).
Rufener, K. S., Oechslin, M. S., Zaehle, T. & Meyer, M. Transcranial alternating current stimulation (tACS) differentially modulates speech perception in young and older adults. Brain Stimul. 9, 560–565 (2016).
Nemcova Elfmarkova, N., Gajdos, M., Rektorova, I., Marecek, R. & Rapcsak, S. Z. Neural evidence for defective top-down control of visual processing in Parkinson’s and Alzheimer’s disease. Neuropsychologia 106, 236–244 (2017).
Rucco, R. et al. Brain networks and cognitive impairment in Parkinson’s disease. Brain Connect. 12, 465–475 (2022).
Srovnalova, H., Marecek, R. & Rektorova, I. The role of the inferior frontal gyri in cognitive processing of patients with Parkinson’s disease: a pilot rTMS study. Mov. Disord. 26, 1545–1548 (2011).
Monastero, R. et al. Transcranial random noise stimulation over the primary motor cortex in PD-MCI patients: a crossover, randomized, sham-controlled study. J. Neural Transm. 127, 1589–1597 (2020).
Madrid, J. & Benninger, D. H. Non-invasive brain stimulation for Parkinson’s disease: clinical evidence, latest concepts and future goals: a systematic review. J. Neurosci. Methods 347, 108957 (2021).
Monte-Silva, K., Liebetanz, D., Grundey, J., Paulus, W. & Nitsche, M. A. Dosage-dependent non-linear effect of L-dopa on human motor cortex plasticity. J. Physiol. 588, 3415–3424 (2010).
Zhang, W. et al. Efficacy of repetitive transcranial magnetic stimulation in Parkinson’s disease: a systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine 52, 101589 (2022).
Li, R. et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil. Neural Repair 36, 395–404 (2022).
Goodwill, A. M. et al. Using non-invasive transcranial stimulation to improve motor and cognitive function in Parkinson’s disease: a systematic review and meta-analysis. Sci. Rep. 7, 14840 (2017).
Pereira, J. B. et al. Modulation of verbal fluency networks by transcranial direct current stimulation (tDCS) in Parkinson’s disease. Brain Stimul. 6, 16–24 (2013).
Manenti, R. et al. Transcranial direct current stimulation combined with cognitive training for the treatment of Parkinson disease: a randomized, placebo-controlled study. Brain Stimul. 11, 1251–1262 (2018).
Cools, R. Dopaminergic modulation of cognitive function-implications for l-DOPA treatment in Parkinson’s disease. Neurosci. Biobehav. Rev. 30, 1–23 (2006).
GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Psychiatry 9, 137–150 (2022).
Ponsford, J. L. et al. Longitudinal follow-up of patients with traumatic brain injury: outcome at two, five, and ten years post-injury. J. Neurotrauma 31, 64–77 (2014).
Feigin, V. L. et al. Pragmatic solutions to reduce the global burden of stroke: a World Stroke Organization–Lancet Neurology Commission. Lancet Neurol. 22, 1160–1206 (2023).
Marshall, S. et al. Updated clinical practice guidelines for concussion/mild traumatic brain injury and persistent symptoms. Brain Inj. 29, 688–700 (2015).
Lefaucheur, J. P. et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin. Neurophysiol. 131, 474–528 (2020).
Antal, A. et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 128, 1774–1809 (2017).
Murase, N., Duque, J., Mazzocchio, R. & Cohen, L. G. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 55, 400–409 (2004).
Morishita, T. & Hummel, F. C. Non-invasive brain stimulation (NIBS) in motor recovery after stroke: concepts to increase efficacy. Curr. Behav. Neurosci. Rep. 4, 280–289 (2017).
Coscia, M. et al. Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain 142, 2182–2197 (2019).
Safdar, A., Smith, M. C., Byblow, W. D. & Stinear, C. M. Applications of repetitive transcranial magnetic stimulation to improve upper limb motor performance after stroke: a systematic review. Neurorehabil. Neural Repair 37, 837–849 (2023).
Savelon, E. C. J., Jordan, H. T., Stinear, C. M. & Byblow, W. D. Noninvasive brain stimulation to improve motor outcomes after stroke. Curr. Opin. Neurol. 37, 621–628 (2024).
Bornheim, S., Croisier, J. L., Maquet, P. & Kaux, J. F. Transcranial direct current stimulation associated with physical-therapy in acute stroke patients — a randomized, triple blind, sham-controlled study. Brain Stimul. 13, 329–336 (2020).
Garrido, M. M. et al. Early transcranial direct current stimulation with modified constraint-induced movement therapy for motor and functional upper limb recovery in hospitalized patients with stroke: a randomized, multicentre, double-blind, clinical trial. Brain Stimul. 16, 40–47 (2023).
Cordes, D. et al. Efficacy and safety of transcranial direct current stimulation to the ipsilesional motor cortex in subacute stroke (NETS): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet Reg. Health Eur. 38, 100825 (2024).
Schlaug, G. et al. Safety and efficacy of transcranial direct current stimulation in addition to constraint-induced movement therapy for post-stroke motor recovery (TRANSPORT2): a phase 2, multicentre, randomised, sham-controlled triple-blind trial. Lancet Neurol. 24, 400–412 (2025).
Harvey, R. L. et al. Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke the NICHE trial. Stroke 49, 2138–2146 (2018).
Luk, K. Y., Ouyang, H. X. & Pang, M. Y. C. Low-Frequency rTMS over contralesional M1 increases ipsilesional cortical excitability and motor function with decreased interhemispheric asymmetry in subacute stroke: a randomized controlled study. Neural Plast. 2022, 3815357 (2022).
Ahmed, I., Yeldan, I. & Mustafaoglu, R. The adjunct of electric neurostimulation to rehabilitation approaches in upper limb stroke rehabilitation: a systematic review with network meta-analysis of randomized controlled trials. Neuromodulation 25, 1197–1214 (2022).
Hofmeijer, J., Ham, F. & Kwakkel, G. Evidence of rTMS for motor or cognitive stroke recovery: hype or hope? Stroke 54, 2500–2511 (2023).
Lu, J. et al. Effect of intermittent theta burst stimulation on upper limb function in stroke patients: a systematic review and meta-analysis. Front. Neurol. 15, 1450435 (2024).
Tang, Z. et al. Efficacy and safety of high-dose TBS on poststroke upper extremity motor impairment: a randomized controlled trial. Stroke 55, 2212–2220 (2024).
Bian, L. et al. Effects of priming intermittent theta burst stimulation with high-definition tDCS on upper limb function in hemiparetic patients with stroke: a randomized controlled study. Neurorehabil. Neural Repair 38, 268–278 (2024).
Vink, J. J. T. et al. Continuous theta-burst stimulation of the contralesional primary motor cortex for promotion of upper limb recovery after stroke: a randomized controlled trial. Stroke 54, 1962–1971 (2023).
Barker-Collo, S. & Feigin, V. The impact of neuropsychological deficits on functional stroke outcomes. Neuropsychol. Rev. 16, 53–64 (2006).
Milosevich, E. T., Moore, M. J., Pendlebury, S. T. & Demeyere, N. Domain-specific cognitive impairment 6 months after stroke: the value of early cognitive screening. Int. J. Stroke 19, 331–341 (2024).
Draaisma, L. R., Wessel, M. J. & Hummel, F. C. Non-invasive brain stimulation to enhance cognitive rehabilitation after stroke. Neurosci. Lett. 719, 133678 (2020).
Stockbridge, M. D. et al. Transcranial direct-current stimulation in subacute aphasia: a randomized controlled trial. Stroke 54, 912–920 (2023).
Jung, I.-Y., Lim, J. Y., Kang, E. K., Sohn, H. M. & Paik, N.-J. The factors associated with good responses to speech therapy combined with transcranial direct current stimulation in post-stroke aphasic patients. Ann. Rehabil. Med. 35, 460 (2011).
Ren, J. et al. Personalized functional imaging-guided rTMS on the superior frontal gyrus for post-stroke aphasia: a randomized sham-controlled trial. Brain Stimul. 16, 1313–1321 (2023).
Biou, E. et al. Transcranial direct current stimulation in post-stroke aphasia rehabilitation: a systematic review. Ann. Phys. Rehabil. Med. 62, 104–121 (2019).
Chai, L. et al. Does SLT combined with NIBS enhance naming recovery in post-stroke aphasia? A meta-analysis and systematic review. NeuroRehabilitation 54, 543–561 (2024).
Elsner, B., Kugler, J., Pohl, M. & Mehrholz, J. Transcranial direct current stimulation (tDCS) for improving aphasia in adults with aphasia after stroke. Cochrane Database Syst. Rev. 5, CD009760 (2019).
Raymer, A. M. & Johnson, R. K. Effectiveness of transcranial direct current stimulation as an adjuvant to aphasia treatment following stroke: evidence from systematic reviews and meta-analyses. Am. J. Speech Lang. Pathol. 33, 3431–3443 (2024).
You, Y. et al. Long-term effects of transcranial direct current stimulation (tDCS) combined with speech language therapy (SLT) on post-stroke aphasia patients: a systematic review and network meta-analysis of randomized controlled trials. NeuroRehabilitation 53, 285–296 (2023).
Longley, V. et al. Non-pharmacological interventions for spatial neglect or inattention following stroke and other non-progressive brain injury. Cochrane Database Syst. Rev. 7, CD003586 (2021).
Nyffeler, T. et al. Theta burst stimulation in neglect after stroke: functional outcome and response variability origins. Brain 142, 992–1008 (2019).
Lin, R. et al. Does repetitive transcranial magnetic stimulation have a beneficial effect on improving unilateral spatial neglect caused by stroke? A meta-analysis. J. Neurol. 271, 6494–6507 (2024).
Wang, Y., Xu, N., Wang, R. & Zai, W. Systematic review and network meta-analysis of effects of noninvasive brain stimulation on post-stroke cognitive impairment. Front. Neurosci. 16, 1082383 (2022).
Gong, C. et al. Therapeutic effects of repetitive transcranial magnetic stimulation on cognitive impairment in stroke patients: a systematic review and meta-analysis. Front. Hum. Neurosci. 17, 1177594 (2023).
Li, Y. et al. The efficacy and safety of post-stroke cognitive impairment therapies: an umbrella review. Front. Pharmacol. 14, 1207075 (2023).
Li, W. et al. Improvement of poststroke cognitive impairment by intermittent theta bursts: a double-blind randomized controlled trial. Brain Behav. 12, e2569 (2022).
Liu, Y. et al. High-frequency rTMS broadly ameliorates working memory and cognitive symptoms in stroke patients: a randomized controlled trial. Neurorehabil. Neural Repair 38, 729–741 (2024).
Liu, Y. W. et al. Explore combined use of transcranial direct current stimulation and cognitive training on executive function after stroke. J. Rehabil. Med. 53, 2766 (2021).
Guggisberg, A. G., Koch, P. J., Hummel, F. C. & Buetefisch, C. M. Brain networks and their relevance for stroke rehabilitation. Clin. Neurophysiol. 130, 1098–1124 (2019).
Koch, P. J. et al. The structural connectome and motor recovery after stroke: predicting natural recovery. Brain 144, 2107–2119 (2021).
Micera, S., Caleo, M., Chisari, C., Hummel, F. C. & Pedrocchi, A. Advanced neurotechnologies for the restoration of motor function. Neuron 105, 604–620 (2020).
Hummel, F. C. et al. Controversy: noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 1, 370–382 (2008).
Di Pino, G. et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat. Rev. Neurol. 10, 597–608 (2014).
Maceira-Elvira, P. et al. Dissecting motor skill acquisition: spatial coordinates take precedence. Sci. Adv. 8, 3505 (2022).
Maceira-Elvira, P. et al. Native learning ability and not age determines the effects of brain stimulation. npj Sci. Learn. 9, 69 (2024).
Jiang, L. et al. Optogenetic inhibition of striatal GABAergic neuronal activity improves outcomes after ischemic brain injury. Stroke 48, 3375–3383 (2017).
Song, M. et al. Optogenetic stimulation of glutamatergic neuronal activity in the striatum enhances neurogenesis in the subventricular zone of normal and stroke mice. Neurobiol. Dis. 98, 9–24 (2017).
Proulx, C. E. & Hummel, F. C. Beyond the surface: advancing neurorehabilitation with transcranial temporal interference stimulation — clinical applications and future prospects. Neural Regen. Res. https://doi.org/10.4103/NRR.NRR-D-24-01573 (2025).
Wessel, M. J. et al. Multi-focal stimulation of the cortico-cerebellar loop during the acquisition of a novel hand motor skill in chronic stroke survivors. Cerebellum 23, 341–354 (2024).
Wessel, M. J. et al. Multifocal stimulation of the cerebro-cerebellar loop during the acquisition of a novel motor skill. Sci. Rep. 11, 1756 (2021).
Bevilacqua, M. et al. Pathway-dependent brain stimulation responses indicate motion processing integrity after stroke. Brain 139, 16–17 (2025).
Bevilacqua, M. et al. Single session cross-frequency bifocal tACS modulates visual motion network activity in young healthy population and stroke patients. Brain Stimul. 17, 660–667 (2024).
Raffin E. B. M. et al. Boosting hemianopia recovery: the power of interareal cross-frequency brain stimulation. Brain (in the press).
Arheix-Parras, S. et al. A systematic review of repetitive transcranial magnetic stimulation in aphasia rehabilitation: leads for future studies. Neurosci. Biobehav. Rev. 127, 212–241 (2021).
Wang, Y. et al. Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials. Ther. Adv. Chronic Dis. 14, 20406223231168754 (2023).
Grover, S., Fayzullina, R., Bullard, B. M., Levina, V. & Reinhart, R. M. G. A meta-analysis suggests that tACS improves cognition in healthy, aging, and psychiatric populations. Sci. Transl. Med. 15, eabo2044 (2023).
Schuhmann, T. et al. Transcranial alternating brain stimulation at alpha frequency reduces hemispatial neglect symptoms in stroke patients. Int. J. Clin. Health Psychol. 22, 100326 (2022).
Chen, J. et al. Efficacy of rTMS combined with cognitive training in TBI with cognition disorder: a systematic review and meta-analysis. Neurol. Sci. 45, 3683–3697 (2024).
Tsai, P. Y., Chen, Y. C., Wang, J. Y., Chung, K. H. & Lai, C. H. Effect of repetitive transcranial magnetic stimulation on depression and cognition in individuals with traumatic brain injury: a systematic review and meta-analysis. Sci. Rep. 11, 16940 (2021).
Franke, L. M. et al. Randomized trial of rTMS in traumatic brain injury: improved subjective neurobehavioral symptoms and increases in EEG delta activity. Brain Inj. 36, 683–692 (2022).
Neville, I. S. et al. Repetitive TMS does not improve cognition in patients with TBI: a randomized double-blind trial. Neurology 93, E190–E199 (2019).
Verisezan Rosu, O. et al. Cerebrolysin and repetitive transcranial magnetic stimulation (rTMS) in patients with traumatic brain injury: a three-arm randomized trial. Front. Neurosci. 17, 1186751 (2023).
Afsharipoor, M. et al. Combined transcranial direct current stimulation with occupational therapy improves activities of daily living in traumatic brain injuries: a pilot randomized clinical trial. J. Mod. Rehabil. 18, 114–120 (2024).
Motes, M. A. et al. High-definition transcranial direct current stimulation to improve verbal retrieval deficits in chronic traumatic brain injury. J. Neurotrauma 37, 170–177 (2020).
Quinn, D. K. et al. Transcranial direct current stimulation modulates working memory and prefrontal-insula connectivity after mild-moderate traumatic brain injury. Front. Hum. Neurosci. 16, 1026639 (2022).
Sacco, K. et al. Concomitant use of transcranial direct current stimulation and computer-assisted training for the rehabilitation of attention in traumatic brain injured patients: behavioral and neuroimaging results. Front. Behav. Neurosci. 10, 57 (2016).
Li, L. M. et al. Traumatic axonal injury influences the cognitive effect of non-invasive brain stimulation. Brain 142, 3280–3293 (2019).
Chiang, H. S., Motes, M., Kraut, M., Vanneste, S. & Hart, J. High-definition transcranial direct current stimulation modulates theta response during a Go-NoGo task in traumatic brain injury. Clin. Neurophysiol. 143, 36–47 (2022).
Galimberti, A., Tik, M., Pellegrino, G. & Schuler, A. L. Effectiveness of rTMS and tDCS treatment for chronic TBI symptoms: a systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 128, 110863 (2024).
Yang, Z. et al. Behavioral effects of repetitive transcranial magnetic stimulation in disorders of consciousness: a systematic review and meta-analysis. Brain Sci. 13, 1362 (2023).
Hoy, K. E. et al. A pilot investigation of repetitive transcranial magnetic stimulation for post-traumatic brain injury depression: safety, tolerability, and efficacy. J. Neurotrauma 36, 2092–2098 (2019).
Kim, W. S., Lee, K., Kim, S., Cho, S. & Paik, N. J. Transcranial direct current stimulation for the treatment of motor impairment following traumatic brain injury. J. Neuroeng. Rehabil. 16, 14 (2019).
Surendrakumar, S. et al. Neuromodulation therapies in pre-clinical models of traumatic brain injury: systematic review and translational applications. J. Neurotrauma 40, 435–448 (2023).
Middleton, A., Fritz, S. L., Liuzzo, D. M., Newman-Norlund, R. & Herter, T. M. Using clinical and robotic assessment tools to examine the feasibility of pairing tDCS with upper extremity physical therapy in patients with stroke and TBI: a consideration-of-concept pilot study. NeuroRehabilitation 35, 741–754 (2014).
Ryan, J. L., Beal, D. S., Levac, D. E., Fehlings, D. L. & Wright, F. V. Integrating transcranial direct current stimulation into an existing inpatient physiotherapy program to enhance motor learning in an adolescent with traumatic brain injury: a case report. Phys. Occup. Ther. Pediatr. 43, 463–481 (2023).
Lutkenhoff, E. S. et al. The subcortical basis of outcome and cognitive impairment in TBI: a longitudinal cohort study. Neurology 95, E2398–E2408 (2020).
Sandry, J. & Dobryakova, E. Global hippocampal and selective thalamic nuclei atrophy differentiate chronic TBI from non-TBI. Cortex 145, 37–56 (2021).
Sharp, D. J., Scott, G. & Leech, R. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 10, 156–166 (2014).
Siegel, J. S., Shulman, G. L. & Corbetta, M. Mapping correlated neurological deficits after stroke to distributed brain networks. Brain Struct. Funct. 227, 3173–3187 (2022).
Talozzi, L. et al. Latent disconnectome prediction of long-term cognitive-behavioural symptoms in stroke. Brain 146, 1963–1978 (2023).
Fox, M. D. Mapping symptoms to brain networks with the human connectome. N. Engl. J. Med. 379, 2237–2245 (2018).
Baldassarre, A., Metcalf, N. V., Shulman, G. L. & Corbetta, M. Brain networks’ functional connectivity separates aphasic deficits in stroke. Neurology 92, E125–E135 (2019).
Siddiqi, S. H., Kording, K. P., Parvizi, J. & Fox, M. D. Causal mapping of human brain function. Nat. Rev. Neurosci. 23, 361–375 (2022).
Lai, M. H. et al. Effectiveness and brain mechanism of multi-target transcranial alternating current stimulation (tACS) on motor learning in stroke patients: study protocol for a randomized controlled trial. Trials 25, 97 (2024).
Tashiro, S., Takemi, M., Yamada, S. & Tsuji, T. Synchronized application of closed-loop NMES and precision tACS in post-stroke hand rehabilitation: a protocol of neurorehabilitation trial. Ther. Adv. Chronic Dis. 15, 20406223241297396 (2024).
Sinisalo, H. et al. Multi-locus transcranial magnetic stimulation with pulse-width modulation. Brain Stimul. 18, 948–956 (2025).
Siddiqi, S. H. & Fox, M. D. Targeting symptom-specific networks with transcranial magnetic stimulation. Biol. Psychiatry 95, 502–509 (2024).
Alekseichuk, I., Turi, Z., Amador de Lara, G., Antal, A. & Paulus, W. Spatial working memory in humans depends on theta and high gamma synchronization in the prefrontal cortex. Curr. Biol. 26, 1513–1521 (2016).
Diedrich, L., Kolhoff, H. I., Bergmann, C., Bähr, M. & Antal, A. Boosting working memory in the elderly: driving prefrontal theta–gamma coupling via repeated neuromodulation. Geroscience 47, 1425–1440 (2024).
Reinhart, R. M. G. & Nguyen, J. A. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat. Neurosci. 22, 820–827 (2019).
Grover, S., Wen, W., Viswanathan, V., Gill, C. T. & Reinhart, R. M. G. Long-lasting, dissociable improvements in working memory and long-term memory in older adults with repetitive neuromodulation. Nat. Neurosci. 25, 1237–1246 (2022).
Xie, X., Hu, P., Tian, Y., Wang, K. & Bai, T. Transcranial alternating current stimulation enhances speech comprehension in chronic post-stroke aphasia patients: a single-blind sham-controlled study. Brain Stimul. 15, 1538–1540 (2022).
Antal, A. & Paulus, W. Transcranial alternating current stimulation (tACS). Front. Hum. Neurosci. 7, 317 (2013).
Yang, S., Yi, Y. G. & Chang, M. C. The effect of transcranial alternating current stimulation on functional recovery in patients with stroke: a narrative review. Front. Neurol. 14, 1327383 (2024).
Gamage, N. N. et al. Theta-gamma transcranial alternating current stimulation enhances ballistic motor performance in healthy young and older adults. Neurobiol. Aging 152, 1–12 (2025).
Grigutsch, L. S. et al. Differential effects of theta-gamma tACS on motor skill acquisition in young individuals and stroke survivors: a double-blind, randomized, sham-controlled study. Brain Stimul. 17, 1076–1085 (2024).
Zrenner, C. & Ziemann, U. Closed-loop brain stimulation. Biol. Psychiatry 95, 545–552 (2024).
Zrenner, B. et al. Brain oscillation-synchronized stimulation of the left dorsolateral prefrontal cortex in depression using real-time EEG-triggered TMS. Brain Stimul. 13, 197–205 (2020).
Lieb, A. et al. Brain-oscillation-synchronized stimulation to enhance motor recovery in early subacute stroke: a randomized controlled double-blind three- arm parallel-group exploratory trial comparing personalized, non-personalized and sham repetitive transcranial magnetic stimulation (acronym: BOSS-STROKE). BMC Neurol. 23, 204 (2023).
Mahmoud, W. et al. Brain state-dependent repetitive transcranial magnetic stimulation for motor stroke rehabilitation: a proof of concept randomized controlled trial. Front. Neurol. 15, 1427198 (2024).
Kahana, M. J. et al. Biomarker-guided neuromodulation aids memory in traumatic brain injury. Brain Stimul. 16, 1086–1093 (2023).
Nojima, I. et al. Gait-combined closed-loop brain stimulation can improve walking dynamics in Parkinsonian gait disturbances: a randomised-control trial. J. Neurol. Neurosurg. Psychiatry 94, 938–944 (2023).
Grossman, N. et al. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell 169, 1029–1041.e16 (2017).
Huang, Z. et al. Low intensity focused ultrasound stimulation in stroke: a phase I safety and feasibility trial. Brain Stimul. 18, 179–187 (2025).
Yuksel, M. M. et al. Low-intensity focused ultrasound neuromodulation for stroke recovery: a novel deep brain stimulation approach for neurorehabilitation? IEEE Open J. Eng. Med. Biol. 4, 300–318 (2023).
Cataldi, S., Stanley, A. T., Miniaci, M. C. & Sulzer, D. Interpreting the role of the striatum during multiple phases of motor learning. FEBS J. 289, 2263–2281 (2022).
Berron, D., van Westen, D., Ossenkoppele, R., Strandberg, O. & Hansson, O. Medial temporal lobe connectivity and its associations with cognition in early Alzheimer’s disease. Brain 143, 1233–1248 (2020).
Dues, D. J., Nguyen, A. P. T., Becker, K., Ma, J. & Moore, D. J. Hippocampal subfield vulnerability to α-synuclein pathology precedes neurodegeneration and cognitive dysfunction. npj Parkinsons Dis. 9, 125 (2023).
Ye, R. et al. Differential vulnerability of hippocampal subfields to amyloid and tau deposition in the Lewy body diseases. Neurology 102, e209460 (2024).
Krajcovicova, L., Klobusiakova, P. & Rektorova, I. Gray matter changes in Parkinson’s and Alzheimer’s disease and relation to cognition. Curr. Neurol. Neurosci. Rep. 19, 85 (2019).
Železníková, Ž et al. Early changes in the locus coeruleus in mild cognitive impairment with Lewy bodies. Mov. Disord. 40, 276–284 (2025).
Lamoš, M. et al. Non-invasive temporal interference stimulation of the subthalamic nucleus in Parkinson’s disease reduces beta activity. Mov. Disord. 40, 1051–1060 (2025).
Yang, C. et al. Transcranial temporal interference subthalamic stimulation for treating motor symptoms in Parkinson’s disease: a pilot study. Brain Stimul. 17, 1250–1252 (2024).
Wang, Y. et al. The safety and efficacy of applying a high-current temporal interference electrical stimulation in humans. Front. Hum. Neurosci. 18, 1484593 (2024).
Antonenko, D. et al. Cognitive training and brain stimulation in patients with cognitive impairment: a randomized controlled trial. Alzheimers Res. Ther. 16, 6 (2024).
O’Flaherty, D. & Ali, K. Recommendations for upper limb motor recovery: an overview of the UK and European rehabilitation after stroke guidelines (2023). Healthcare 12, 1433 (2024).
Lee, S. H. & Yoo, Y. J. A literature review on optimal stimulation parameters of transcranial direct current stimulation for motor recovery after stroke. Brain Neurorehabil. 17, e24 (2024).
Cole, E., O’Sullivan, S. J., Tik, M. & Williams, N. R. Accelerated theta burst stimulation: safety, efficacy, and future advancements. Biol. Psychiatry 95, 523–535 (2024).
Sonmez, A. I. et al. Accelerated TMS for depression: a systematic review and meta-analysis. Psychiatry Res. 273, 770–781 (2019).
Pastore-Wapp, M., Nyffeler, T., Nef, T., Bohlhalter, S. & Vanbellingen, T. Non-invasive brain stimulation in limb praxis and apraxia: a scoping review in healthy subjects and patients with stroke. Cortex 138, 152–164 (2021).
Pastore-Wapp, M. et al. Feasibility of a combined intermittent theta-burst stimulation and video game-based dexterity training in Parkinson’s disease. J. Neuroeng. Rehabil. 20, 2 (2023).
Lefaucheur, J. P. et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 125, 2150–2206 (2014).
Fox, M. D., Halko, M. A., Eldaief, M. C. & Pascual-Leone, A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 62, 2232–2243 (2012).
Siebner, H. R. et al. Transcranial magnetic stimulation of the brain: What is stimulated? – a consensus and critical position paper. Clin. Neurophysiol. 140, 59–97 (2022).
Liu, A. et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 9, 5092 (2018).
Reato, D., Rahman, A., Bikson, M. & Parra, L. C. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J. Neurosci. 30, 15067–15079 (2010).
Moret, B., Donato, R., Nucci, M., Cona, G. & Campana, G. Transcranial random noise stimulation (tRNS): a wide range of frequencies is needed for increasing cortical excitability. Sci. Rep. 9, 15150 (2019).
Acknowledgements
F.C.H., I.R. and M.P. were supported by a grant of the Swiss National Science Foundation (SNSF) project 320030L_197899 in cooperation with the Czech Science Foundation (GAČR) GF21-13462L (Novel individualized brain stimulation network-based approaches to improve cognition in healthy seniors and patients with mild cognitive impairment). I.R., M.P. and L.B. were supported from the Czech Ministry of Health project no. NW25-04-00142 (Non-invasive stimulation of deep brain structures for modulation of working memory) and project no. LX22NPO5107 (MEYS), financed by the European Union – Next Generation EU. F.C.H. was supported by the Defitech Foundation (NIN-PARK, Morges, Switzerland), the Bertarelli Foundation—Catalyst programme (Deep-MCI-T, Gstaad, Switzerland), the Wyss Center for Bio and Neuroengineering (the Lighthouse Partnership for AI-guided Neuromodulation, Geneva, Switzerland) and the AKIVA Foundation (nDBS-TBI, Crans-Montana, Switzerland).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, wrote the article and reviewed and/or edited the manuscript before submission. I.R. and F.C.H. contributed substantially to discussion of the content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks L. Bréchet, S. Jaberzadeh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Acetylcholinesterase inhibitors
-
A class of drugs that increase levels of the neurotransmitter acetylcholine by inhibiting its breakdown. These drugs are commonly used to treat cognitive symptoms in Alzheimer disease.
- Closed-loop NIBS
-
A non-invasive brain stimulation approach in which stimulation is adapted in real time on the basis of ongoing brain activity, thereby increasing personalization and, potentially, effectiveness of neuromodulation.
- Deep brain stimulation (DBS)
-
A neurosurgical procedure involving the implantation of electrodes into specific brain regions to modulate abnormal activity, commonly used to treat Parkinson disease.
- Default-mode network (DMN)
-
A brain network that is active during rest and is involved in self-referential thinking and mind wandering.
- Dementia Rating Scale-2
-
A clinical tool used to assess the severity of cognitive dysfunction and progression of dementia.
- Diffusion tensor imaging
-
An MRI technique that maps white matter tracts by measuring the diffusion of water molecules in tissue.
- Dorsal anterior cingulate cortex
-
A brain region involved in cognitive control, conflict monitoring and emotion regulation, forming part of the brain’s salience network.
- Dorsolateral prefrontal cortex
-
A region of the frontal lobe that is involved in executive functions such as working memory, decision-making and cognitive control.
- Frontotemporal dementia (FTD)
-
A group of disorders caused by progressive damage to the frontal and/or temporal lobes, often resulting in personality changes and language deficits.
- Functional MRI (fMRI)
-
A technique for measuring and mapping brain activity on the basis of changes in blood flow.
- Gamma-frequency stimulation
-
Stimulation in the range of ~40 Hz, linked to memory and attention and used in transcranial alternating current stimulation studies to enhance cognitive function.
- Hypokinetic dysarthria
-
A speech disorder in people with Parkinson disease, characterized by reduced voice volume, monotone speech and articulation problems.
- Long-term depression
-
A long-lasting decrease in synaptic strength, important for forgetting and learning flexibility.
- Long-term potentiation
-
A cellular mechanism comprising persistent strengthening of synapses that is considered to underlie learning and memory.
- Mild cognitive impairment (MCI)
-
A subtle but noticeable cognitive decline that is greater than expected for age but not severe enough to interfere substantially with daily life.
- Mini-Mental State Examination
-
A brief clinical test that is used to assess cognitive function and screen for dementia.
- Montreal Cognitive Assessment
-
A widely used cognitive screening test that is designed to detect mild cognitive impairment.
- Phase–amplitude coupling
-
A neural mechanism whereby the amplitude of high-frequency brain waves is modulated by the phase of lower-frequency rhythms.
- Primary motor cortex (M1)
-
A brain region that is responsible for voluntary muscle movements and represents a frequent target in motor rehabilitation and brain stimulation.
- Resting motor threshold
-
The minimum intensity of transcranial magnetic stimulation that is needed to produce a motor-evoked potential in a target muscle while the muscle is at rest. It is used to individualize stimulation parameters and ensure safety and efficacy in transcranial magnetic stimulation protocols.
- Stochastic resonance
-
A phenomenon whereby random noise enhances the brain’s ability to detect weak signals, possibly explaining the effects of transcranial random noise stimulation.
- Supplementary motor area
-
A region in the medial frontal cortex that is involved in the planning and initiation of voluntary movements.
- Theta-burst stimulation (TBS)
-
Theta-burst stimulation is a rapid form of repetitive transcranial magnetic stimulation that delivers bursts of stimulation at theta frequency to modulate cortical excitability. The bursts can be delivered intermittently (iTBS) or in a continuous train (cTBS).
- Transcranial temporal interference stimulation (tTIS)
-
A relatively new technique that uses intersecting high-frequency currents to target deep brain regions non-invasively.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rektorová, I., Pupíková, M., Fleury, L. et al. Non-invasive brain stimulation: current and future applications in neurology. Nat Rev Neurol 21, 669–686 (2025). https://doi.org/10.1038/s41582-025-01137-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-025-01137-z