Abstract
Rheumatoid arthritis (RA) is a potentially devastating autoimmune disease. The great majority of patients with RA are seropositive for anti-citrullinated protein antibodies (ACPAs), rheumatoid factors, or other autoantibodies. The onset of clinically apparent inflammatory arthritis meeting classification criteria (clinical RA) is preceded by ACPA seropositivity for an average of 3–5 years, a period that is designated as ‘at-risk’ of RA for ACPA-positive individuals who do not display signs of arthritis, or ‘pre-RA’ for individuals who are known to have progressed to developing clinical RA. Prior studies of individuals at-risk of RA have associated pulmonary mucosal inflammation with local production of ACPAs and rheumatoid factors, leading to development of the ‘mucosal origins hypothesis’. Recent work now suggests the presence of multiple distinct mucosal site-specific mechanisms that drive RA evolution. Indicatively, subsets of individuals at-risk of RA and patients with RA harbour a faecal bacterial strain that has exhibited arthritogenic activity in animal models and that favours T helper 17 (TH17) cell responses in patients. Periodontal inflammation and oral microbiota have also been suggested to promote the development of arthritis through breaches in the mucosal barrier. Herein, we argue that mucosal sites and their associated microbial strains can contribute to RA evolution via distinct pathogenic mechanisms, which can be considered causal mucosal endotypes. Future therapies instituted for prevention in the at-risk period, or, perhaps, during clinical RA as therapeutics for active arthritis, will possibly have to address these individual mechanisms as part of precision medicine approaches.
Key points
-
The onset of seropositive rheumatoid arthritis (RA) is typically preceded by the presence of RA-related autoantibodies (ACPAs, rheumatoid factors, AMPAs) in the peripheral blood for 3–5 years prior to the onset of symptoms and histologically or imaging-identified joint inflammation.
-
The period of time prior to clinical RA onset can be designated a period ‘at-risk of RA’ prospectively, or a ‘pre-RA’ period retrospectively.
-
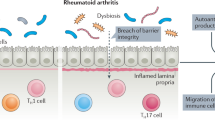
An increasing number of studies have identified immune and microbial alterations at mucosal sites in subsets of individuals at-risk of RA or with pre-RA. The primary sites studied include the lung, gut and oral or periodontal regions.
-
Each of the mucosal sites seems to elaborate unique phenotypes and mechanisms that might drive the development of pre-RA and generation of local or systemic autoantibodies. These apparent mechanistic differences underlie the causal mucosal endotype hypothesis.
-
The inter-relationships and relative timing of changes at each of these mucosal sites is not yet understood but is the object of ongoing studies in conjunction with work to understand the underlying mechanisms of loss of tolerance.
-
If confirmed, the presence of distinct causal mucosal endotypes is likely to influence the design of RA prevention trials as well as potentially underlie differences in therapeutic responses in patients with clinical RA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lee, D. M. & Weinblatt, M. E. Rheumatoid arthritis. Lancet 359, 903–911 (2001).
Gravallese, E. M. & Firestein, G. S. Rheumatoid arthritis — common origins, divergent mechanisms. N. Engl. J. Med. 388, 529–542 (2023).
Karlson, E. W. & Costenbader, K. H. Epidemiology: interpreting studies of interactions between RA risk factors. Nat. Rev. Rheum. 6, 72–73 (2010).
Karlson, E. W. & Deane, K. D. Environmental and gene-environment interactions and risk of rheumatoid arthritis. Rheum. Dis. Clin. North. Am. 38, 405–426 (2012).
Silman, A. J. Epidemiology of rheumatoid arthritis. APMIS 102, 721–728 (1994).
Peschken, C. A. & Esdaile, J. M. Rheumatic diseases in North America’s indigenous peoples. Semin. Arthritis Rheum. 28, 368–391 (1999).
Aletaha, D. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 (2010).
Arnett, F. C. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31, 314–324 (1998).
Fuchs, H. A. & Sergent, J. S. in Arthritis and Allied Conditions: A Textbook of Rheumatology. (ed Koopman W. J.) 1041–1070 (Williams and Wilkins, 1997).
Trouw, L. A., Rispens, T. & Toes, R. E. M. Beyond citrullination: other post-translational protein modifications in rheumatoid arthritis. Nat. Rev. Rheum. 13, 331–339 (2017).
Emery, P. Review of health economics modelling in rheumatoid arthritis. Pharmacoeconomics 22, 55–69 (2004).
Deane, K. D., Norris, J. M. & Holers, V. M. Pre-clinical rheumatoid arthritis: identification, evaluation and future directions for investigation. Rheum. Dis. Clin. North Am. 36, 236–241 (2010).
Malmström, V., Catrina, A. I. & Klareskog, L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat. Rev. Immunol. 17, 60–75 (2017).
Kelmenson, L. B. et al. Timing of elevations of autoantibody isotypes in rheumatoid arthritis prior to disease diagnosis. Arthritis Rheumatol. 72, 251–261 (2019).
Gerlag, D. M. et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann. Rheum. Dis. 71, 638–641 (2012).
Sokolove, J. et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS ONE 7, e35296 (2012).
Deane, K. D. et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 62, 3161–3172 (2010).
Suwannalai, P. et al. Avidity maturation of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum. 64, 1323–1328 (2012).
Brink, M. et al. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to the development of rheumatoid arthritis. Arthritis Rheum. 65, 899–910 (2013).
Whiting, P. F. et al. Systematic review: accuracy of anti-citrullinated peptide antibodies for diagnosing rheumatoid arthritis. Ann. Intern. Med. 152, 456–464 (2010).
Deane, K. D. & Holers, V. M. Rheumatoid arthritis pathogenesis, prediction, and prevention: an emerging paradigm shift. Arthritis Rheumatol. 73, 181–193 (2021).
Ponchel, F. et al. T-cell subset abnormalities predict progression along the inflammatory arthritis disease continuum: implications for management. Sci. Rep. 10, 3669 (2020).
Nepom, G. T. & Nepom, B. S. Prediction of susceptibility to rheumatoid arthritis by human leukocyte antigen phenotyping. Rheum. Dis. Clin. North. Am. 18, 785–792 (1992).
Gregersen, P. K., Silver, J. & Winchester, R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30, 1205–1213 (1987).
Reveille, J. D. The genetic contribution to the pathogenesis of rheumatoid arthritis. Curr. Opin. Rheum. 10, 187–200 (1998).
Coenen, M. J. & Gregersen, P. K. Rheumatoid arthritis: a view of the current genetic landscape. Genes. Immun. 10, 101–111 (2009).
Viatte, S., Plant, D. & Raychaudhuri, S. Genetics and epigenetics of rheumatoid arthritis. Nat. Rev. Rheum. 9, 141–153 (2013).
Ishigaki, K. et al. Multi-ancestry genome-wide association analyses identify novel genetic mechanisms in rheumatoid arthritis. Nat. Genet. 54, 1640–1651 (2022).
Lufkin, L., Budišić, M., Mondal, S. & Sur, S. A Bayesian model to analyze the association of rheumatoid arthritis with risk factors and their interactions. Front. Public. Health 9, 693830 (2021).
Terao, C., Raychaudhuri, S. & Gregersen, P. K. Recent advances in defining the genetic basis of rheumatoid arthritis. Ann. Rev. Genomics Hum. Genet. 17, 273–301 (2016).
Goldmann, K. et al. Expression quantitative trait loci analysis in rheumatoid arthritis identifies tissue specific variants associated with severity and outcome. Ann. Rheum. Dis. 83, 288–299 (2023).
Holers, V. M. Autoimmunity to citrullinated proteins and the initiation of rheumatoid arthritis. Curr. Opin. Immunol. 25, 728–735 (2013).
Holers, V. M. et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat. Rev. Rheum. 14, 542–557 (2018).
Holers, V. M. et al. Mechanism-driven strategies for the prevention of rheumatoid arthritis. Rheumatol. Autoimmun. 2, 109–119 (2022).
Kinslow, J. D. et al. Elevated IgA plasmablast levels in subjects at risk of developing rheumatoid arthritis. Arthritis Rheumatol. 68, 2372–2383 (2016).
Elliott, S. E. et al. Affinity maturation drives epitope spreading and generation of proinflammatory anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheumatol. 70, 1946–1958 (2018).
Sparks, J. A. et al. Association of fish intake and smoking with risk of rheumatoid arthritis and age of onset: a prospective cohort study. BMC Musculoskelet. Disord. 20, 2 (2019).
Klareskog, L., Malmstrom, V., Lundberg, K., Padyukov, L. & Alfredsson, L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Semin. Immunol. 23, 92–98 (2011).
Ljungberg, K. R., Martinsson, K., Wetterö, J., Svärd, A. & Kastbom, A. Circulating anti-citrullinated protein antibodies containing secretory component are prognostic for arthritis onset in at-risk patients. Clin. Exp. Immunol. 204, 344–351 (2021).
Johansson, L. et al. Concentration of antibodies against Porphyromonas gingivalis is increased before the onset of symptoms of rheumatoid arthritis. Arthritis Res. Ther. 18, 201 (2016).
Cheng, Z. et al. Dysbiosis in the oral microbiomes of anti-CCP positive individuals at risk of developing rheumatoid arthritis. Ann. Rheum. Dis. 80, 162–168 (2021).
Luo, Y. et al. Alteration of gut microbiota in individuals at high-risk for rheumatoid arthritis associated with disturbed metabolome and the initiation of arthritis through the triggering of mucosal immunity imbalance. Arthritis Rheumatol. 75, 1736–1748 (2023).
Wells, P. M. et al. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: a cross-sectional study. Lancet Rheumatol. 25, e418–e427 (2020).
Nii, T. et al. Genomic repertoires linked with pathogenic potency of arthritogenic Prevotella copri isolated from the gut of patients with rheumatoid arthritis. Ann. Rheum. Dis. 82, 621–629 (2023).
Lin, L. et al. Gut microbiota in pre-clinical rheumatoid arthritis: from pathogenesis to preventing progression. J. Autoimmun. 141, 103001 (2023).
Chriswell, M. et al. Dual IgA/IgG family autoantibodies from individuals at-risk for rheumatoid arthritis identify an arthritogenic strain of Subdoligranulum. Sci. Trans. Med. 14, eabn5166 (2022).
van Steenbergen, H. W., Cope, A. P. & van der Helm-van Mil, A. H. M. Rheumatoid arthritis prevention in arthralgia: fantasy or reality? Nat. Rev. Rheum. 19, 767–777 (2023).
Yunt, Z. X. & Solomon, J. J. Lung disease in rheumatoid arthritis. Rheum. Dis. Clin. North Am. 41, 225–236 (2015).
Rangel-Moreno, J. et al. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J. Clin. Invest. 116, 3183–3194 (2006).
Zaccardelli, A. et al. Elevated anti-citrullinated protein antibodies prior to rheumatoid arthritis diagnosis and risks for chronic obstructive pulmonary disease or asthma. Arthritis Res. Care 73, 498–509 (2019).
Kronzer, V. L., Crowson, C. S., Sparks, J. A., Vassallo, R. & Davis, J. M. III Investigating asthma, allergic disease, passive smoke exposure, and risk of rheumatoid arthritis. Arthritis Rheumatol. 71, 1217–1224 (2019).
Joshua, V. et al. Rheumatoid arthritis specific autoimmunity in the lung before and at the onset of disease. Arthritis Rheumatol. 75, 1910–1922 (2023)
Reynisdottir, G. et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann. Rheum. Dis. 75, 1722–1727 (2016).
Scher, J. U. et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome 4, 60 (2016).
Demoruelle, M. K. et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 64, 1756–1761 (2012).
Demoruelle, M. K. et al. Antibody responses to citrullinated and non-citrullinated antigens in the sputum of subjects with and at-risk for rheumatoid arthritis. Arthritis Rheumatol. 70, 516–527 (2017).
Demoruelle, M. K. et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol. 69, 1165–1175 (2017).
Willis, V. C. et al. Sputa autoantibodies in patients with established rheumatoid arthritis and subjects at-risk for future clinically apparent disease. Arthritis Rheum. 65, 2545–2554 (2013).
Okamoto, Y. et al. Association of sputum neutrophil extracellular trap subsets with IgA anti-citrullinated protein antibodies in subjects at risk for rheumatoid arthritis. Arthritis Rheumatol. 74, 38–48 (2022).
Wilson, T. et al. Sputum RA-associated autoantibodies independently associate with future development of classified RA in an at-risk cohort of individuals with systemic anti-CCP positivity. Arthritis Rheumatol. 10.1002/art.42355 (2022).
Thompson, K. N. et al. Alterations in the gut microbiome implicate key taxa and metabolic pathways across inflammatory arthritis phenotypes. Sci. Trans. Med. 15, eabn4722 (2023).
Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202 (2013).
Zhang, X. et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 21, 895–905 (2015).
Chen, J. et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 8, 43 (2016).
Pianta, A. et al. Evidence of the immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 69, 964–975 (2017).
Seifert, J. A. et al. Association of antibodies to Prevotella copri in anti-cyclic citrullinated peptide-positive individuals at risk of developing rheumatoid arthritis and in patients with early or established rheumatoid arthritis. Arthritis Rheumatol. 75, 507–516 (2023).
Alpizar-Rodriguez, D. et al. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 78, 590–593 (2019).
Tajik, N. et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 11, 1995 (2020).
Rooney, C. M. et al. Perturbations of the gut microbiome in anti-CCP positive individuals at risk of developing rheumatoid arthritis. Rheumatology 60, 3380–3387 (2021).
Eriksson, K. et al. Prevalence of periodontitis in patients with established rheumatoid arthritis: a Swedish population based case-control study. PLoS ONE https://doi.org/10.1371/journal.pone.0155956 (2016).
Li, Y. et al. The relationship between Porphyromonas gingivalis and rheumatoid arthritis: a meta-analysis. Front. Cell Infect. Microbiol. 12, 956417 (2022).
Mankia, K. et al. Prevalence of periodontal disease and periodontopathic bacteria in anti-cyclic citrullinated protein antibody-positive at-risk adults without arthritis. JAMA Netw. Open. 5, e195394 (2019).
Gómez-Bañuelos, E., Mukherjee, A., Darrah, E. & Andrade, F. Rheumatoid arthritis-associated mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J. Clin. Med. 8, 1309 (2019).
Kharlamova, N. et al. Antibodies to Porphyromonas gingivalis indicate interaction between oral infection, smoking, and risk genes in rheumatoid arthritis etiology. Arthritis Rheumatol. 68, 604–613 (2016).
Lundberg, K., Wegner, N., Yucel-Lindberg, T. & Venables, P. J. Periodontitis in RA — the citrullinated enolase connection. Nat. Rev. Rheum. 6, 727–730 (2010).
Brewer, C. et al. Oral mucosal breaks trigger unique systemic immune responses that mediate rheumatoid arthritis. Sci. Trans. Med 15, eabq8476 (2022).
Kronzer, V. L. et al. Timing of sinusitis and other respiratory tract diseases and risk of rheumatoid arthritis. Semin. Arthritis Rheum. 52, 151937 (2022).
Young, K. A. et al. Relatives without rheumatoid arthritis show reactivity to anti-citrullinated protein/peptide antibodies that are associated with arthritis-related traits: studies of the etiology of rheumatoid arthritis. Arthritis Rheum. 65, 1995–2004 (2013).
Li, G. et al. Identification and characterization of the lactating mouse mammary gland citrullinome. Int. J. Mol. Sci. 21, 2634 (2020).
Ebringer, A. & Rashid, T. Rheumatoid arthritis is caused by a Proteus urinary tract infection. APMIS 122, 363–368 (2013).
Lamacchia, C. et al. A potential role for chlamydial infection in rheumatoid arthritis development. Rheumatology 13, kead682 (2023).
Berglin, E. et al. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA-DRB1 locus antigens is strongly associated with the future onset of rheumatoid arthritis. Arthritis Res. Ther. 6, R303–R308 (2004).
Nielen, M. M. J. et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis. Arthritis Rheum. 50, 380–386 (2004).
Gan, R. W. et al. Anti-carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. J. Rheumatol. 42, 572–579 (2015).
Costenbader, K. H., Feskanich, D., Mandl, L. A. & Karlson, E. W. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am. J. Med. 119, e1–e9 (2006).
O’Neil, L. J. et al. Association of a serum protein signature with rheumatoid arthritis development. Arthritis Rheumatol. 73, 78–88 (2021).
Kissel, K. et al. Surface Ig variable domain glycosylation affects autoantigen binding and acts as threshold for human autoreactive B cell activation. Sci. Adv. 8, eabm1759 (2022).
Pfeifle, R. et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat. Immunol. 18, 104–113 (2017).
Aslam, A. et al. Emergence of proinflammatory autoreactive T-cell responses in preclinical rheumatoid arthritis. Lancet https://doi.org/10.1016/S0140-6736(14)60285-3 (2014).
Hunt, L. et al. T cell subsets: an immunological biomarker to predict progression to clinical arthritis in ACPA-positive individuals. Ann. Rheum. Dis. 75, 1884–1889 (2016).
James, E. A. et al. Multifaceted immune dysregulation characterizes individuals at-risk for rheumatoid arthritis. Nat. Commun. 14, 7637 (2023).
Takada, H. et al. Expansion of HLA-DR positive peripheral helper T and naive B cells in anticitrullinated protein antibody-positive individuals at risk for rheumatoid arthritis. Arthritis Rheumatol. 76, 1023–1035 (2024).
Chang, H. H. et al. A molecular timeline of preclinical rheumatoid arthritis defined by dysregulated PTPN22, hypercitrullination, aberrant cytokine and metabolic profiles in PBMC of at-risk individuals. J. Clin. Invest. Insight 1, e90045 (2016).
Okamato, Y. et al. Subjects at-risk for future development of rheumatoid arthritis demonstrate a PAD4- and TLR-dependent enhanced histone H3 citrullination and proinflammatory cytokine production in CD14hi monocytes. J. Autoimmun. 117, 102581 (2021).
Pattison, D. J. et al. Dietary risk factors for the development of inflammatory polyarthritis: evidence for a role of high level of red meat consumption. Arthritis Rheum. 50, 3804–3812 (2004).
Hu, Y. et al. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am. J. Clin. Nutr. 100, 959–967 (2014).
Castro-Webb, N. et al. Association of macronutrients and dietary patterns with risk of systemic lupus erythematosus in the Black Women’s Health Study. Am. J. Clin. Nutr. 114, 1486–1494 (2021).
Choi, M. Y. et al. Association of a combination of healthy lifestyle behaviors with reduced risk of incident systemic lupus erythematosus. Arthritis Rheumatol. 74, 274–283 (2022).
Gan, R. W. et al. Lower omega-3 fatty acids are associated with the presence of anti-cyclic citrullinated peptide autoantibodies in a population at risk for future rheumatoid arthritis: a nested case-control study. Rheumatology 55, 367–376 (2016).
Gan, R. W. et al. Omega-3 fatty acids are associated with a lower prevalence of autoantibodies in shared epitope-positive subjects at risk for rheumatoid arthritis. Ann. Rheum. Dis. 76, 147–152 (2017).
Gan, R. W. et al. The association between omega-3 fatty acid biomarkers and inflammatory arthritis in an anti-citrullinated protein antibody positive population. Rheumatology 56, 2229–2236 (2017).
Vanderlinden, L. A. et al. Relationship between a vitamin D genetic risk score and autoantibodies among first-degree relatives of probands with rheumatoid arthritis and systemic lupus erythematosus. Front. Immunol. 13, 881332 (2022).
Deane, K. D. et al. Genetic and environmental risk factors for rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 31, 3–18 (2017).
Parks, C. G., de Souza Espindola Santos, A., Barbhaiya, M. & Costenbader, K. H. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best. Pract. Res. Clin. Rheumatol. 31, 306–320 (2017).
Gan, R. W. et al. Relationship between air pollution and positivity of RA-related autoantibodies in individuals without established RA: a report on SERA. Ann. Rheum. Dis. 72, 2002–2005 (2013).
Zhang, J. et al. Association of combined exposure to ambient air pollutants, genetic risk, and incident rheumatoid arthritis: a prospective cohort study in the UK biobank. Environ. Health Perspect. 131, 37008 (2023).
Polinski, K. J. et al. Perceived stress and inflammatory arthritis: a prospective investigation in the studies of the etiologies of rheumatoid arthritis cohort. Arthritis Care Res. 72, 1766–1771 (2020).
Kuhn, K. A. et al. Antibodies to citrullinated proteins enhance tissue injury in experimental arthritis. J. Clin. Invest. 116, 961–973 (2006).
Catrina, A. I., Svensson, C. I., Malmström, V., Schett, G. & Klareskog, L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat. Rev. Rheum. 13, 79–86 (2017).
Corthésy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 4, 185 (2013).
Raposo, B. et al. Divergent and dominant anti-inflammatory effects of patient-derived anticitrullinated protein antibodies (ACPA) in arthritis development. Ann. Rheum. Dis. 82, 724–726 (2023).
Gomez, A. M. et al. Anti-citrullinated protein antibodies with multiple specificities ameliorate collagen antibody-induced arthritis in a time-dependent manner. Arthritis Rheumatol. 76, 181–191 (2023).
Lu, D. R. et al. T cell-dependent affinity maturation and innate immune pathways differentially drive autoreactive B cell responses in rheumatoid arthritis. Arthritis Rheumatol. 70, 1732–1744 (2018).
Kanjana, K. et al. Autoimmunity to synovial extracellular matrix proteins in patients with postinfectious Lyme arthritis. J. Clin. Invest. 133, e161170 (2023).
Lochhead, R. B., Strle, K., Arvikar, S. L., Weis, J. J. & Steere, A. C. Lyme arthritis: linking infection, inflammation, and autoimmunity. Nat. Rev. Rheum. 17, 449–461 (2021).
Rouse, J. R. et al. HLA-DR-expressing fibroblast-like synoviocytes are inducible antigen presenting cells that present autoantigens in Lyme arthritis. ACR Open Rheumatol. https://doi.org/10.1002/acr2.11710 (2024).
English, J., Patrick, S. & Stewart, L. D. The potential role of molecular mimicry by the anaerobic microbiota in the aetiology of autoimmune disease. Anaerobe 80, 102721 (2023).
Albani, S. & Carson, D. A multistep molecular mimicry hypothesis for the pathogenesis of rheumatoid arthritis. Immunol. Today 17, 466–470 (1996).
Mangalea, M. R. et al. Individuals at risk for rheumatoid arthritis harbor differential intestinal bacteriophage communities with distinct metabolic potential. Cell Host Microbe 29, 726–739 (2021).
Kongpachith, S. et al. Affinity maturation of the anti-citrullinated protein antibody paratope drives epitope spreading and polyreactivity in rheumatoid arthritis. Arthritis Rheumatol. 71, 507–517 (2019).
Wang, P. et al. A systematic assessment of MHC Class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput. Biol. 4, e100048 (2008).
Lefferts, A. R., Norman, E., Claypool, D. J., Kantheti, U. & Kuhn, K. A. Cytokine competent gut-joint migratory T cells contribute to inflammation in the joint. Front. Immunol. 13, 932393 (2022).
Jubair, W. K. et al. Modulation of inflammatory arthritis by gut microbiota through mucosal inflammation and autoantibody generation. Arthritis Rheumatol. 70, 1220–1233 (2018).
Mosconi, I. et al. Intestinal bacteria induce TSLP to promote mutualistic T-cell responses. Mucosal Immunol. 6, 1157–1167 (2013).
Thomson, C. A., Nibbs, R. J., McCoy, K. D. & Mowat, A. M. Immunological roles of intestinal mesenchymal cells. Immunol 160, 313–324 (2020).
Serhan, C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 (2014).
Polinski, K. J. et al. Association of lipid mediators with development of future incident inflammatory arthritis in an anti-citrullinated protein antibody-positive population. Arthritis Rheumatol. 73, 955–962 (2021).
Seymour, B. J. et al. Microbiota-dependent indole production stimulates the development of collagen-induced arthritis in mice. J. Clin. Invest. 134, e167671 (2023).
Dürholz, K. et al. Dietary short-term fiber interventions in arthritis patients increase systemic SCFA levels and regulate inflammation. Nutrients 12, 3207 (2020).
Kang, N., Liu, X., Haneef, K. & Liu, W. Old and new damage-associated molecular patterns (DAMPs) in autoimmune diseases. Rheumatol. Autoimmun. https://doi.org/10.1002/rai2.12046 (2022).
Holers, V. M., Carroll, M. C. & Holers Innate autoimmunity. Adv. Immunol. 86, 137–157 (2005).
Ge, Y. et al. Interdental oral hygiene interventions elicit varying compositional microbiome changes in naturally occurring gingivitis: secondary data analysis from a clinical trial. J. Clin. Periodont. https://doi.org/10.1111/jcpe.13899 (2023).
Al-Bawardy, B., Shivashankar, R. & Proctor, D. D. Novel and emerging therapies for inflammatory bowel disease. Front. Pharmacol. 12, 651415 (2021).
Demoruelle, M. K. et al. Airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 64, 1756–1761 (2012).
Klareskog, L. et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 54, 38–46 (2006).
Kinloch, A. et al. Identification of citrullinated α-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res. Ther. 7, R1421–R1429 (2005).
Zhang, F. et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature 623, 616–624 (2023).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Greiling, T. M. et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci. Transl. Med. 10, eaan2306 (2018).
Azzouz, D. F. et al. Longitudinal gut microbiome analyses and blooms of pathogenic strains during lupus disease flares. Ann. Rheum. Dis. 82, 1315–1327 (2023).
Brandsma, C.-A., Van den Berge, M., Hackett, T.-L., Brusselle, G. & Timens, W. Recent advances in chronic obstructive pulmonary disease pathogenesis: from disease mechanisms to precision medicine. J. Pathol. 250, 624–635 (2020).
Petersen, C. & Round, J. L. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 16, 1024–1033 (2014).
Kadura, W. & Raghu, G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur. Resp. Rev. 30, 210011 (2021).
Wegner, N. et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 62, 2662–2672 (2010).
Jung, H. et al. Arthritic role of Porphyromonas gingivalis in collagen-induced arthritis mice. PLoS ONE 12, e0188698 (2017).
Acknowledgements
V.M.H.’s work is supported by grants U01 AI101981, T32 AR07534, P30 AR079369, UM1 AI110503, R01 AR051394, U19 AI50864 and Rheumatology Research Foundation; K.D.D.’s work is supported by NIH P30 AR079369; K.A.K.’s work is supported by Pfizer ASPIRE, K08 DK107905 and R01 AR075033; J.M.N.’s work is supported by R01 AR081812.
Author information
Authors and Affiliations
Contributions
V.M.H. wrote the first draft of the manuscript based on concepts devised through years of interactions and discussions with all the other authors, who also all then reviewed, revised and contributed substantially to its content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Axel Finckh, Vanessa Kronzer, Renuka Nayak and Mario Zaiss for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Chronic obstructive pulmonary disease
-
Lung alveolar and airway disease characterized by tissue destruction and defective oxygenation often associated with cigarette and other deleterious environmental exposures141.
- Dysbiosis
-
Alterations of the normal composition of the microbiome at any specific site142.
- Inducible bronchus-associated lymphatic tissue
-
Immune aggregates found in the lung in the interstitial and peri-bronchial sites of patients with clinical rheumatoid arthritis, as well as other immune lung diseases49.
- Non-specific interstitial pneumonitis
-
Form of interstitial lung disease in patients with clinical rheumatoid arthritis that is characterized radiographically by predominant traction bronchiectasis and extensive ground-glass opacities with sub-pleural sparing143.
- Usual interstitial pneumonia
-
Form of interstitial lung disease rheumatoid arthritis characterized by basal predominant reticular opacities in the sub-pleural site, as well as honeycombing, sites of ground-glass opacity, and evidence of traction bronchiectasis143.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Holers, V.M., Demoruelle, K.M., Buckner, J.H. et al. Distinct mucosal endotypes as initiators and drivers of rheumatoid arthritis. Nat Rev Rheumatol 20, 601–613 (2024). https://doi.org/10.1038/s41584-024-01154-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41584-024-01154-0
This article is cited by
-
Targeting macrophage JAK3/STAT3 signaling with tectochrysin: a novel therapeutic strategy to ameliorate bone erosion and synovitis in rheumatoid arthritis
Journal of Orthopaedic Surgery and Research (2025)
-
The senescence-like activity of BMS-470539 is associated with anti-fibrotic actions in models of dermal fibrosis
Arthritis Research & Therapy (2025)
-
CXCR4-targeted PET imaging in rheumatoid arthritis: a novel approach for monitoring disease activity and therapeutic response
EJNMMI Research (2025)
-
More than a leaky gut: how gut priming shapes arthritis
Nature Reviews Rheumatology (2025)